The Prevalence of Developmental Enamel Defects in Israeli Children and Its Association with Perinatal Conditions: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Training
2.3. Clinical Examination
2.4. Questionnaire
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamunadevi, A.; Pratibha, R.; Rajmohan, M.; Mahendraperumal, S.; Ganapathy, N.; Srivandhana, R. First Molars in Permanent Dentition and their Malformations in Various Pathologies: A Review. J. Pharm. Bioallied. Sci. 2021, 13, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Stoica, S.N.; Nimigean, V.; Vîrlan, M.J.R.; Nimigean, V.R. The Pathology of the First Permanent Molar during the Mixed Dentition Stage—Review. Appl. Sci. 2023, 13, 483. [Google Scholar] [CrossRef]
- Weerheijm, K.L.; Jälevik, B.; Alaluusua, S. Molar-incisor hypomineralisation. Caries Res. 2001, 35, 390–391. [Google Scholar] [CrossRef]
- Weerheijm, K.L. Molar incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2003, 4, 114–120. [Google Scholar] [PubMed]
- Garot, E.; Rouas, P.; Somani, C.; Taylor, G.D.; Wong, F.; Lygidakis, N.A. An update of the aetiological factors involved in molar incisor hypomineralisation (MIH): A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2022, 23, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Fatturi, A.L.; Wambier, L.M.; Chibinski, A.C.; Assunção, L.; Brancher, J.A.; Reis, A.; Souza, J.F. A systematic review and meta-analysis of systemic exposure associated with molar incisor hypomineralization. Community Dent. Oral Epidemiol. 2019, 47, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Weerheijm, K.L.; Duggal, M.; Mejàre, I.; Papagiannoulis, L.; Koch, G.; Martens, L.C.; Hallonsten, A.L. Judgement criteria for molar incisor hypomineralisation (MIH) in epidemiologic studies: A summary of the European meeting on MIH held in Athens, 2003. Eur. J. Paediatr. Dent. 2003, 4, 110–113. [Google Scholar]
- Kotsanos, N.; Kaklamanos, E.G.; Arapostathis, K. Treatment management of first permanent molars in children with Molar-Incisor Hypomineralisation. Eur. J. Paediatr. Dent. 2005, 6, 179–184. [Google Scholar]
- Fagrell, T.G.; Lingström, P.; Olsson, S.; Steiniger, F.; Norén, J.G. Bacterial invasion of dentinal tubules beneath apparently intact but hypomineralized enamel in molar teeth with molar incisor hypomineralization. Int. J. Paediatr. Dent. 2008, 18, 333–340. [Google Scholar] [CrossRef]
- Rodd, H.D.; Boissonade, F.M.; Day, P.F. Pulpal status of hypomineralized permanent molars. Pediatr. Dent. 2007, 29, 514–520. [Google Scholar]
- Crombie, F.; Manton, D.; Kilpatrick, N. Aetiology of molar-incisor hypomineralization: A critical review. Int. J. Paediatr. Dent. 2009, 19, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lygidakis, N.A.; Dimou, G.; Marinou, D. Molar-incisor-hypomineralisation (MIH). A retrospective clinical study in Greek children. II. Possible medical aetiological factors. Eur. Arch. Paediatr. Dent. 2008, 9, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Beentjes, V.E.; Weerheijm, K.L.; Groen, H.J. Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2002, 3, 9–13. [Google Scholar]
- Jälevik, B.; Norén, J.G.; Klingberg, G.; Barregård, L. Etiologic factors influencing the prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Eur. J. Oral Sci. 2001, 109, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Pitiphat, W.; Luangchaichaweng, S.; Pungchanchaikul, P.; Angwaravong, O.; Chansamak, N. Factors associated with molar incisor hypomineralization in Thai children. Eur. J. Oral Sci. 2014, 122, 265–270. [Google Scholar] [CrossRef]
