Abstract
Background: Obstructive sleep apnea can have a negative impact on children’s and adolescents’ neurocognitive abilities and hinder their academic and adaptive progress in academic, social, and/or behavioral dimensions. In this retrospective cross-sectional study, we investigated the influence of body weight conditions and allergy status on long-term mental health, cognitive development, and quality of life in children and adolescents who snored. Methods: The study sample included 47 subjects (age range 4.1 to 15.3 years) who exhibited high levels of snoring and underwent home-based polysomnography between 2015 and 2019. Follow-up assessments (3 years on average between baseline and follow-up) entailed phone interviews with the subject’s parents/caregivers who completed three validated questionnaires investigating sleep, quality of life, and parental ratings. Results: We found a correlation between age at diagnosis and being retrospectively overweight and high levels of snoring. In addition to a higher risk of developing emotional symptoms (8.2% increase in retrospective overweight status for each unit increase in the emotional score at follow-up) and oppositional behavior (9% increase in retrospective overweight status for each unit of oppositional T points at follow-up), we also noted reduced long-term social symptoms (11% decrease in retrospective overweight status for each unit increase in the social score at follow-up) and cognitive symptoms (10.6% decrease in retrospective overweight status for each unit increase in the cognitive score at follow-up), as well as a 6.1% increase in retrospective allergy status for each unit increase in academic performance at follow-up. Conclusions: Snoring can have negative impacts on mental health and cognitive development in the long term. Early detection and intervention for neuropsychological disorders is important in children and adolescents who score high on snoring. In the long term, the effects of snoring on neuropsychological disorders may vary based on previous body weight and allergy status.
1. Introduction
The prevalence of obstructive sleep apnea syndrome (OSAS) in children varies depending on the population studied and the diagnostic criteria used. It is estimated that OSAS affects approximately 1% to 5% of children [1]. The percentage of individuals under the age of 18 who report regular heavy snoring ranges between 8% and 12%. Initial studies have estimated the prevalence of pediatric OSAS to be between 1% and 3% [2]. Sleep-disordered breathing (SDB) can lead to morbidities of the central nervous system (CNS), cardiovascular [3] and metabolic systems, body growth [4], and diminished quality of life (QoL) [5]. There is also evidence for an association between OSA and hyperactivity, inattentive behaviors, cognitive deficits, hyperactivity, and aggressive behaviors, albeit to a lesser extent in children who habitually snore [5]. OSA can have a negative effect on neurocognitive and neurological development, hindering a child’s academic and learning abilities [6]. Furthermore, SDB symptoms may adversely affect academic performance in children with overweight/obesity [7]. Behavioral functioning was noted to be worse in children originally diagnosed with SDB than controls. Nevertheless, the long-term cognitive and behavioral outcomes were found to be minimally affected by the resolution of SDB in preschool-aged children [8]. Accordingly, there is an ongoing search for clinically useful tools that can identify children at risk for developing cognitive and behavioral deficits [9].
Polysomnography (PSG), a multi-parameter sleep study performed overnight, is the gold standard for diagnosing SDB [10], while simpler tools for OSA diagnosis and screening have been developed and approved for settings with limited resources [11]. For example, questionnaires can be administered [12,13] to screen for SDB and evaluate the neuropsychological impact of OSA on children [14].
Childhood obesity is the second leading cause of snoring [15,16] and children who habitually snore are often noted to have an allergy [17]. Pediatric obesity remains an issue of global concern, affecting approximately 17% of children and adolescents in the United States [18]. In the short term, overweight children are more likely to suffer from depression, anxiety, low self-esteem, and a range of emotional and behavioral disorders [19]. Obesity in children with SDB during late childhood and adolescence is correlated with behavioral functioning, particularly inattention and learning difficulties, which ensues functional difficulties at school [20].
The increasing prevalence of allergies among children raises concern. Allergic diseases in children have significantly increased in recent years and now affect up to 35% of children [21]. Allergic rhinitis, for instance, affects approximately 400 million people worldwide [22,23]. The prevalence of wheezing in the preschool population was 23.7%. Among the children included in a study, 13.7% were classified as overweight and 5.7% as obese [24]. Allergic rhinitis appears to be present in 35% of children with primary snoring and in 6% with OSA [25]. Rhinitis can seriously disturb sleep in patients with OSA [26] and is a recognized risk factor for habitual snoring and for OSA in children [25].
Cognitive functions have been found to be affected during the pollen season in children with allergy, and the more symptoms an allergic child has, the longer the reaction time on cognitive tests [27].
Determining the differences between obese and normal-weight individuals, as well as between allergic and non-allergic children and adolescents who exhibit high levels of snoring, and understanding their long-term neuropsychological outcomes, remains a challenge. We hypothesize that overweight and allergy status have a significant impact on the long-term neuropsychological outcomes in children and adolescents who snore, with higher rates of adverse outcomes observed in those with overweight and allergies compared to those without these conditions. To address this issue, we conducted assessments at two distinct time points. The first assessment occurred during the retrospective enrollment of children, while the second assessment served as a follow-up.
2. Materials and Methods
In our retrospective cohort study, we recruited children and adolescents with a history of snoring. The study sample included both male and female Caucasian subjects who underwent cardiorespiratory PSG between 2015 and 2019 at the Department of Pediatrics, University of Verona, Italy. Recruitment was based on the availability of retrospectively collected data and the voluntary participation of the children’s parents/caregivers. Children and adolescents who retrospectively had incomplete or missing PSG recordings lasting less than 6 h, or who had comorbidities, such as neurological or neuromuscular diseases, genetic syndromes, or neuropsychiatric syndromes, were not included in the study. The protocol for clinical studies was approved by the ethics committee of our Integrated University Hospital (CESC601; 12 August 2015) Informed consent for the scientific use of data was obtained from the parents or guardians of the subjects.
