Comparison of a Telehealth-Based Intensive Treatment Program with a Rewarding App vs. On-Site Care for Youth with Obesity: A Historical Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Outcomes
2.4. The Telehealth Program
2.5. Variables
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R. Planning for the worst: Estimates of obesity and comorbidities in school-age children in 2025. Pediatr. Obes. 2016, 11, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Kobes, A.; Kretschmer, T.; Timmerman, G.; Schreuder, P. Interventions aimed at preventing and reducing overweight/obesity among children and adolescents: A meta-synthesis. Obes. Rev. 2018, 19, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Ells, L.J.; Rees, K.; Brown, T.; Mead, E.; Al-Khudairy, L.; Azevedo, L.; McGeechan, G.J.; Baur, L.; Loveman, E.; Clements, H.; et al. Interventions for treating children and adolescents with overweight and obesity: An overview of Cochrane reviews. Int. J. Obes. 2018, 42, 1823–1833. [Google Scholar] [CrossRef]
- Gates, A.; Elliott, S.A.; Shulhan-Kilroy, J.; Ball, G.D.C.; Hartling, L. Effectiveness and safety of interventions to manage childhood overweight and obesity: An Overview of Cochrane systematic reviews. Paediatr. Child Health 2020, 26, 310–316. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Grossman, D.C.; Bibbins-Domingo, K.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; Krist, A.H.; et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 2417–2426. [Google Scholar]
- Pietrobelli, A.; Pecoraro, L.; Ferruzzi, A.; Heo, M.; Faith, M.; Zoller, T.; Antoniazzi, F.; Piacentini, G.; Fearnbach, S.N.; Heymsfield, S.B. Effects of COVID-19 Lockdown on Lifestyle Behaviors in Children with Obesity Living in Verona, Italy: A Longitudinal Study. Obesity 2020, 28, 1382–1385. [Google Scholar] [CrossRef]
- Ruíz-Roso, M.B.; de Carvalho Padilha, P.; Matilla-Escalante, D.C.; Brun, P.; Ulloa, N.; Acevedo-Correa, D.; Ferreira Peres, W.A.; Martorell, M.; Bousquet Carrilho, T.R.; De Oliveira Cardoso, L.; et al. Changes of physical activity and ultra-processed food consumption in adolescents from different countries during COVID-19 pandemic: An observational study. Nutrients 2020, 12, 2289. [Google Scholar] [CrossRef]
- Neshteruk, C.D.; Zizzi, A.; Suarez, L.; Erickson, E.; Kraus, W.E.; Li, J.S.; Skinner, A.C.; Story, M.; Zucker, N.; Armstrong, S.C. Weight-Related Behaviors of Children with Obesity during the COVID-19 Pandemic. Child. Obes. 2021, 17, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Kwon, Y.; Choe, Y.H.; Kim, M.J. COVID-19-related school closing aggravate obesity and glucose intolerance in pediatric patients with obesity. Sci. Rep. 2021, 11, 5494. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, B.P.; Kelly, M.K.; Powell, M.; Bouchelle, Z.; Mayne, S.L.; Fiks, A.G. COVID-19 and Changes in Child Obesity. Pediatrics 2021, 147, e2021050123. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Maor, S.; Ziv-Baran, T. Pediatric obesity and body weight following the COVID-19 pandemic. Child Care Health Dev. 2022, 48, 881–885. [Google Scholar] [CrossRef]
- Anderson, L.N.; Yoshida-Montezuma, Y.; Dewart, N.; Jalil, E.; Khattar, J.; De Rubeis, V.; Carsley, S.; Griffith, L.E.; Mbuagbaw, L. Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13550. [Google Scholar] [CrossRef]
- Moorman, E.L.; Koskela-Staples, N.C.; Mathai, B.B.; Fedele, D.A.; Janicke, D.M. Pediatric Obesity Treatment via Telehealth: Current Evidence and Future Directions. Curr. Obes. Rep. 2021, 10, 371–384. [Google Scholar] [CrossRef]
- Whitley, A.; Yahia, N. Efficacy of Clinic-Based Telehealth vs. Face-to-Face Interventions for Obesity Treatment in Children and Adolescents in the United States and Canada: A Systematic Review. Child. Obes. 2021, 17, 299–310. [Google Scholar] [CrossRef]
- World Health Organization. Growth Reference Data for 5–19 Years. BMI-for-Age. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 12 June 2023).