- Tourino, L.F.; Corrêa-Faria, P.; Ferreira, R.C.; Bendo, C.B.; Zarzar, P.M.; Vale, M.P. Association between Molar Incisor Hypomineralization in Schoolchildren and Both Prenatal and Postnatal Factors: A Population-Based Study. PLoS ONE 2016, 11, e0156332. [Google Scholar] [CrossRef] [PubMed]
- Né, Y.G.S.; Frazão, D.R.; Lopes, G.O.; Fagundes, N.C.F.; Souza-Rodrigues, R.D.; Paula-Silva, F.W.G.; Maia, L.C.; Lima, R.R. Association between respiratory diseases and molar-incisor hypomineralization: A systematic review and meta-analysis. Front. Med. 2022, 9, 990421. [Google Scholar] [CrossRef] [PubMed]
- Wuollet, E.; Laisi, S.; Salmela, E.; Ess, A.; Alaluusua, S. Molar-incisor hypomineralization and the association with childhood illnesses and antibiotics in a group of Finnish children. Acta Odontol. Scand. 2016, 74, 416–422. [Google Scholar] [CrossRef]
- Alaluusua, S. Aetiology of Molar-Incisor Hypomineralisation: A systematic review. Eur. Arch. Paediatr. Dent. 2010, 11, 53–58. [Google Scholar] [CrossRef]
- Chawla, N.; Messer, L.B.; Silva, M. Clinical studies on molar-incisor-hypomineralisation part 1: Distribution and putative associations. Eur. Arch. Paediatr. Dent. 2008, 9, 180–190. [Google Scholar] [CrossRef]
- Koruyucu, M.; Özel, S.; Tuna, E.B. Prevalence and etiology of molar-incisor hypomineralization (MIH) in the city of Istanbul. J. Dent Sci. 2018, 13, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Schmalfuss, A.J.; Sehic, A.; Brusevold, I.J. Effects of antibiotics on the developing enamel in neonatal mice. Eur. Arch. Paediatr. Dent. 2022, 23, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; Marciano, C.; Loiodice, S.; Boudalia, S.; Canivenc Lavier, M.C.; Berdal, A.; Babajko, S. Enamel hypomineralization due to endocrine disruptors. Connect. Tissue Res. 2014, 55 (Suppl. S1), 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kuscu, O.O.; Caglar, E.; Aslan, S.; Durmusoglu, E.; Karademir, A.; Sandalli, N. The prevalence of molar incisor hypomineralization (MIH) in a group of children in a highly polluted urban region and a windfarm-green energy island. Int. J. Paediatr. Dent. 2009, 19, 176–185. [Google Scholar] [CrossRef]
- Laisi, S.; Kiviranta, H.; Lukinmaa, P.L.; Vartiainen, T.; Alaluusua, S. Molar-incisor-hypomineralisation and dioxins: New findings. Eur. Arch. Paediatr. Dent. 2008, 9, 224–227. [Google Scholar] [CrossRef]
- Biondi, A.M.; Cortese, S.G.; Martínez, K.; Ortolani, A.M.; Sebelli, P.M.; Ienco, M.; Paván, V.H.; Mendel, N.; Bertolino, M.; Hecht, P. Prevalence of molar incisor hypomineralization in the city of Buenos Aires. Acta Odontol. Latinoam. 2011, 24, 81–85. [Google Scholar]
- Brejawi, M.S.; Venkiteswaran, A.; Ergieg, S.M.O.; Sabri, B.M. Correlation between Molar-Incisor Hypomineralization, Stress, and Family Functioning. J. Int. Soc. Prev. Community Dent. 2022, 12, 547–553. [Google Scholar] [CrossRef]
- Ghanim, A.; Manton, D.; Mariño, R.; Morgan, M.; Bailey, D. Prevalence of demarcated hypomineralisation defects in second primary molars in Iraqi children. Int. J. Paediatr. Dent. 2013, 23, 48–55. [Google Scholar] [CrossRef]
- Dantas-Neta, N.B.; Moura, L.F.; Cruz, P.F.; Moura, M.S.; Paiva, S.M.; Martins, C.C.; Lima, M.D. Impact of molar-incisor hypomineralization on oral health-related quality of life in schoolchildren. Braz. Oral Res. 2016, 30, e117. [Google Scholar] [CrossRef]
- Jawdekar, A.M.; Kamath, S.; Kale, S.; Mistry, L. Assessment of oral health-related quality of life (OHRQoL) in children with molar incisor hypomineralization (MIH)—A systematic review and meta-analysis of observational studies. J. Indian Soc. Pedod. Prev. Dent. 2022, 40, 368–376. [Google Scholar] [CrossRef]
