1. Introduction
Since April 2020, after the worldwide spread of the coronavirus SARS-CoV-2 infection, numerous reports have described a novel condition associated with coronavirus disease 2019 (COVID-19) and named Multisystem Inflammatory Syndrome in Children (MIS-C) or Pediatric Inflammatory Multisystem Syndrome temporarily related to SARS-CoV-2 (PIMS-TS) [
1,
2,
3]. This condition showed overlapping clinical features with previously known diseases including Kawasaki Disease (KD), Toxic Shock Syndrome (TSS), and Macrophage Activation Syndrome (MAS). MIS-C is a severe complication of COVID-19 that often requires intensive care unit (ICU) admittance due to severe cardiac involvement and shock at onset, but also multiple organ failure that may develop in the disease course [
2,
4,
5]. MIS-C usually starts 2–6 weeks after a benign SARS-CoV-2 infection. The prevalence of the condition has been estimated at about 45 cases per 100,000 (0.045%) infected children during the first pandemic wave, with lowering incidence through the following SARS-CoV-2-variants waves and a mortality rate below 2% [
6]. Case definitions for this syndrome have been developed by the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), the European Centre for Disease Prevention and Control (ECDC), and the Royal College of Pediatrics and Child Health (RCPCH) [
7,
8,
9]. The diagnostic criteria are based on the presence of fever, clinical symptoms of different organs dysfunction, signs of systemic inflammation in laboratory tests (such as increased ferritin, C-Reactive Protein (CRP), Troponin T (TnT), Brain Natriuretic Peptide test (BNP or NT-proBNP)), and the association with SARS-CoV-2 infection in subjects 21-year-old or less. The CDC’s case definition is the most used of the four available, and probably includes a wider group of patients as it defines a smaller fever period as criteria (24 h). The condition appears to be more frequent in male children over five, even though some cases have been diagnosed in younger infants. Fever must be present in all patients, variably associated with gastrointestinal, respiratory, neurological, or renal signs and symptoms, and cardiac dysfunction. First-line therapy usually includes a single dose of intravenous immunoglobulin and high-dose glucocorticoids; additionally, anakinra has been used successfully in the case of critically ill or refractory MIS-C patients. Low-dose aspirin is usually started in all patients and continued for at least 4 weeks in those with coronary artery abnormalities (CAAs); anticoagulation with Enoxaparin is indicated in selected cases of documented thrombosis or with an ejection fraction below 35% [
10,
11].
The pathogenesis of MIS-C has been largely investigated. The disease is considered a post-infectious syndrome caused by the upregulation of different pro-inflammatory mediators, such as the monocyte-derived cytokines IL-1RA, IL-6, IL-10, IL-18, MCP1 (CCL2), and TNF, or the interferons (IFNs). It is still not clear if the hyperinflammatory state is induced by SARSCoV-2 acting as a superantigen, or by a mechanism of antibody-dependent enhancement.
There is now evidence that the SARS-CoV-2 spike protein may contain a superantigen-like motif, which can bind both MHC class I molecules and T cell receptors [
12]. Case descriptions reported of lymphopenia in MIS-C patients, and the investigation of the immunological behavior of MIS-C patients has shown, in ~75% of patients, the polyclonal expansion of CD4+ and CD8+ T cells bearing the Vβ21.3 segment and TCR V11-2 skewing in association with HLA A*02, B*35 and C*04 [
13]. All these findings point to a possible superantigen conveying the disease [
14].
SARS-CoV-2 infection is characterized by extreme inter-individual clinical variability. In an effort to understand the underlying causes of this variability, molecular and genetic studies have revealed inborn errors of type I interferon (IFN) immunity and their phenocopies—autoantibodies against type I IFNs—collectively in at least 15% of cases of critical COVID-19 pneumonia in unvaccinated patients. Similarly, genetic studies have been conducted in MIS-C patients to identify monogenic inborn errors of immunity to SARS-CoV-2 that could explain the occurrence of the syndrome [
15,
16,
17]. Recently, autosomal recessive deficiencies of
OAS1,
OAS2, or
RNASEL have been identified in five unrelated children with MIS-C [
18].
Therefore, MIS-C may represent a disease model of a hyperinflammatory condition secondary to a viral infection. The comprehension of the underlying immunological and genetic mechanisms may guide clinicians to better management and to target therapeutic options.
We conducted a longitudinal study to analyze a single-center cohort of MIS-C patients to characterize retrospectively their immune response during the acute phase of the disease and the outpatient long-term follow-up.
2. Materials and Methods
We conducted a longitudinal study at the Pediatric Department of ASST Spedali Civili of Brescia. We included all children and adolescents who were diagnosed with MIS-C, in accordance with the CDC definition [
8] and our internal MIS-C diagnostic and therapeutic protocol, from April 2020 to June 2022. The CDC’s criteria considered the patient’s age defined as <21 years; fever defined as ≥38 °C, and present for at least 24 h; laboratory signs of inflammation; and the evidence of SARS-CoV-2 infection that could be current or even recent, as demonstrated by molecular biology detection (RT-PCR), positive serology, and antigenic test or COVID-19 exposure within the prior 4 weeks.