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Berry, E.M. What paediatric obesity treatment programmes work, and how can we measure their success? Acta Paediatr. 2017, 106, 1724–1726. [Google Scholar] [CrossRef]
- Irby, M.B.; Boles, K.A.; Jordan, C.; Skelton, J.A. TeleFIT: Adapting a Multidisciplinary, Tertiary-Care Pediatric Obesity Clinic to Rural Populations. Telemed. e-Health 2012, 18, 247–249. [Google Scholar] [CrossRef]
- Slusser, W.; Whitley, M.; Izadpanah, N.; Kim, S.L.; Ponturo, D. Multidisciplinary Pediatric Obesity Clinic via Telemedicine within the Los Angeles Metropolitan Area. Clin. Pediatr. 2016, 55, 251–259. [Google Scholar] [CrossRef]
- Reschke, F.; Galuschka, L.; Landsberg, S.; Weiner, C.; Guntermann, C.; Sadeghian, E.; Lange, K.; Danne, T. Successful telehealth transformation of a pediatric outpatient obesity teaching program due to the COVID-19 pandemic—The “Video KiCK” program. J. Pediatr. Endocrinol. Metab. 2022, 35, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Fidjeland, T.G.; Øen, K.G. Parents’ Experiences Using Digital Health Technologies in Paediatric Overweight and Obesity Support: An Integrative Review. Int. J. Environ. Res. Public Health 2022, 20, 410. [Google Scholar] [CrossRef] [PubMed]
- Kodjebacheva, G.D.; Tang, C.; Groesbeck, F.; Walker, L.; Woodworth, J.; Schindler-Ruwisch, J. Telehealth Use in Pediatric Care during the COVID-19 Pandemic: A Qualitative Study on the Perspectives of Caregivers. Children 2023, 10, 311. [Google Scholar] [CrossRef]
- Tully, L.; Sorensen, J.; O’Malley, G. Pediatric Weight Management Through mHealth Compared to Face-to-Face Care: Cost Analysis of a Randomized Control Trial. JMIR mHealth uHealth 2021, 9, e31621. [Google Scholar] [CrossRef]
- Azevedo, L.B.; Stephenson, J.; Ells, L.; Adu-Ntiamoah, S.; DeSmet, A.; Giles, E.L.; Haste, A.; O’Malley, C.; Jones, D.; Chai, L.K.; et al. The effectiveness of e-health interventions for the treatment of overweight or obesity in children and adolescents: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13373. [Google Scholar] [CrossRef]
- Davis, A.M.; Sampilo, M.; Gallagher, K.S.; Dean, K.; Saroja, M.B.; Yu, Q.; He, J.; Sporn, N. Treating rural paediatric obesity through telemedicine vs. telephone: Outcomes from a cluster randomized controlled trial. J. Telemed. Telecare 2016, 22, 86–95. [Google Scholar] [CrossRef]
- Siegel, R.; Stackpole, K.; Kirk, S.; Kharofa, R. Families Chose In-Person Visits over Telehealth for Pediatric Weight Management during the COVID-19 Pandemic. Child. Obes. 2022, 18, 572–575. [Google Scholar] [CrossRef]