- De Barros, L.V.C.; Vale, M.P.; Tourino, L.; Bittencourt, J.M.; Bendo, C.B. Determination of dental caries, molar-incisor hypomineralization, and oral health-related quality of life in schoolchildren: A structural equation modeling approach. Int. J. Paediatr. Dent. 2022, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jälevik, B.; Odelius, H.; Dietz, W.; Norén, J. Secondary ion mass spectrometry and X-ray microanalysis of hypomineralized enamel in human permanent first molars. Arch. Oral Biol. 2001, 46, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Ramesh, M. Molar incisor hypomineralisation: A review of its current concepts and management. SRM J. Res. Dent. Sci. 2014, 5, 248. [Google Scholar] [CrossRef]
- Padavala, S.; Sukumaran, G. Molar Incisor Hypomineralization and Its Prevalence. Contemp. Clin. Dent. 2018, 9, S246–S250. [Google Scholar] [CrossRef]
- Elfrink, M.E.; Veerkamp, J.S.; Aartman, I.H.; Moll, H.A.; Ten Cate, J.M. Validity of scoring caries and primary molar hypomineralization (DMH) on intraoral photographs. Eur. Arch. Paediatr. Dent. 2009, 10 (Suppl. S1), 5–10. [Google Scholar] [CrossRef]
- Petrou, M.A.; Giraki, M.; Bissar, A.R.; Basner, R.; Wempe, C.; Altarabulsi, M.B.; Schäfer, M.; Schiffner, U.; Beikler, T.; Schulte, A.G.; et al. Prevalence of Molar-Incisor-Hypomineralisation among school children in four German cities. Int. J. Paediatr. Dent. 2014, 24, 434–440. [Google Scholar] [CrossRef]
- Lygidakis, N.A.; Dimou, G.; Briseniou, E. Molar-incisor-hypomineralisation (MIH). Retrospective clinical study in Greek children. I. Prevalence and defect characteristics. Eur. Arch. Paediatr. Dent. 2008, 9, 200–206. [Google Scholar] [CrossRef]
- Glodkowska, N.; Emerich, K. Molar Incisor Hypomineralization: Prevalence and severity among children from Nothern Poland. Eur. J. Paediatr. Dent. 2019, 20, 59–66. [Google Scholar] [CrossRef]
- Allazzam, S.M.; Alaki, S.M.; El Meligy, O.A. Molar incisor hypomineralization, prevalence, and etiology. Int. J. Dent. 2014, 2014, 234508. [Google Scholar] [CrossRef]
- Calderara, P.C.; Gerthoux, P.M.; Mocarelli, P.; Lukinmaa, P.L.; Tramacere, P.L.; Alaluusua, S. The prevalence of Molar Incisor Hypomineralisation (MIH) in a group of Italian school children. Eur. J. Paediatr. Dent. 2005, 6, 79–83. [Google Scholar]
- Jälevik, B.; Klingberg, G.; Barregård, L.; Norén, J.G. The prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Acta Odontol. Scand. 2001, 59, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Leppäniemi, A.; Lukinmaa, P.L.; Alaluusua, S. Nonfluoride hypomineralizations in the permanent first molars and their impact on the treatment need. Caries Res. 2001, 35, 36–40. [Google Scholar] [CrossRef]
- Da Costa-Silva, C.M.; Jeremias, F.; de Souza, J.F.; Cordeiro Rde, C.; Santos-Pinto, L.; Zuanon, A.C. Molar incisor hypomineralization: Prevalence, severity and clinical consequences in Brazilian children. Int. J. Paediatr. Dent. 2010, 20, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.T.; Alhasan, H.A.; Qari, M.T.; Sabbagh, H.J.; Farsi, N.M. Prevalence and risk factors of molar incisor hypomineralization in the Middle East: A systematic review and meta-analysis. J. Taibah. Univ. Med. Sci. 2023, 18, 696–710. [Google Scholar] [CrossRef]
- Leibowiz Haviv, S.; Zilberman, U.; Aboud, M. MIH Survey among Youths in the City of Ashkelon, Israel. In Proceedings of the IADR/PER Congress. Abstract 0241, Jerusalem, Israel, 20 September 2016; Available online: https://iadr.abstractarchives.com/abstract/per16-2529502/mih-survey-among-youths-in-the-city-of-ashkelon-israel (accessed on 18 April 2023).