2.1. Study Population
Figure 1 presents the study chart divided into two sections that illustrate the retrospective collection of clinical and medical history and the results of at-home overnight PSG recording, and the collection of telephone questionnaire responses (February to June 2021), respectively. The study sample included 47 children and adolescents with a history of snoring and had undergone at-home overnight PSG between 2015 and 2019 (Figure 1). At follow-up (February to June 2021), they were contacted via phone and they agreed to participate in the survey. The children’s or adolescents’ parents/caregivers were also asked whether the subjects also had a history of respiratory allergies.
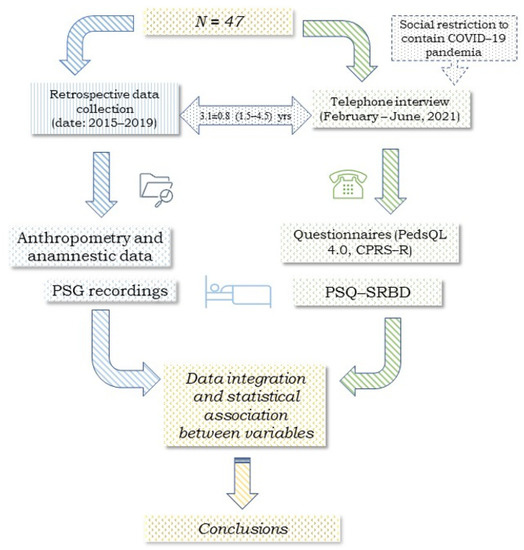
Figure 1.
Flow chart of this retrospective cross-sectional study involving 47 children and adolescents. Legend: pediatric sleep questionnaire (PSQ-SRBD), the pediatric quality of life inventory (PedsQL 4.0), and the short version of Conners’ parent rating scale-revised (CPRS-R).
2.2. Anthropometry
We retrospectively collected the height and weight of the enrolled subjects (Figure 1). They were measured using a precision medical scale (WUNDER C201, Wunder Sa.Bi. srl, Milan, Italy) and a telescopic stadiometer. Trained healthcare professionals conducted the measurements on lightly dressed subjects without shoes. Weight was recorded with an accuracy of 0.1 kg, and height with an accuracy of 0.1 cm. To calculate body mass index (BMI), BMI percentiles, and BMI z-scores, we utilized an online tool (http://www.bcm.edu/bodycomplab/BMIapp/BMI-calculator-kids.html, accessed on 30 June 2021) based on CDC growth charts for children and adolescents aged 2–19 years (https://www.cdc.gov/healthyweight/bmi/calculator.html, accessed on 30 June 2021). The subjects were categorized as underweight/normal weight when the BMI z-score was ≤0.99 and overweight/obese when it was ≥1.
2.3. Respiratory Polysomnography
We retrospectively collected the data of PSG recordings (Figure 1). The overnight PSG recordings were made using a portable ambulatory device (SOM-NOscreenTM PSG, SOMNOmedics GmbH, Randersacker, Germany) at the subjects’ home. The device continuously monitors physiological parameters, including nasal airflow through a cannula, respiratory movements of the chest and abdomen by means of belts, and oxygen saturation (SpO2) by heart rate, body position, and respiratory sounds, as previously described [14]. The children’s or adolescents’ parents/caregivers were trained to correctly use the device and taught to record the diary entries for the subjects’ bedtime, nighttime awakenings, and the child’s or adolescents’ wake-up time in the morning. The recordings were analyzed using DOMINO software (Somnomedics v. 2.6.0) by one of the investigators (MZ). MZ has experience in standardized reading and in continuous training, and follows recognized diagnostic criteria and guidelines for the interpretation of PSG recordings. The estimated total sleep time (eTST) was calculated [28]. Respiratory events were assessed following the guidelines established by the American Academy of Sleep Medicine [10]. The obstructive apnea–hypopnea index (oAHI) was defined as the sum of obstructive apneas, mixed apneas, and hypopneas divided by the eTST [28]. The ODI3% (oxygen desaturation index ≤3%) was determined by dividing the total number of desaturations by the eTST (events per hour). The average and minimum SpO2 (%) were computed automatically. Additionally, snoring events were assessed as a percentage of the eTST in the comprehensive overnight recording.
2.4. Telephone Interviews
At follow-up, the parents/caregivers of the children or adolescents were contacted via phone between February and June 2021 (Figure 1). Those who consented to participate shared their medical background, which included details about the child’s respiratory allergies and any past adenoid or tonsil surgeries that occurred after the examination. The parents/caregivers completed three validated questionnaires: the 22-item abbreviated version of the Pediatric Sleep Questionnaire (PSQ-SRBD), the Pediatric Quality of Life Inventory (PedsQL 4.0), and the abbreviated version of Conners’ Parent Rating Scale-Revised (CPRS-R).
2.5. Questionnaires
2.5.1. Pediatric Sleep Questionnaire
The PSQ is employed as a screening tool for obstructive sleep apnea syndrome (OSAS) and to evaluate the quality of life of the children and adolescents [29,30]. It consists of 22 items with a “yes,” “no,” or “do not know” response. The overall score is calculated from the percentage of affirmative (“yes”) responses. A score is considered significant if the number of positive responses exceeds 33% of the total.