Patient care and research were conducted in compliance with the Declaration of Helsinki. Informed consent was acquired from patients’ parents, according to ASST Spedali Civili of Brescia protocols.
For all patients, we recorded demographics, length of hospital stays, need for pediatric intensive care unit (PICU), comorbidities, clinical presentation, and laboratory data that included full blood count and inflammatory markers: C-Reactive Protein (CRP), ferritin, Troponin T (TnT), and N-Terminal pro B-type Natriuretic Peptide (NT-proBNP). A cardiological evaluation was also carried out and included echocardiographic examination and electrocardiogram.
We considered as basal values the biochemical and blood count parameters that had been evaluated within 48 h from hospital admission and prior to the initiation of systemic therapy. These parameters had been monitored with daily frequency during the acute phase and at reduced frequency during the subacute phase, according to amelioration of the patients’ clinical conditions, until discharge.
A detailed multiparametric flow cytometry evaluation of immunological markers had been performed at baseline, and during the hospitalization in the acute phase of the disease and follow-up. Flow cytometry analyses were performed on fresh blood samples using four different multiparametric panels including appropriate mixtures of monoclonal antibodies (mAbs) according to manufacturer’s protocols. A standard TBNK panel was used on Becton Dickinson Trucount™ tubes to determine the percentages and counts of main lymphocyte populations (CD3+, CD4+, CD8+ T lymphocytes and their HLA-DR+ activated fraction, CD19+ B lymphocytes, and CD3-CD56/CD16+ NK cells). T-cell differentiation was assessed by using anti-GammaDelta TCR, CD45RA, CCR7, CD31 mAbs, on CD3+, CD4+, CD8+, and CD4-CD8- double negative cells. This strategy allowed for identifying CD4+CD45RA+CCR7+CD31+ Recent Thymic Emigrants (RTEs), and different maturation stages of T cells according to CD45RA and CCR7 expression: CD45RA+CCR7+ naïve, CD45RA+CCR7- central memory, CD45RA-CCR7- effector memory, and CD45RA+CCR7- terminal differentiated cells. Analyses of B-cell differentiation was assessed by using anti-CD19, CD20, IgM, IgD, CD38, CD27, CD21, and CD10 mAbs, which allowed for identifying CD19+CD20+ subsets such as Recent Bone Marrow Emigrants (RBE) (CD38++CD10+), naïve (CD38+IgD+IgM+CD27-), switched memory (CD38+/-IgD-IgM-CD27+), unswitched memory (CD38+/-IgD+IgM+CD27+), and CD38low-CD21low- B cells, along with CD38+++CD27+CD20- terminally differentiated B cells. Plasmacytoid (pDC) and myeloid (mDC) dendritic cells were identified as CD45dimCD4dimCD123+BDCA2+ cells and CD45dimCD4dimCD1c+CD14- cells, respectively, while basophils were gated as CD45dimCD4-CD123+BDCA2- cells. Expression of CD4 on monocytes was evaluated by considering both the percentage of CD4- cells and a Mean Fluorescence Intensity (MFI) ratio between CD4 expression on resting T helper cells and on monocytes, as previously described [
19].
Multiparametric flow cytometry samples were acquired on FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA) flow cytometers, while data analyses were performed using FACSDiva 9.0 software (BD Biosciences).
For data analyses, we calculated and analyzed normalized values obtained by dividing patients’ values by the median of their own age-matched reference range (herein termed as normalization ratio). For this purpose, we used datasets previously obtained from pools of healthy donors at our laboratory. For the main lymphocyte subsets (total CD3+ T lymphocytes, CD3+CD4+ and CD3+CD8+ T lymphocytes, total CD19+ B lymphocytes, NK cells), this calculation was made considering data of absolute counts, while for T- and B-cell subsets considering their percentages with respect to their parental populations. Patients’ data were categorized into four longitudinal groups: (1) the first 5 days of hospitalization (16 determinations); (2) the second phase of hospitalization, the subacute phase, up to discharge (from day +6 to day +16, 15 determinations); (3) short-term follow-up at 20–60 days (21 determinations); (4) long-term follow-up at 100–414 days (31 determinations).