- Elfrink, M.E.; Schuller, A.A.; Weerheijm, K.L.; Veerkamp, J.S. Hypomineralized second primary molars: Prevalence data in Dutch 5-year-olds. Caries Res. 2008, 42, 282–285. [Google Scholar] [CrossRef]
- Zakirulla, M.; Alasiri, M.A.; Alshahrani, M.R.; Alkhairy, M.I.; Laheq, M.T.; Althuqbi, A.A.; Asiri, H.I.A.; Almalki, A.Y. Prevalence of hypomineralization in second primary molars (HSPM) in 7 to 10-year-old Saudi children. J. Res. Med. Dent. Sci. 2020, 8, 124–127. [Google Scholar]
- Lygidakis, N.A.; Garot, E.; Somani, C.; Taylor, G.D.; Rouas, P.; Wong, F.S.L. Best clinical practice guidance for clinicians dealing with children presenting with molar-incisor-hypomineralisation (MIH): An updated European Academy of Paediatric Dentistry policy document. Eur. Arch. Paediatr. Dent. 2022, 23, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.; Moll, H.A.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; Hofman, A.; ten Cate, J.M.; Veerkamp, J.S. Pre- and postnatal determinants of deciduous molar hypomineralisation in 6-year-old children. The generation R study. PLoS ONE 2014, 9, e91057. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.; ten Cate, J.M.; Jaddoe, V.W.; Hofman, A.; Moll, H.A.; Veerkamp, J.S. Deciduous molar hypomineralization and molar incisor hypomineralization. J. Dent. Res. 2012, 91, 551–555. [Google Scholar] [CrossRef]
- Elger, W.; Illge, C.; Kiess, W.; Körner, A.; Kratzsch, J.; Schrock, A.; Hirsch, C. Relationship between deciduous molar hypomineralisation and parameters of bone metabolism in preschool children. Int. Dent. J. 2020, 70, 303–307. [Google Scholar] [CrossRef]
- Temilola, O.D.; Folayan, M.O.; Oyedele, T. The prevalence and pattern of deciduous molar hypomineralization and molar-incisor hypomineralization in children from a suburban population in Nigeria. BMC Oral. Health 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
| DMH N = 72 | MIH N = 68 | MIH + DMH N = 15 | χ2 (df) | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Child’s sex, n (%) | |||||
| Female | 37 (52.1%) | 29 (42.7%) | 11 (73.3%) | 4.86 (2) | 0.09 |
| Male | 34 (47. 9%) | 39 (57.4%) | 4 (26.7%) | ||
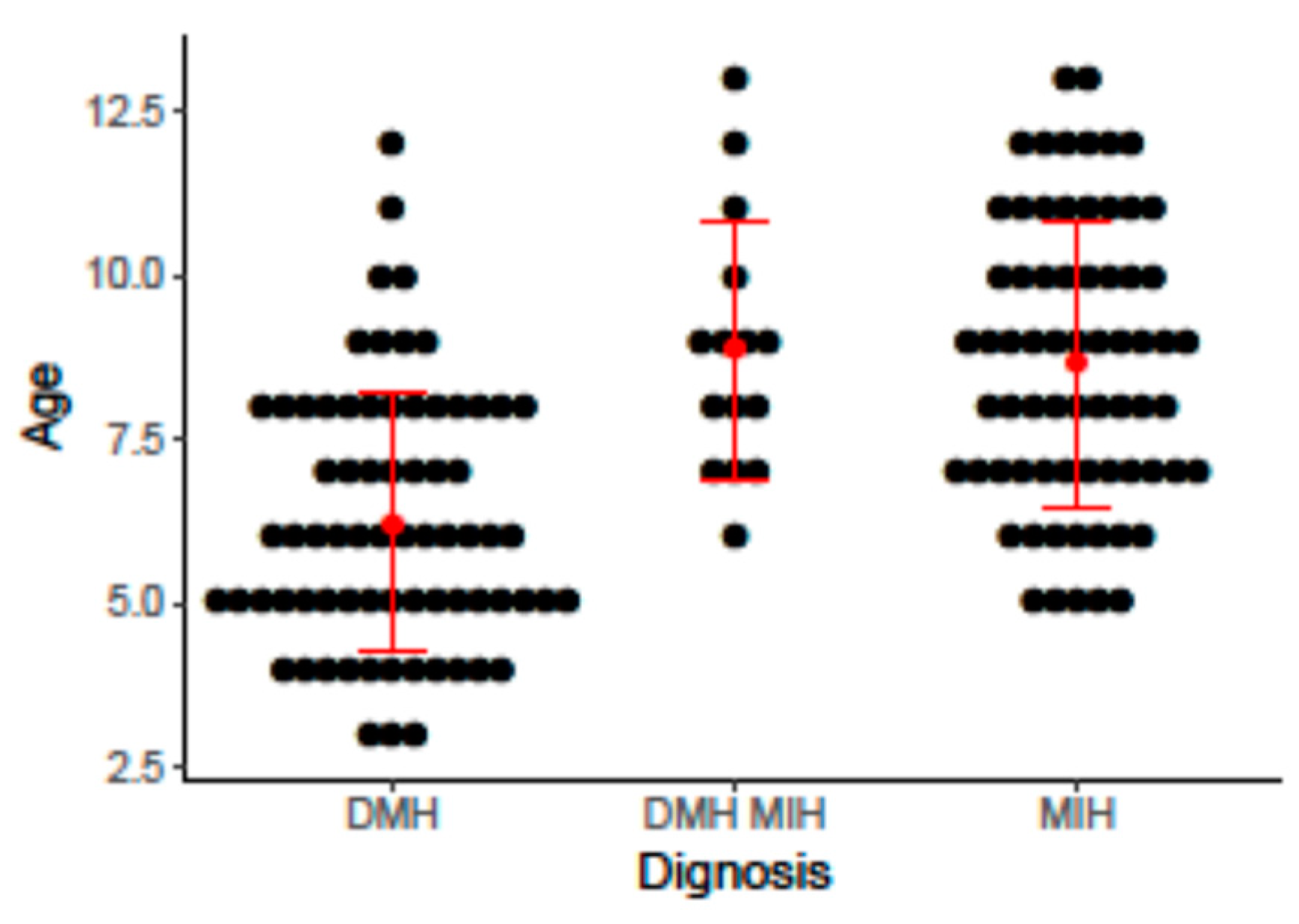