2.5.2. Pediatric Quality of Life Inventory
The PedsQL is used to assess the quality of life in children and adolescents between the ages of 2 and 18 years [31,32]. It has been validated in Italian [32] and is age-related, with items categorized by age. The questionnaire consists of 23 items that investigate four domains: physical health and activity (8 items), emotions (5 items), social relationships (5 items), and school (5 items). The first 8 items pertain to physical health, while the remaining 15 investigate psychosocial health. Parents/caregivers are asked to reflect on the child’s and adolescent’s life in the month up to questionnaire administration [33,34]. A higher score corresponds to a better quality of life.
2.5.3. Conners’ Parent Rating Scales Revised
This questionnaire is utilized to evaluate potential behavioral and cognitive changes in children and adolescents aged 3 to 17 years. The areas of examination encompass hyperactivity, aggressive behavior, impulsivity, oppositional behavior, challenges in behavioral regulation, attention deficits, and modifications in working memory. Parents who complete the questionnaire should provide information about their child’s and adolescent’s behavior over the past month. Each questionnaire item has four possible responses. Scores range from 0 to 3. A score lower than 60 is not considered indicative of pathology, a score between 60 and 70 suggests pathology, and a score higher than 70 indicates pathology [35].
The questionnaire also investigates four conditions: oppositional behavior, cognitive difficulty, hyperactivity, and attention-deficit hyperactivity disorder (ADHD) index. Oppositional behavior is related to rule-breaking behaviors, problems with authority figures, and easy irritability. Cognitive difficulty concerns children and adolescents with learning problems, organization, task completion, and concentration. Hyperactivity concerns subjects with difficulty sitting for long periods and who are restless and impulsive. Finally, the CPRS-R is a screening tool to identify children and adolescents at risk for ADHD. The raw scores obtained from the questionnaire undergo standardization by calculating the T variable, enabling comparison with reference values from the general population. The profile sheet utilized for scoring and the subsequent conversion into standardized T scores takes into account gender diversity. Higher T scores indicate more pronounced behavioral difficulties within each specific category [36].
2.6. Statistical Analysis
Statistical analysis compared differences between the two groups of subjects (dependent variables) according to retrospective body weight status categorized as normal weight and overweight (including obesity) and allergy status (yes, and no). Independent variables (or covariate) were as follows: age at PSG recording, years; retrospective body weight status, normal or overweight; allergy status, no or yes; oAHI; ODI; minimum SpO2; SpO2 time < 90%; snoring (% TST); age at follow-up; follow-up time; and PSQ (%); physical score; emotional score; psychosocial score; total QoL; oppositional behavior (T); cognitive disorders (T); hyperactivity (T); and ADHD index (T) at follow-up.
The data present the number of subjects in each group, followed by descriptive statistics (mean, standard deviation, minimum and maximum) for each variable. The non-parametric Mann–Whitney U test was used to compare the differences between the two groups. The chi-square test was used for categorical variables and Fisher’s exact test was used if values were <5. The p-value was reported for each outcome measure. A p-value < 0.05 was considered statistically significant. Of note is that p-values from 0.05 to 0.1 in a study with a relatively small sample size may require more careful and cautious evaluation. Since they can lead to greater uncertainty and a higher risk of type I errors [37,38] they are discussed in the text and highlighted in green in the tables.
The results of binary logistic regression analysis of retrospective weight status (normal weight versus overweight) and allergy status (yes, and no) are presented in a table. The T column presents the t-test values for the independent variable in the model, while the S.E. column is the standard error for that variable. The Wald column presents the Wald test statistic, which is used to test the null hypothesis that the independent variable is not associated with the dependent variable. The Significance (p) column presents the test’s significance level, i.e., the probability that the observed association between the independent and the dependent variables is random. The Exp(B) column presents the odds ratio for the independent variable, i.e., the percentage increase in the odds of the dependent variable associated with a unit increase in the independent variable. Finally, the 95% CI for B (lower limit) and 95% CI for B (upper limit) columns present the 95% confidence intervals for the odds ratio of the independent variable.
The data was documented in a Microsoft® Excel® database compatible with Windows 11 and subjected to statistical analysis using IBM SPSS version 22.0 specifically designed for Windows operating system. (IBM Corp., 2013, Armonk, NY, USA). Figures were implemented using IBM SPSS version 22.0 for Windows (IBM Corp.).
Graphics were created by drawing on the mean scores of PSQ positivity, physical, emotional, social, academic performance, psychosocial, total QoL, oppositional behavior, cognitive disorders, hyperactivity, and ADHD index at follow-up of the children and adolescents divided by retrospective body weight category and allergy status.
3. Results
Table 1 summarizes the retrospective clinical data of enrolled subjects. In particular, the table shows mean, standard deviation (SD), and range for the variables age, weight, height, BMI, BMI Z-score, and BMI percentile for the four groups based on weight category (Figure 2): normal weight (n = 29) and overweight (n = 18), and on allergy status (Figure 3): non-allergic (n = 34) and allergic (allergic rhinitis, n = 13). No statistically significant differences were found for sex distribution between the four groups. There was a difference in age at PSG recording between the retrospective normal weight and the overweight status, with a higher average noted for the overweight group. There were no differences between the retrospective non-allergic and the allergic groups in age at PSG, and retrospective weight, height, BMI, BMI z-score, and BMI percentile. The retrospective higher mean body weight and height in the allergic group compared to the non-allergic group approached statistical significance (73.1 vs. 54.2) (highlighted in green).