The database was formatted by using Microsoft Excel® software (365 version) and later imported from IBM-SPSS® software ver. 28.0.1 (IBM SPSS Inc., Chicago, IL, USA). Use of Stata® software ver. 17.0 (Stata Corporation, College Station, TX, USA) was also considered for comparisons or implementations of test output. Normality of the distributions was assessed using the Kolmogorov–Smirnov test. Categorical variables were presented as frequencies or percentages and compared with the use of the chi-square test and Fisher’s exact test, as appropriate; associations of the cross-tabulations were verified using standardized adjusted residuals. Continuous variables were presented as means ±SD (in case of a normal distribution), or medians and min/max (in case of a skewed distribution) and compared with the use of Student’s T-test, Anova, or the Mann–Whitney test and Kruskal–Wallis test, and also with Wilcoxon and Friedman tests; correlations among variables were analyzed by the Pearson’s or Spearman’s rank correlation test. A two-sided α level of 0.05 was used for all tests. The authors had full access to the data and assume full responsibility for their integrity.
4. Discussion
Multisystem inflammatory syndrome in children is a rare but severe complication of COVID-19, known since 2020, that results from a hyper-reactive dysregulated immune response. From April 2020 to June 2022, at the Department of Pediatrics of the ASST Spedali Civili of Brescia, 40 patients were admitted for MIS-C.
All patients were treated according to our standard protocol [
3], based on an updated review of the literature and a multidisciplinary expert consensus. In most of the patients (75%), the cardinal treatment consisted of IVIG at 2 g/kg and corticosteroids (intravenous methylprednisolone 2 mg/kg, followed by tapering with oral prednisone). A small group of five very young patients with a milder clinical picture and milder abnormalities in the biochemical and immunological parameters (CRP and lymphocyte/neutrophil counts) rapidly improved with sole IVIG, while four other patients were treated only with corticosteroids.
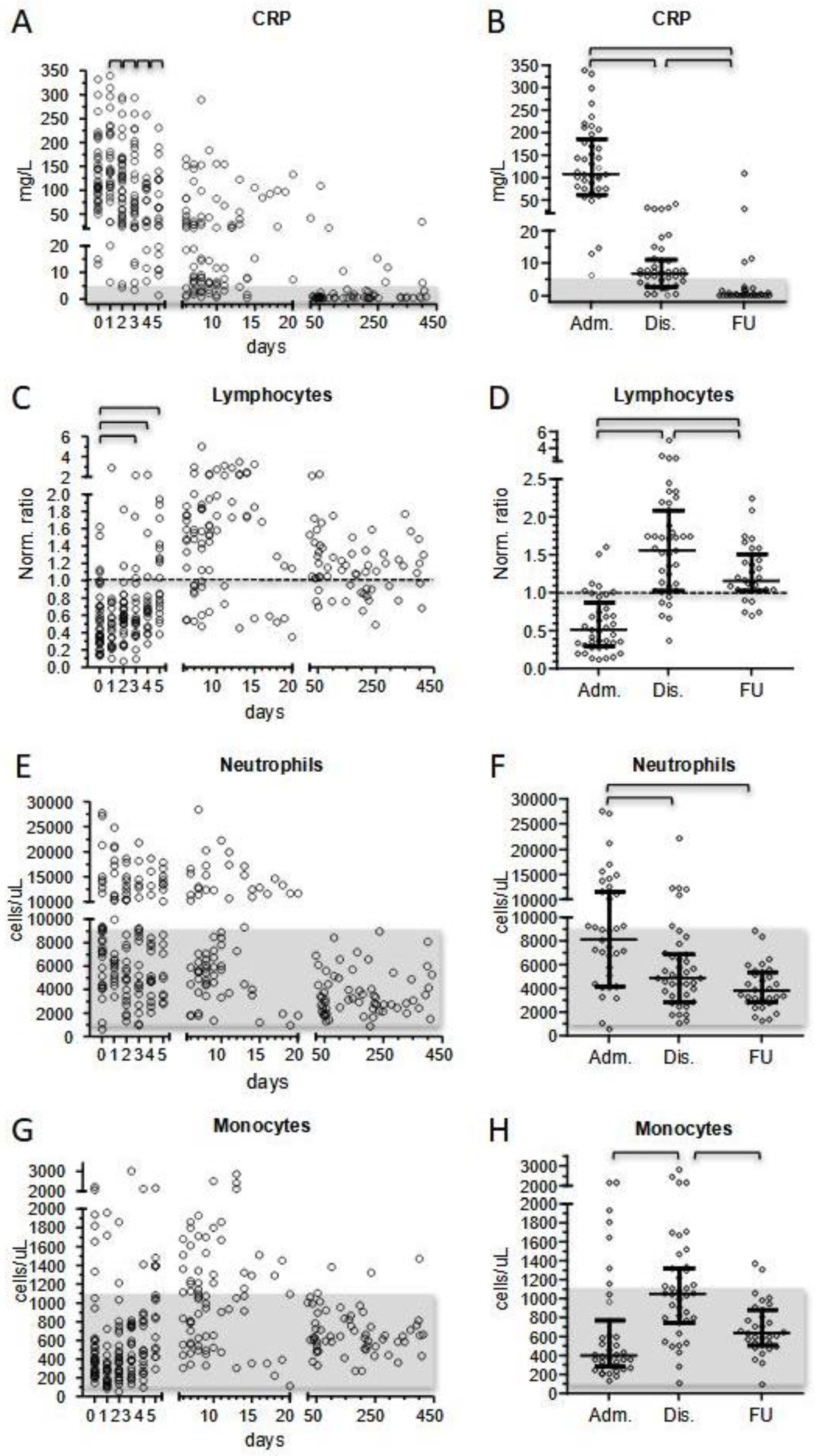
MIS-C patients exhibited increased inflammatory markers at presentation, with CRP as the most extensively measured marker. Although slightly heterogeneous, we did not observe significantly different CRP values comparing patients’ subgroups at first measurement. After the beginning of treatment, CRP displayed a day-by-day significant decrease that reduced the initial value by half on day +5 and normalized before discharge in one-third of patients (13/39) and in all patients at short-term follow-up.