| Child’s age at MIH/DMH diagnosis, years, mean (SD) [range] | 6.23 (1.97) [3,4,5,6,7,8,9,10,11,12] | 8.65 (2.18) [5,6,7,8,9,10,11,12,13] | 8.87 (1.96) [6,7,8,9,10,11,12,13] | <0.01 | |
| Age categories, n (%) | |||||
| ≤5 years | 31 (43.7%) | 5 (7.4%) | 0 (0%) | 30.63 (2) | <0.01 |
| >5 years | 40 (56.3%) | 63 (92.7%) | 15 (100.0%) | ||
| Ethnicity, n (%) | |||||
| Jewish | 69 (97.2%) | 62 (91.2%) | 13 (86.7%) | 3.34 (2) | 0.19 |
| Arab | 2 (2.8%) | 6 (8.8%) | 2 (13.3%) | ||
| Parents’ place of residence, n (%) | |||||
| Urban | 68 (95.8%) | 59 (86.8%) | 13 (86.7%) | 3.77 (2) | 0.15 |
| Rural | 3 (4.2%) | 9 (13.2%) | 2 (13.3%) | ||
| Pregnancy characteristics | |||||
| Conception, n (%) | |||||
| Spontaneous | 68 (95.8%) | 64 (94.1%) | 12 (80.0%) | 5.15 (2) | 0.08 |
| In vitro fertilization | 3 (4.2%) | 4 (5.9%) | 3 (20.0%) | ||
| Illness during pregnancy, n (%) | 7 (9.9%) | 3 (4.4%) | 1 (6.7%) | 1.56 (2) | 0.46 |
| High-risk pregnancy, n (%) | 5 (7.0%) | 6 (8.8%) | 2 (13.3%) | 0.66 (2) | 0.72 |
| Any medication during the pregnancy, n (%) | 6 (8.5%) | 2 (2.9%) | 1 (7.7%) | 1.94 (2) | 0.04 |
| Delivery, n (%) | |||||
| Regular | 60 (84.5%) | 50 (73.5%) | 12 (80.0%) | 3.95 (4) | 0.41 |
| Cesarean section | 7 (9.8%) | 13 (19.1%) | 3 (20.0%) | ||
| Vacuum | 4 (5.6%) | 5 (7.4%) | 0 | ||
| Analgesics during delivery, n (%) | |||||
| Epidural | 66 (93.0%) | 61 (89.7%) | 14 (93.3%) | 1.82 (4) | 0.77 |
| Nitrous oxide | 2 (2.8%) | 1 (1.5%) | 0 | ||
| None | 3 (4.2%) | 6 (8.8%) | 1 (6.7%) | ||
| Gestational week at birth, n (%) | |||||
| 24–37 | 3 (4.2%) | 5 (7.5%) | 0 | 1.60 (2) | 0.45 |
| >37 | 68 (95.8%) | 63 (92.7%) | 15 (100.0%) | ||
| Birth weight, kg, n (%) | |||||
| <1 | 1 (1.4%) | 1 (1.5%) | 0 | 1.70 (4) | 0.79 |
| 1–2.5 | 5 (7.0%) | 6 (8.8%) | 0 | ||
| >2.5 | 65 (91.6%) | 61 (89.7%) | 15 (100.0%) | ||
| Child’s hospitalization, n (%) | |||||
| During the first year of life | 13 (18.3%) | 6 (8.8%) | 2 (13.3%) | 2.65 (2) | 0.26 |
| During the second year of life | 5 (7.0%) | 4 (5.9%) | 3 (20.0%) | 3.51 (2) | 0.17 |
| During the third year of life | 1 (1.4%) | 4 (6.0%) | 0 | 2.83 (2) | 0.24 |
| Child’s illness, n (%) | |||||
| During the first year of life | 20 (28.2%) | 12 (17.7%) | 4 (26.7%) | 2.25 (2) | 0.32 |