- Paul-Ebhohimhen, V.; Avenell, A. Systematic review of the use of financial incentives in treatments for obesity and overweight. Obes. Rev. 2008, 9, 355–367. [Google Scholar] [CrossRef]
- Ananthapavan, J.; Peeterson, A.; Sacks, G. Paying people to lose weight: The effectiveness of financial incentives provided by health insurers for the prevention and management of overweight and obesity—A systematic review. Obes. Rev. 2018, 19, 605–613. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Waddell, K.J.; Small, D.S.; Evans, C.; Harrington, T.O.; Djaraher, R.; Oon, A.L.; Patel, M.S. Effect of Gamification with and without Financial Incentives to Increase Physical Activity among Veterans Classified as Having Obesity or Overweight: A randomized clinical trial. JAMA Netw. Open 2021, 4, e2116256. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Tan, Y.-T.; Malhotra, R.; Lee, C.-F.; Goh, S.-S.; Saw, S.-M. A Cluster Randomized Controlled Trial of an Incentive-Based Outdoor Physical Activity Program. J. Pediatr. 2013, 163, 167–172.e1. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Chadwick, J.Q.; Cannady, T.K.; Branam, D.E.; Wharton, D.F.; Tullier, M.A.; Thompson, D.M.; Copeland, K.C. Using financial incentives to promote physical activity in American Indian adolescents: A randomized controlled trial. PLoS ONE 2018, 13, e0198390. [Google Scholar] [CrossRef]
- Karatzi, K.; Poulia, K.-A.; Papakonstantinou, E.; Zampelas, A. The Impact of Nutritional and Lifestyle Changes on Body Weight, Body Composition and Cardiometabolic Risk Factors in Children and Adolescents during the Pandemic of COVID-19: A Systematic Review. Children 2021, 8, 1130. [Google Scholar] [CrossRef]
| Characteristic | Comparison Group | Telehealth Group | p-Value |
|---|---|---|---|
| Age (years) | 14.1 ± 2.1 | 12.9 ± 1.75 | <0.001 |
| Males (n, %) | 46 (53%) | 35 (55%) | 0.825 |
| Height (cm) | 161.2 ± 10.9 | 160.0 ± 11.2 | 0.212 |
| Weight (kg) | 86.6 ± 23.4 | 77.1 ± 19.7 | 0.04 |
| BMI (kg/m2) | 32.9 ± 7.0 | 29.7 ± 4.7 | 0.008 |
| zBMI | 2.12 ± 0.49 | 2.03 ± 0.42 | 0.124 |
| Fat percent (%) | 39.0 ± 8.1 | 38.3 ± 8.0 | 0.625 |
| z fat percent | 2.55 ± 0.68 | 2.34 ± 0.62 | 0.063 |
| Characteristic | Comparison Group—Observed (n = 52) | Telehealth Group—Observed (n = 55) | p-Value | Comparison Group— LOCF (n = 87) | Telehealth Group— LOCF (n = 64) | p-Value |
|---|---|---|---|---|---|---|
| BMI change (kg/m2) | −0.20 ± 1.38 | −0.25 ± 1.80 | 0.876 | −0.21 ± 1.26 | −0.19 ± 1.72 | 0.923 |