Neutrophil activation is a distinct feature of Kawasaki disease and has already been reported in patients with MIS-C [
21,
22]. In our cohort, neutrophilia affected half of the patients at presentation and was more evident in more severe cases (median 10,290 vs. 7810 cells/μL, respectively). During treatment, neutrophil counts tended to normalize, with only six patients (15%) presenting with neutrophilia at discharge.
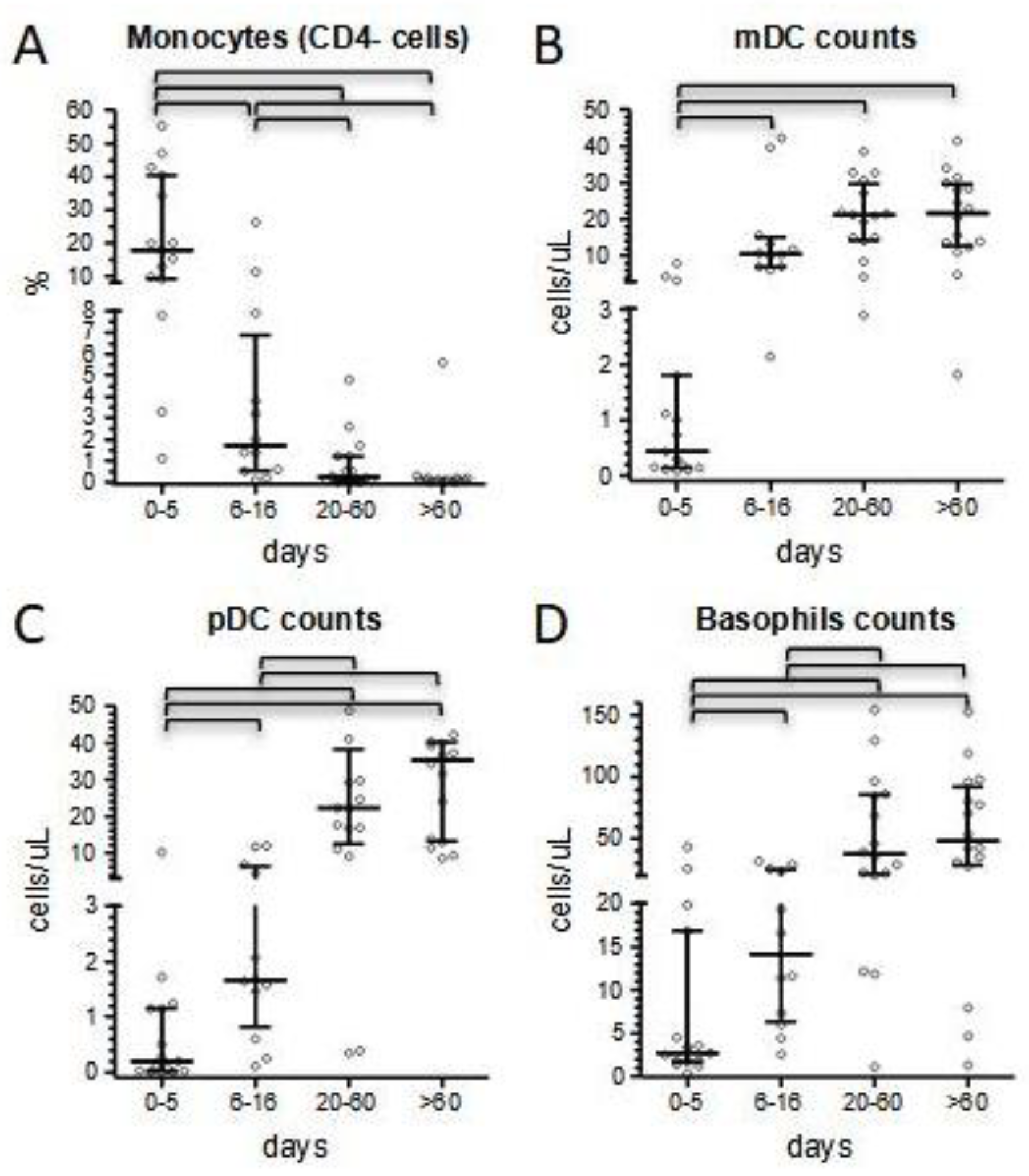
Conversely to neutrophils, monocyte count was initially normal in 78% of the patients, then increased in the subacute phase of hospitalization (median values 400 vs. 1050 cells/μL). Although the total number of monocytes has been reported to be normal in MIS-C, there is also evidence of the active recruitment of this cell population in some MIS-C patients, as shown by Gruber et al. [
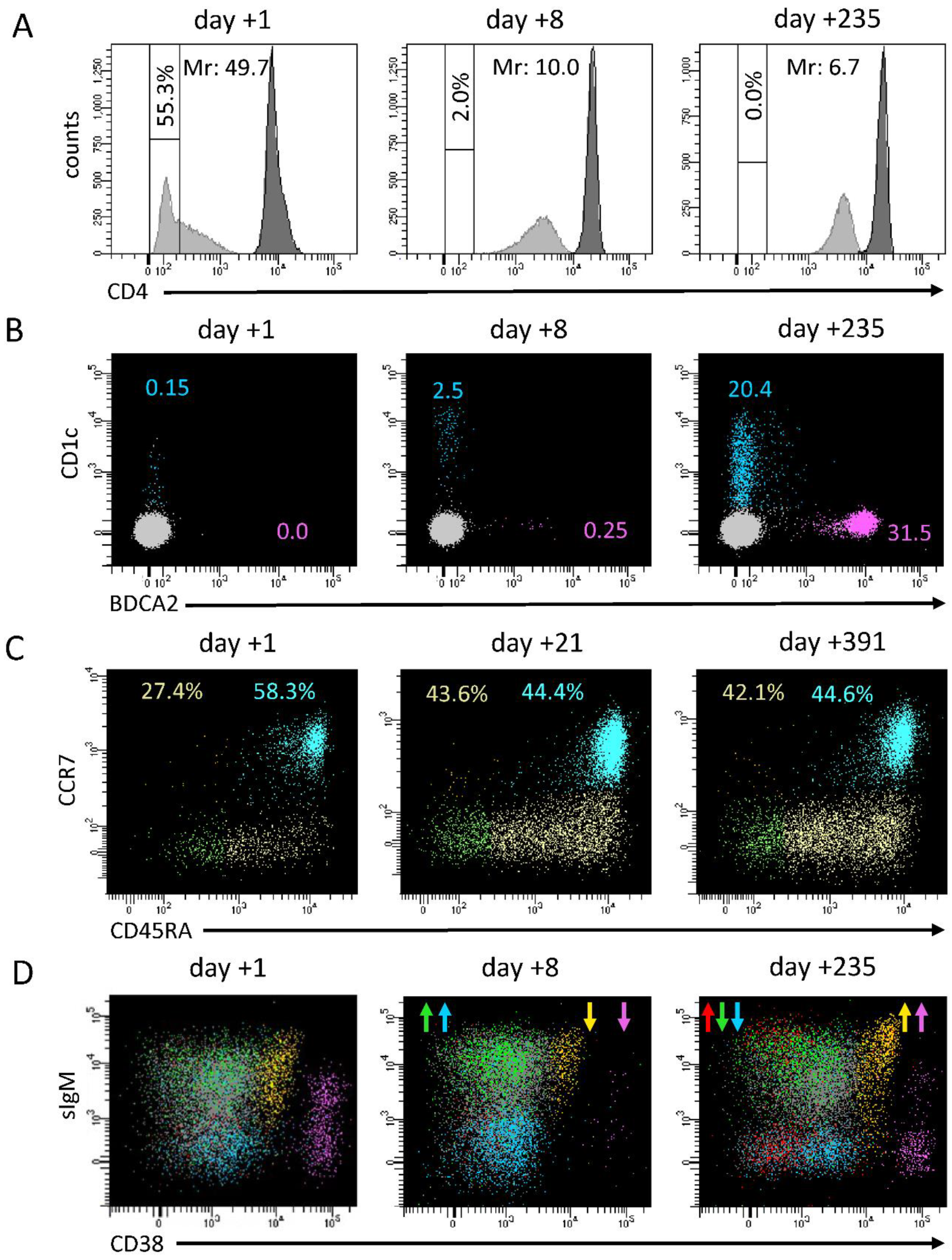
21]. A shift in monocyte counts paralleled with normalization of the expression of surface-specific markers. We focused on the expression of CD4, because of its ease to evaluate in very common flow cytometric panels and its strict temporal correlation with the amelioration of CRP values and lymphocyte counts; in fact, the median of CD4- monocytes dropped from 18% in the acute phase of the disease to 1.7% from day +5 onward, before slightly disappearing at the last follow-up determinations (
Figure 5A). Moreover, in our experience, CD4 expression may correlate with clinical conditions at presentation, as suggested by its increase in three of our most severe patients. A similar trend was observed also in dendritic cells and basophils counts. Consistent with literature and our previous results [
23], we confirmed a profound depletion of dendritic cells at admission, which was more marked in the most severe patients and that we herein reported also for basophils. The normalization of these cell subsets’ counts was slower compared to the rapid improvement we conversely observed for lymphocyte subsets, and completed only at long-term follow-up (
Figure 5B). In particular, median pDC count remained below 1/20 of long-term follow-up values for the entire duration of the hospital stay.