Table 1.
This table shows the descriptive statistics of clinical data collected retrospectively. It represents the mean, standard deviation, minimum and maximum for the variables of interest in the two groups of subjects who snore categorized by retrospective body weight (normal weight vs. overweight) and allergy status (non-allergic vs. allergic). Legend: BMI, body mass index. Legend: orange box = p < 0.05; green box = 0.05 < p < 0.1.
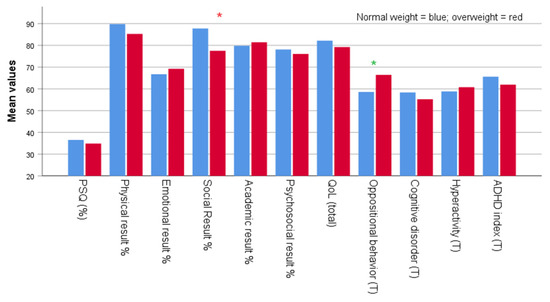
Figure 2.
Mean scores for PSQ positivity, physical, emotional, social, academic performance, psychosocial, total QoL, oppositional behavior, cognitive disorders, hyperactivity, and ADHD index at follow-up according to retrospective body weight status. Legend: * = p < 0.05; * = 0.05 < p < 0.1.
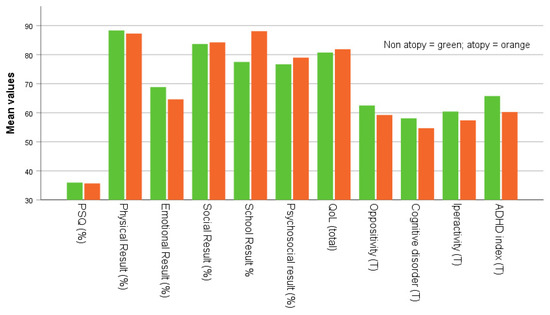
Figure 3.
Mean PSQ positivity scores, physical, emotional, social, academic performance, psychosocial, total QoL, oppositional behavior, cognitive disorders, hyperactivity, and ADHD index at follow-up in subject with history of non-allergic and allergic status.
Table 2 presents the Mann–Whitney U test of retrospective PSG data for the two groups: normal weight (n = 29) and overweight (n = 18) as mean, SD, and range. Overall, there were a few differences between the two groups: a lower mean duration of retrospective PSG data for the overweight compared to the normal weight group (9.1 ± 0.7 h vs. 9.4 ± 1.0 h.) (highlighted in green) and no differences in retrospective OSA, oAHI, and ODI, although the mean oAHI (3.9 ± 3.3 events/h vs. 5.5 ± 6.8 events/h) and the ODI (2.8 ± 2.5 events/h vs. 3.5 ± 4.9) were slightly lower in the retrospective overweight compared to the normal weight status. Furthermore, there was no significant difference in the percentage of time spent retrospectively with SpO2 < 90% during the eTST: lower mean minimum SpO2 (86.6 ± 10.1% and 89.4 ± 4.5%) in the overweight and the normal weight group, respectively. Finally, the retrospectively percentage of time spent snoring was slightly higher in the retrospective overweight compared to the normal weight status (1.7 ± 5.0% vs. 1.5 ± 4.4%). Finally, there were no significant differences between the non-allergic and the allergic group.

Table 2.
Statistical analysis comparing the retrospective PSG recordings between two groups categorized by retrospective body weight (normal weight and overweight) and allergy (non-allergic and allergic, allergic rhinitis) status. The data indicate the number of subjects in each group, followed by descriptive statistics (mean, SD, and range) for the baseline variables of interest (eTST, estimated total sleep time; h, hours; OA, obstructive apnea; oAHI, obstructive apnea–hypopnea index; ODI, oxygen desaturation index; SD, standard deviation). Legend: green box = 0.05 < p < 0.1.
Table 3 presents a comparison between child’s and adolescent’s age at follow-up, length of follow-up, PSQ positivity, physical, emotional, social, and academic performance, psychosocial and total quality of life, oppositional behavior, cognitive disorders, hyperactivity, and ADHD index as mean (SD) and range for each group at follow-up. The analysis was conducted on two independent sample groups: 29 were normal weight and 18 were overweight subjects. There was a significantly higher follow-up age in the retrospectively overweight compared to the normal weight status (10.1 ± 2.5 vs. 8.2 ± 2.6) years, respectively (p < 0.001). There were no significant differences in PSQ positivity (%) at follow-up. The length of follow-up was slightly shorter for the retrospective overweight than the normal weight status (3.0 ± 0.9 vs. 3.2 ± 0.7 years) (highlighted in green). At follow-up, there were no statistically significant differences between the two groups for physical, emotional, psychosocial, and total quality of life outcomes at follow-up. The subjects who were normal weight, scored significantly higher on social variables compared to the subjects who were overweight (87.8 ± 16.2 vs. 77.5 ± 20.1) (p = 0.039), while the subjects who were overweight scored higher on oppositional behavior as measured based on the T score (66.4 ± 15.1 vs. 58.6 ± 13.4) (highlighted in green) at follow-up. There were no significant differences in cognitive disorders, hyperactivity, and ADHD index between the two groups at follow-up.

Table 3.
The number of subjects in each group (history of normal weight versus overweight status and not allergic versus allergic status) and descriptive statistics, including mean, SD, range for the variables of interest at follow-up (questionnaires result). The Mann–Whitney U test was used to compare differences between the compared groups. Legend: orange box = p < 0.05; green box = 0.05 < p < 0.1.