| Reduced BMI (n, %) | 27 (52%) | 31 (56%) | 0.572 | 45 (52%) | 35 (55%) | 0.718 |
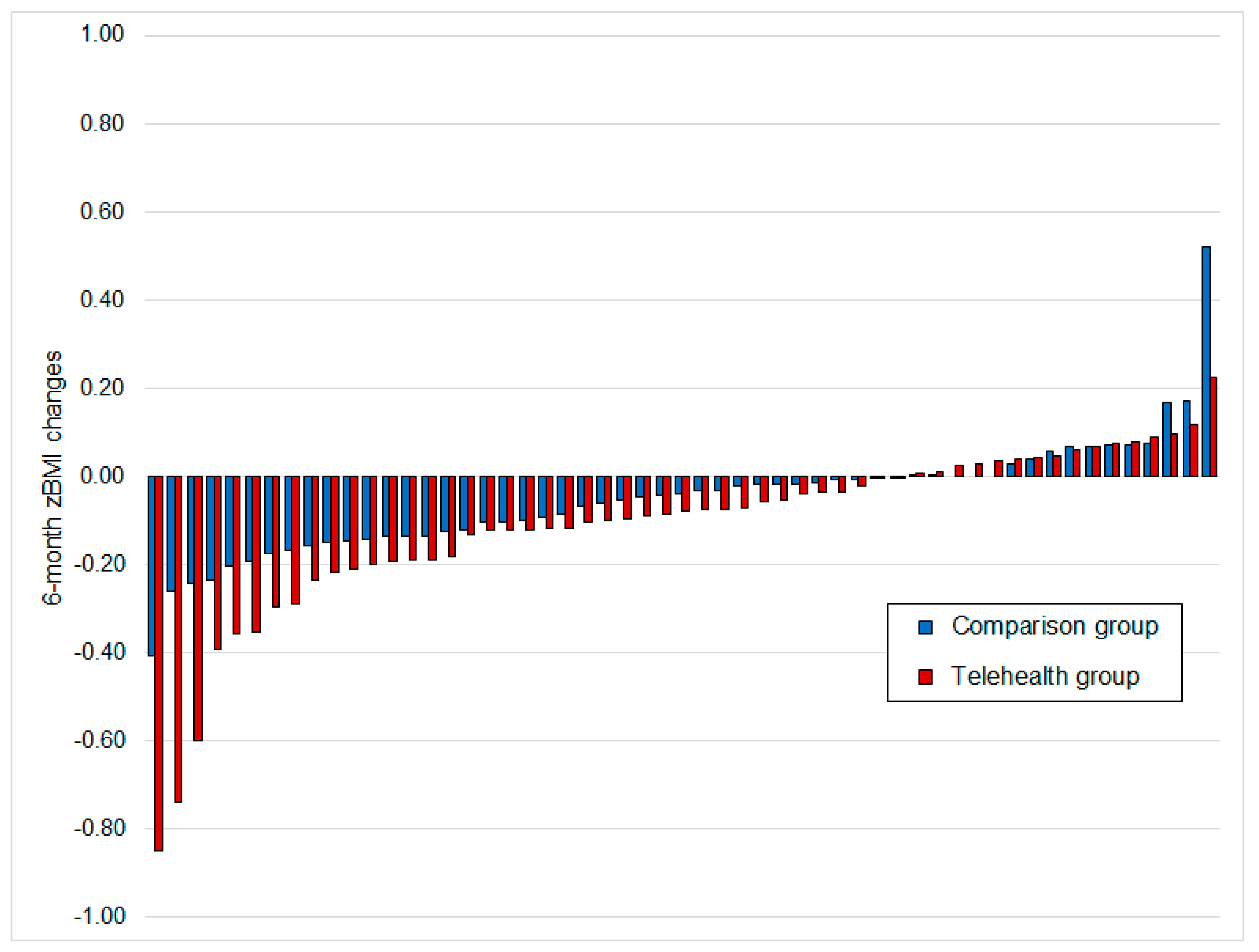
| zBMI change | −0.011 ± 0.337 | −0.113 ± 0.198 | 0.298 | −0.022 ± 0.271 | −0.099 ± 0.189 | 0.234 |
| Reduced zBMI (n, %) | 39 (75%) | 39 (71%) | 0.756 | 60 (69%) | 44 (69%) | 0.977 |
| Reduced zBMI > 0.2 | 5 (9.6%) | 11 (20.0%) | 0.122 | 8 (9.2%) | 11 (17.2%) | 0.143 |
| Reduced zBMI > 0.25 | 2 (3.8%) | 8 (14.5%) | 0.094 | 3 (3.4%) | 8 (12.5%) | 0.054 |
| Fat percent change (%) | −0.85 ± 3.37 | −1.05 ± 3.33 | 0.774 | −0.63 ± 3.08 | −0.76 ± 3.28 | 0.817 |
| Reduced fat percent (n, %) | 29 (56%) | 34 (62%) | 0.863 | 43 (49%) | 38 (59%) | 0.226 |
| z fat percent change | −0.07 ± 0.32 | −0.06 ± 0.29 | 0.856 | −0.05 ± 0.30 | −0.04 ± 0.28 | 0.768 |
| Reduced z fat precent (n, %) | 25 (48%) | 32 (58%) | 0.401 | 40 (46%) | 35 (55%) | 0.290 |
| Glucose | Hemoglobin A1c | ALT | Triglycerides | LDL | HDL | |
|---|---|---|---|---|---|---|
| Baseline | 89.6 ± 7.0 | 5.2 ± 0.4 | 26.6 ± 24.4 | 98.5 ± 39.9 | 90.9 ± 23.8 | 46.8 ± 9.1 |
| End | 92.2 ± 7.7 | 5.3 ± 0.3 | 23.3 ± 24.3 | 95.2 ± 48.6 | 96.3 ± 24.3 | 47.1 ± 7.6 |
| Change | −1.8 ± 6.9 | −0.1 ± 0.2 | 0.7 ± 8.2 | −5.9 ± 44.8 | −2.5 ± 16.8 | 0.9 ± 5.7 |
| p-Value | 0.122 | 0.044 | 0.269 | 0.910 | 0.388 | 0.527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sela Peremen, K.; Maor, S.; Yaniv, A.; Aloni, I.; Ziv-Baran, T.; Dubnov-Raz, G. Comparison of a Telehealth-Based Intensive Treatment Program with a Rewarding App vs. On-Site Care for Youth with Obesity: A Historical Cohort Study. Children 2023, 10, 1117. https://doi.org/10.3390/children10071117
Sela Peremen K, Maor S, Yaniv A, Aloni I, Ziv-Baran T, Dubnov-Raz G. Comparison of a Telehealth-Based Intensive Treatment Program with a Rewarding App vs. On-Site Care for Youth with Obesity: A Historical Cohort Study. Children. 2023; 10(7):1117. https://doi.org/10.3390/children10071117
Chicago/Turabian StyleSela Peremen, Khen, Shay Maor, Amit Yaniv, Ishai Aloni, Tomer Ziv-Baran, and Gal Dubnov-Raz. 2023. "Comparison of a Telehealth-Based Intensive Treatment Program with a Rewarding App vs. On-Site Care for Youth with Obesity: A Historical Cohort Study" Children 10, no. 7: 1117. https://doi.org/10.3390/children10071117
APA StyleSela Peremen, K., Maor, S., Yaniv, A., Aloni, I., Ziv-Baran, T., & Dubnov-Raz, G. (2023). Comparison of a Telehealth-Based Intensive Treatment Program with a Rewarding App vs. On-Site Care for Youth with Obesity: A Historical Cohort Study. Children, 10(7), 1117. https://doi.org/10.3390/children10071117