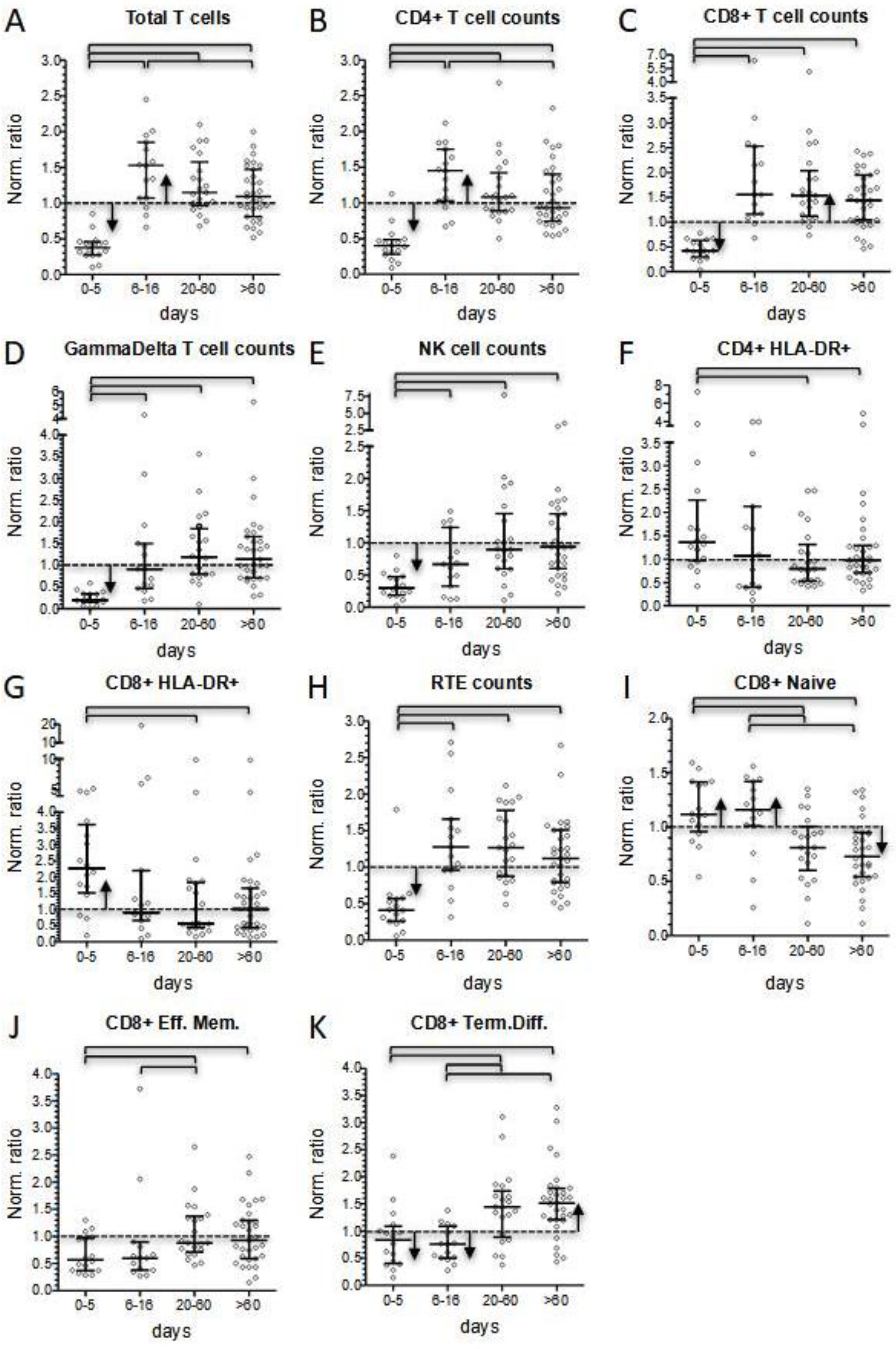
It is recognized that lymphopenia represents the most evident immunological hallmark of MIS-C. Considering normalized values, we reported that 51% of patients displayed lymphopenia at onset, while 87% presented with total lymphocyte counts below the median of the reference value for their age. Interestingly, the prevalence of lymphocyte counts below the normalized median disappeared after 5 days of hospitalization. Conversely to what expected, after day +5 total lymphocyte counts kept on increasing. Thus, at discharge 25% of patients presented with lymphocytosis, while 85% of them displayed lymphocyte counts above the normalized median. The lymphocytosis phase was temporary and at short-term follow-up total lymphocyte counts were significantly lower and much closer to the normal range, despite a tendency to remain above the normalized median value. No significant differences in total lymphocyte counts were observed between patients’ subgroups according to age, treatment, and severity. More severe patients did not display a higher degree of lymphopenia at presentation, or a different incidence of lymphocytosis in the subacute phase of the disease. The acute phase of the disease was characterized by a significant reduction in T- and NK-cells, with GammaDelta+ cells as the T subset most dramatically affected by the disease (normalized median 0.201 vs. 0.379 of the whole T-cell compartment), followed by NK cells (normalized median 0.298). Our finding confirms the reduction in these populations in acute MIS-C patients already observed by Gruber et al. [
21].