Furthermore, we found no significant differences between subjects that retrospectively declared the non-allergic and the allergic status for other variables (age and length of follow-up, PSQ positivity, physical, emotional, social, psychosocial, academic results, total QoL, oppositional behavior, cognitive disorders, hyperactivity, and ADHD index) at follow-up. This lack of difference suggests that previous respiratory allergies did not appear to affect long term quality of life or neuropsychological functions differently at follow-up in these children and adolescent.
Table 4 presents the results of a binary logistic regression analysis in which the independent variable was retrospective normal body weight (0) and overweight (1) condition. The model included two groups of variables: (1) retrospective age at PSG recording, oAHI, ODI, minimum SpO2 saturation, mean SpO2, time of SpO2 < 90%, and snoring (% of total sleep time); and (2) age at follow-up, follow-up length, physical, emotional, psychosocial, and total quality of life outcomes, oppositional behavior, cognitive disorders, hyperactivity, and ADHD score index at follow-up.

Table 4.
Binary logistic regression analysis. The “T” column presents the t-test value for the independent variable in the model, while the “S.E.” column presents the standard error for that variable. The “Wald” column presents the Wald test statistic, which was used to test the null hypothesis that the independent variable was not associated with the dependent variable. The “p-value” column presents the test significance, i.e., the probability that the observed association between the independent and the dependent variables is random. The “Exp(B)” column presents the odds ratio for the independent variable, i.e., the percentage increase in the odds of the dependent variable associated with a unit increase in the independent variable. Finally, the “95% CI for B (Lower Bound)” and the “95% CI for B (Upper Bound)” columns present the 95% confidence intervals for the odds ratio of the independent variable. Legend: orange box = p < 0.05; green box = 0.05 < p < 0.1.
There was a significant and positive association between age at PSG recording and the retrospective body weight category, indicating an increased likelihood of being overweight with increasing age at PSG recording. The Exp(B) was 1.358, which means that for each year older at PSG recording, the likelihood of being obese increased by 35.8%. Furthermore, the 95% confidence interval for Exp(B) was 1.055 to 1.747, suggesting that we can be reasonably confident that the actual Exp(B) value falls within this range with 95% confidence.
The associated odds ratio for the emotional disorders at follow-up was 1.082, indicating that for each unit increase in emotional disorder at follow-up, the likelihood of being retrospectively obese increased by approximately 8.2% compared to being retrospectively normal weight. For the oppositional behavior T points at follow-up, the associated odds ratio was 1.090, indicating that for each unit increase in oppositional behavior T point at follow-up, the likelihood of being retrospectively obese increased by approximately 9% compared to the retrospective normal weight status. The associated odds ratio for the social disorder was 0.890 at follow-up, indicating that for each unit increase in social disorders at follow-up, the likelihood of being retrospectively overweight decreased by 11%. The odds ratio for the cognitive disorder T scores was 0.894 at follow-up, indicating that for each unit increase in cognitive outcomes at follow-up, the likelihood of being retrospectively overweight decreased by 10.6%. Finally, the likelihood of being retrospectively allergic approached statistical significance in children and adolescents, scoring retrospectively high on body weight and snoring (p = 0.090) status.
There was no significant association between emotional disorders at follow-up and retrospective allergy status (p = 0.070), whereas there was a significant association between allergy status and school performance at follow-up (p = 0.032). The odds ratio for school performance at follow-up was 1.061, indicating that for each unit increase in school performance at follow-up, the odds of retrospective allergic status increased by 6.1%. The confidence intervals of the odds ratio for both variables do not encompass 1, indicating that these data are statistically significant.
4. Discussion
We specifically evaluated the influence of body weight and allergy history on the mental health, cognitive development, and quality of life in children and adolescents who snore. This approach allowed us to examine the potential associations and outcomes over time in relation to snoring, body weight, and allergy status. Our study findings have clinical implications for assessing the mental health and cognitive performance at follow-up of children and adolescents who scored high on retrospective snoring and differed in body weight and allergy status. The associations between neuropsychological disorders at follow-up and retrospective body weight and allergy status may help to better understand the mechanisms underlying SDB and identify treatments that may improve the mental and cognitive health of the affected individuals. Finally, snoring can have negative impacts on mental health and cognitive development in the long term. Early detection and intervention for neuropsychological disorders is important in children and adolescents who snore. In the long term, the effects of snoring on neuropsychological disorders may vary based on previous body weight and allergy status.
No significant differences were found for baseline PSG respiratory parameters or PSQ (%) scores at follow-up between the groups scoring high on snoring (i.e., retrospectively normal weight and overweight status, and allergic and non-allergic status). The age at follow-up was significantly older for the retrospectively overweight and snoring group compared to the normal weight group. Unfortunately, we do not have anthropometric data at the follow-up. However, the age at follow-up was significantly older in the retrospectively overweight group compared to the normal weight and snoring group. This difference suggests that subjects with retrospectively overweight status and high snoring scores may be older at the follow-up assessment. The absence of anthropometric data at the follow-up precludes drawing definitive conclusions about the physical differences between the groups at that specific time point.
There were minor differences in neurocognitive scores at follow-up between the overweight subjects retrospectively scoring high on snoring (emotional and oppositional disorders) and the normal weight subjects (social and cognitive disorders). Additionally, significant differences were found between the two groups that retrospectively scored high on snoring, regardless of allergy status, in terms of school performance at follow-up, which showed a significant association with allergy status.
Children with SDB are noted to show deficits in neurocognitive performance, behavioral impairment, and school performance [39]. Previous studies report worse school performance, lower neurocognitive test scores, and behavioral abnormalities in children with SDB [9]. Children with a higher AHI were found to have more impairments than those with a lower AHI, indicating a reduction in grey matter and a dose–response impact of SDB [6].
A systematic review found a negative association between obesity and neurocognitive functions, such as executive functions, attention, visuospatial performance, and motor skills [40]. Childhood obesity has been associated with a lower ability to modulate the executive function network that supports visuospatial attention [41]. The BMI mediates the relationship between environmental degradation and reduction in global cortical and prefrontal volume, as well as the performance on cognitive tasks, such as working memory and cognitive flexibility [42]. Students with severe obesity show a delay in cognitive functions compared to those with a normal BMI. Reductions in cognitive function related to attention, memory, intelligence, and cognitive flexibility were observed in children with severe obesity [43].
Adolescents with OSAS were noted to show worse executive function and attention and were at greater risk of depression and externalizing symptoms compared to non-obese controls. Obese adolescents with OSAS may present significant neurobehavioral deficits that may persist into adulthood [44]. Adolescents with obesity and OSAS were found to have worse executive function than the normative sample. A strong association between obesity, OSAS, and cognitive impairment has been reported [45]. In addition, the severity of SDB may affect brain health and school performance in overweight children. A previous study suggested that the severity of SDB may impact school performance in overweight children but does not appear to affect brain structure [7].
High BMI and younger age at the time of testing are the two factors that may negatively affect outcomes in some domains [46]. In our study, emotional and oppositional behavior (T-scores) were significantly associated with overweight in children and adolescent scoring high on snoring. The two variables, social outcomes and cognitive disorders, were inversely associated with the condition, i.e., they were significantly associated with normal weight.
Previous studies have suggested a correlation between inhalant allergy and various neurocognitive functions in children scoring high on snoring. Multiple pediatric studies, based on surveys of parents or teachers, have found an association between SDB in childhood and aggressive behavior, impulsivity, hyperactivity, and decreased attention [39]. Inhalant allergy has emerged as one of the most critical risk factors for habitual snoring in children. It seems to increase the risk of OSA, with a significant and independent association between the severity of inhalant allergy and the severity of pediatric OSA [25].
In a cross-sectional study, the total cognitive function composite score among children who habitually snored was significantly lower. When adjusted for demographic, anthropometric, and socioeconomic characteristics, the association was substantially attenuated [47]. Our findings suggest that an increase in BMI percentile in the allergic group could constitute a risk factor for OSA. Furthermore, school performance at follow-up was significantly associated with retrospective allergy status, which could be helpful in assessing the quality of life of allergic patients in terms of their academic performance. In contrast, our study found no significant associations between emotional disorders at follow-up and retrospective allergy status.
This study has some limitations, including the small sample size, which may affect the validity and generalizability of the results. In addition, there may have been a difference in participant selection or follow-up duration between the two groups. Nonetheless, we were able to test the associations between neurobehavioral disorders and body weight, which is relevant for identifying interventions that may improve the mental and cognitive health of children and adolescents with SDB.
Various studies have examined neuropsychological and behavioral functioning in children with SDB [48,49,50] or snoring [47,51], through questionnaire-based designs [52] or before and after adenotonsillectomy [53]. Our retrospective cohort study addressed some additional key points in the current literature. Firstly, children and adolescents who scored high on snoring and had a history of overweight may develop emotional and oppositional symptoms, whereas normal weight children scoring high on snoring may exhibit more social and cognitive symptoms. Secondly, school performance at follow-up was significantly associated with a history of allergy in children and adolescents who scored high on snoring. Finally, in the long term, the effects of snoring on neuropsychological disorders may vary based on the history of overweight and allergy status.
In summary, we observed differences in neuropsychological disorders at follow-up between children and adolescents with retrospectively classified overweight status and those with normal weight who scored high on snoring. Emotional and oppositional symptoms at follow-up were associated with being overweight. Social outcomes and cognitive disorders variables at follow-up were inversely associated with retrospectively classified overweight status, meaning they were associated with normal body weight. Lastly, no differences were found in the quality of life and neuropsychological functions at follow-up between children who scored high on retrospective snoring and those with allergic rhinitis, although it is worth noting that such symptoms in academic settings were significantly associated with allergy.
5. Conclusions
Our study findings may have practical implications in the clinical setting. We found a correlation between age at diagnosis of SDB and obesity history in children and adolescents scoring high on snoring. Retrospective obesity status associated with snoring may become increasingly challenging to manage as affected subjects grow older. Additionally, retrospective overweight status may be at greater risk of developing emotional and oppositional symptoms, whereas normal weight status scoring high on snoring may exhibit more social and cognitive symptoms at follow-up. Furthermore, assessing the quality of life at follow-up in subjects scoring high on retrospective allergy status and snoring may be helpful, especially regarding academic performance at follow-up. These findings suggest early detection and intervention for neuropsychological disorders in children and adolescents scoring high on snoring, as snoring may negatively impact their mental health and cognitive development, though the effects may vary based on body weight and allergy status.