CD4+ and CD8+ T cells were also significantly reduced at onset (normalized median 0.399 and 0.427, respectively). As already reported by Carter et al. [
22], both subsets displayed a significant increase in activated cells compared to normalized medians. In contrast, while CD4+ maturation profile was consistent with the expected distribution in healthy condition, CD8+ cells showed a reduction in more differentiated subsets, especially in the three patients requiring admission to the intensive care unit (
Figure 5C).
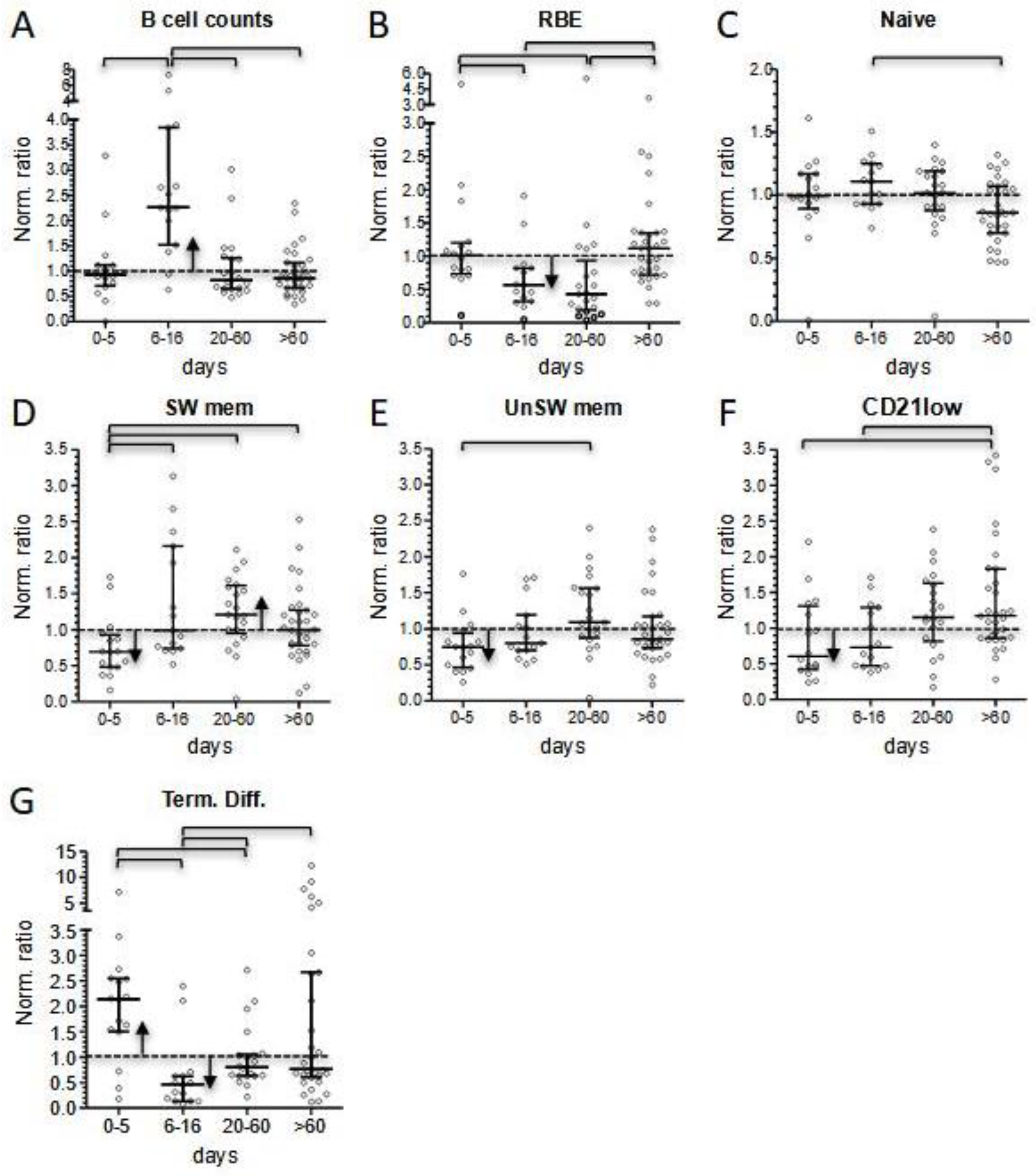
Interestingly, the same three patients displayed a significant reduction in total B cell counts at onset, a condition limited to few cases in our cohort. At the level of B cells, the inflammatory status in the acute phase of the disease matched with a general expansion of terminally differentiated cells, counterbalanced by a reduction in the memory compartment (
Figure 5D).