Author Contributions
Conceptualization, M.Z. and A.P.; methodology, L.Z. and L.T.; software, M.P.; validation, M.L.C., L.N. and G.P.; formal analysis, M.Z.; investigation, M.Z. and L.Z.; data curation, M.P.; writing—original draft preparation, M.Z., G.F. and L.T.; writing—review and editing, A.P., M.L.C., G.F. and L.N.; supervision, G.P. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Verona and Rovigo at the Integrated University Hospital (CESC601; 12 August 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data is unavailable due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brunetti, L.; Rana, S.; Lospalluti, M.L.; Pietrafesa, A.; Francavilla, R.; Fanelli, M.; Armenio, L. Prevalence of obstructive sleep apnea syndrome in a cohort of 1207 children of Southern Italy. Chest 2001, 120, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Lee, J.H.; Chan, A. Pediatric obstructive sleep apnea syndrome. Arch. Pediatr. Adolesc. Med. 2005, 159, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J. Hypertens. 2016, 35, 125–131. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. Beyond the growth delay in children with sleep-related breathing disorders: A systematic review. Panminerva Med. 2020, 62, 164–175. [Google Scholar] [CrossRef]
- Capdevila, O.S.; Kheirandish-Gozal, L.; Dayyat, E.; Gozal, D. Pediatric obstructive sleep apnea: Complications, management, and long-term outcomes. Proc. Am. Thorac. Soc. 2008, 5, 274–282. [Google Scholar] [CrossRef]
- Hunter, S.J.; Gozal, D.; Smith, D.L.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am. J. Respir. Crit. Care Med. 2016, 194, 739–747. [Google Scholar] [CrossRef]
- Torres-Lopez, L.V.; Cadenas-Sanchez, C.; Migueles, J.H.; Esteban-Cornejo, I.; Molina-Garcia, P.; Hillman, C.; Catena, A.; Ortega, F.B. Does sleep-disordered breathing add to impairments in academic performance and brain structure usually observed in children with overweight/obesity? Eur. J. Pediatr. 2022, 181, 2055–2065. [Google Scholar] [CrossRef]
- Biggs, S.N.; Walter, L.M.; Jackman, A.R.; Nisbet, L.C.; Weichard, A.J.; Hollis, S.L.; Davey, M.J.; Anderson, V.; Nixon, G.M.; Horne, R.S. Long-Term Cognitive and Behavioral Outcomes following Resolution of Sleep Disordered Breathing in Preschool Children. PLoS ONE 2015, 10, e0139142. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children 2022, 9, 1278. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Villa, M.P.; Pietropaoli, N.; Supino, M.C.; Vitelli, O.; Rabasco, J.; Evangelisti, M.; Del Pozzo, M.; Kaditis, A.G. Diagnosis of Pediatric Obstructive Sleep Apnea Syndrome in Settings With Limited Resources. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 990–996. [Google Scholar] [CrossRef]
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Dillon, J.E.; Guire, K.E. Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Incerti Parenti, S.; Fiordelli, A.; Bartolucci, M.L.; Martina, S.; D’Antò, V.; Alessandri-Bonetti, G. Diagnostic accuracy of screening questionnaires for obstructive sleep apnea in children: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101464. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Ferrante, G.; Zoccante, L.; Ciceri, M.L.; Nosetti, L.; Tenero, L.; Piazza, M.; Piacentini, G. Predictive Power of Oxygen Desaturation Index (ODI) and Apnea-Hypopnea Index (AHI) in Detecting Long-Term Neurocognitive and Psychosocial Outcomes of Sleep-Disordered Breathing in Children: A Questionnaire-Based Study. J. Clin. Med. 2023, 12, 3060. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, S.L.; Van Gaal, L.; De Backer, W.; Desager, K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med. Rev. 2008, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vlastos, I.M.; Hajiioannou, J.K. Clinical practice: Diagnosis and treatment of childhood snoring. Eur. J. Pediatr. 2010, 169, 261–267. [Google Scholar] [CrossRef] [PubMed]
- McColley, S.A.; Carroll, J.L.; Curtis, S.; Loughlin, G.M.; Sampson, H.A. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest 1997, 111, 170–173. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef]
- Beebe, D.W.; Ris, M.D.; Kramer, M.E.; Long, E.; Amin, R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep 2010, 33, 1447–1456. [Google Scholar] [CrossRef]
- Chad, Z. Allergies in children. Paediatr. Child Health 2001, 6, 555–566. [Google Scholar] [CrossRef]
- Nur Husna, S.M.; Tan, H.T.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 2022, 9, 874114. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Volkmer, R.; Burton, A. Association between asthma symptoms and obesity in preschool (4–5 year old) children. J. Asthma 2009, 46, 362–365. [Google Scholar] [CrossRef]
- D’Elia, C.; Gozal, D.; Bruni, O.; Goudouris, E.; Meira, E.C.M. Allergic rhinitis and sleep disorders in children—Coexistence and reciprocal interactions. J. Pediatr. 2022, 98, 444–454. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Ge, S.; Gu, Y.; Ding, X.; Zhang, Y.; Ye, J.; Zhang, L. Allergic and Non-Allergic Rhinitis Are Common in Obstructive Sleep Apnea but Not Associated With Disease Severity. J. Clin. Sleep Med. 2017, 13, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Papapostolou, G.; Kiotseridis, H.; Romberg, K.; Dahl, Å.; Bjermer, L.; Lindgren, M.; Aronsson, D.; Tunsäter, A.; Tufvesson, E. Cognitive dysfunction and quality of life during pollen season in children with seasonal allergic rhinitis. Pediatr. Allergy Immunol. 2021, 32, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dehlink, E.; Tan, H.L. Update on paediatric obstructive sleep apnoea. J. Thorac. Dis. 2016, 8, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Ward, T.M.; Chen, M.L.; Landis, C.A.; Ringold, S.; Beebe, D.W.; Pike, K.C.; Wallace, C.A. Congruence between polysomnography obstructive sleep apnea and the pediatric sleep questionnaire: Fatigue and health-related quality of life in juvenile idiopathic arthritis. Qual. Life Res. 2017, 26, 779–788. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Hullmann, S.E.; Ryan, J.L.; Ramsey, R.R.; Chaney, J.M.; Mullins, L.L. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res. 2011, 63 (Suppl. S11), S420–S430. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; AbdollahiFakhim, S.; Lotfi, A.; Bayazian, G.; Sohrabpour, M.; Hemmatjoo, T. Effect of adenotonsillectomy on ADHD symptoms of children with adenotonsillar hypertrophy and sleep disordered breathing. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1213–1217. [Google Scholar] [CrossRef]
- Bergeron, M.; Duggins, A.L.; Cohen, A.P.; Ishman, S.L. Comparison of Patient- and Parent-Reported Quality of Life for Patients Treated for Persistent Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2018, 159, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Isaiah, A.; Shikara, M.; Pereira, K.D.; Das, G. Refining screening questionnaires for prediction of sleep apnea severity in children. Sleep Breath 2020, 24, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.; Epstein, J.N. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998, 26, 257–268. [Google Scholar] [CrossRef]
- Dekking, F.M. A Modern Introduction to Probability and Statistics: Understanding Why and How; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, p values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Trosman, I.; Trosman, S.J. Cognitive and Behavioral Consequences of Sleep Disordered Breathing in Children. Med. Sci. 2017, 5, 30. [Google Scholar] [CrossRef]
- Liang, J.; Matheson, B.E.; Kaye, W.H.; Boutelle, K.N. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int. J. Obes. 2014, 38, 494–506. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chen, F.C.; Pan, C.Y.; Tseng, Y.T. The Neurocognitive Performance of Visuospatial Attention in Children with Obesity. Front. Psychol. 2016, 7, 1033. [Google Scholar] [CrossRef]
- Dennis, E.; Manza, P.; Volkow, N.D. Socioeconomic status, BMI, and brain development in children. Transl. Psychiatry 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Altuwaym, A.A.; Alfallaj, R.M.; Alduraibi, K.A.; Alhamoudi, A.M.; Alghamdi, S.M.; Akram, A. Effect of Obesity on Cognitive Function among School Adolescents: A Cross-Sectional Study. Obes. Facts 2019, 12, 150–156. [Google Scholar] [CrossRef]
- Konstantinopoulou, S.; Tapia, I.E.; Kim, J.Y.; Xanthopoulos, M.S.; Radcliffe, J.; Cohen, M.S.; Hanna, B.D.; Pipan, M.; Cielo, C.; Thomas, A.J.; et al. Relationship between obstructive sleep apnea cardiac complications and sleepiness in children with Down syndrome. Sleep Med. 2016, 17, 18–24. [Google Scholar] [CrossRef]
- Watach, A.J.; Radcliffe, J.; Xanthopoulos, M.S.; Novick, M.B.; Sawyer, A.M. Executive Function Impairments in Adolescents With Obesity and Obstructive Sleep Apnea Syndrome. Biol. Res. Nurs. 2019, 21, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.; Teng, A.; Burns, M.; Lah, S. Neurocognitive outcomes of children with sleep disordered breathing: A systematic review with meta-analysis. Sleep Med. Rev. 2022, 63, 101629. [Google Scholar] [CrossRef]
- Isaiah, A.; Ernst, T.; Cloak, C.C.; Clark, D.B.; Chang, L. Association Between Habitual Snoring and Cognitive Performance Among a Large Sample of Preadolescent Children. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Hodges, E.K.; Guire, K.E.; Ruzicka, D.L.; Dillon, J.E.; Weatherly, R.A.; Garetz, S.L.; Chervin, R.D. Neuropsychological and behavioral functioning in children with and without obstructive sleep apnea referred for tonsillectomy. J. Int. Neuropsychol. Soc. 2008, 14, 571–581. [Google Scholar] [CrossRef]
- Calhoun, S.L.; Mayes, S.D.; Vgontzas, A.N.; Tsaoussoglou, M.; Shifflett, L.J.; Bixler, E.O. No relationship between neurocognitive functioning and mild sleep disordered breathing in a community sample of children. J. Clin. Sleep Med. 2009, 5, 228–234. [Google Scholar] [CrossRef]
- Csábi, E.; Gaál, V.; Hallgató, E.; Schulcz, R.A.; Katona, G.; Benedek, P. Increased behavioral problems in children with sleep-disordered breathing. Ital. J. Pediatr. 2022, 48, 173. [Google Scholar] [CrossRef]
- Hagström, K.; Saarenpää-Heikkilä, O.; Himanen, S.L.; Lampinlampi, A.M.; Rantanen, K. Neurobehavioral Outcomes in School-Aged Children with Primary Snoring. Arch. Clin. Neuropsychol. 2020, 35, 401–412. [Google Scholar] [CrossRef]
- Blunden, S.; Lushington, K.; Lorenzen, B.; Martin, J.; Kennedy, D. Neuropsychological and psychosocial function in children with a history of snoring or behavioral sleep problems. J. Pediatr. 2005, 146, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Spirito, A.; Marcotte, A.; McGuinn, M.; Berkelhammer, L. Neuropsychological and Behavioral Correlates of Obstructive Sleep Apnea Syndrome in Children: A Preliminary Study. Sleep Breath 2000, 4, 67–78. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).