The subacute phase of the disease was associated with marked changes in lymphocyte counts. We observed a four-fold increase in CD4+, CD8+, and GammaDelta+ T-cell subsets within a few days (0.399–1.447, 0.427–1.553, and 0.201–0.911, respectively), while B lymphocytes markedly expanded to a maximum seven-fold increase. Instead, the increase in NK cell count was less evident (0.298–0.667) and remained below the normalized median. In this phase of the disease, T-cell subsets showed a significant decrease in HLA-DR+ activated cells, but only minimal changes in the distribution of subsets associated with different maturation stages. We also observed rapid changes in the level of B-cell subsets. RBE cells and terminally differentiated cells dropped. This last shift, together with the reduced activation status of T cells, may correspond to the switching-off of the hyperactivation phase of the disease. Naïve B cells displayed their highest peak in the subacute phase, indicating that they were the greatest contributors to B-cell count increase, together with the rise in the proportion of switched memory cells, especially observed in the more severe patients. In contrast, a T-cell increase may be ascribable to a general rise in all the subsets, perhaps due to a peripheral expansion or, more reasonably, to a mobilization of T lymphocytes from other districts.
At follow-up, the apparent quiet trend toward normal counts of most of the main lymphocyte populations hid a constant evolution in both B- and CD8+ T-cell subset distribution (
Figure 5C,D). In fact, at the level of the B-cell compartment, during the short-term follow-up, the depletion in the RBE subset became more pronounced (normalized median of 0.435), while median values for both switched and unswitched memory B cells displayed their highest points. These alterations progressively attenuated during follow-up. Of note, we observed a constant increase in atypical CD21low during follow-up, which in some cases reached more than a three-fold increase compared to reference medians in the last determinations.
CD8+ T cells constituted the lymphocyte subset more consistently altered at follow-up. In fact, their counts remained elevated even at long-term follow-up (normalized median 1.64 at least 300 days after hospital admission). Persistence of elevated CD8+ T cell counts was mainly sustained by terminally differentiated cells, which remained significantly above the normalized median even at longer time points (normalized median 1.61 at least 300 days after hospital admission). In contrast, percentages of naïve cells were significantly lower compared to normalized medians.
Of note, an even more pronounced long-term increase in terminally differentiated cells could also be observed in CD4+ T lymphocytes (normalized median of 2.65 at least 300 days after hospital admission), although this population has much less relevance than its counterpart at the level of CD8+ cells.
All the observations reported above represent an effort to depict a clear picture of the longitudinal changes in the immunological cell subset in a relatively small group of patients with MIS-C treated in our center according to standard criteria. Moreover, the distribution observed among the different cell subsets suggests the involvement of both innate and adaptive immunities in the pathogenesis of MIS-C. The perturbations described at presentation clearly resemble those immunological characteristics observed in acute COVID-19 adult patients [
19,
20]. Lymphopenia and neutrophilia, together with an increase in biochemical inflammatory markers and lymphocyte-activated subsets, are among the most known hallmarks of the disease. Additionally, the profound depletion of dendritic cells and the marked alteration in monocyte surface markers, particularly dramatic in MIS-C patients displaying the most severe course of the disease, may correlate with severe COVID-19 in adults. Of note, from the lymphocyte point of view, B-cell lymphopenia, and a higher reduction in terminally differentiated CD8+ T cells, characterized the most severe cases in the acute phase of MIS-C.
While the immunological changes observed in the first days after the initiation of the pharmacological treatment, such as increase in lymphocyte counts, drop of activated subsets, and bone marrow release of newly generated B cells, may depend on the immunomodulatory treatment on the acquired immune system, the latter abnormalities, such as long-term persistence of terminally differentiated T cells and increase in atypical CD21low and memory B cells, can be classed as inheritance of the inflammatory response (
Figure 5C,D).
Limitations of the study include the limited sample size that impairs the strength of the analysis, and the retrospective nature of the immunological evaluation that caused missing data during hospitalization and follow-up. Moreover, most MIS-C patients were treated with IVIG alone or in association with glucocorticoids early in the course of the disease, and it was difficult to discriminate the role of the medical therapies on the evolution of the distribution of lymphocyte subsets. Nevertheless, our data may contribute to exploring the immune response of hyperinflammatory complications that may occur after viral triggers.