| During the second year of life | 18 (25.4%) | 10 (14.7%) | 3 (20.0%) | 2.45 (2) | 0.29 |
| During the third year of life | 13 (18.3%) | 11 (16.2%) | 1 (6.7%) | 1.23 (2) | 0.54 |
| The child received antibiotics, n (%) | |||||
| During the first year of life | 35 (49.3%) | 27 (39.7%) | 7 (46.7%) | 1.31 (2) | 0.51 |
| During the second year of life | 24 (33.8%) | 25 (36.8%) | 7 (46.7%) | 0.89 (2) | 0.64 |
| During the third year of life | 19 (26.8%) | 20 (29.4%) | 5 (33.3%) | 0.30 (2) | 0.86 |
| The child had an ear infection, n (%) | |||||
| During the first year of life | 21 (29.6%) | 17 (25.0%) | 4 (26.7%) | 0.37 (2) | 0.83 |
| During the second year of life | 16 (22.5%) | 14 (20.6%) | 4 (26.7%) | 0.28 (2) | 0.87 |
| During the third year of life | 12 (16.9%) | 10 (14.7%) | 4 (26.7%) | 1.25 (2) | 0.53 |
| MIH/DMH severity, n (%) | |||||
| Mild | 30 (42.3%) | 42 (61.8%) | 4 (26.7%) | 8.71 (2) | 0.01 |
| Severe | 41 (57.8%) | 26 (38.2%) | 11 (73.3%) |
| Variable | B | SE | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|
| Child’s age at diagnosis: >5 years (vs. <5 years) | 0.35 | 0.14 | 1.42 (1.10–1.90) | 0.01 |
| Severity of hypomineralization: severe (vs. mild) | 1.43 | 0.65 | 4.18 (1.26–17.16) | 0.03 |
| Medications during pregnancy: yes (vs. no) | 0.59 | 1.14 | 1.80 (0.09–12.54) | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenstein Ajzman, G.; Dagon, N.; Iraqi, R.; Blumer, S.; Fadela, S. The Prevalence of Developmental Enamel Defects in Israeli Children and Its Association with Perinatal Conditions: A Cross-Sectional Study. Children 2023, 10, 903. https://doi.org/10.3390/children10050903
Berenstein Ajzman G, Dagon N, Iraqi R, Blumer S, Fadela S. The Prevalence of Developmental Enamel Defects in Israeli Children and Its Association with Perinatal Conditions: A Cross-Sectional Study. Children. 2023; 10(5):903. https://doi.org/10.3390/children10050903
Chicago/Turabian StyleBerenstein Ajzman, Gisela, Nurit Dagon, Rabea Iraqi, Sigalit Blumer, and Shada Fadela. 2023. "The Prevalence of Developmental Enamel Defects in Israeli Children and Its Association with Perinatal Conditions: A Cross-Sectional Study" Children 10, no. 5: 903. https://doi.org/10.3390/children10050903
APA StyleBerenstein Ajzman, G., Dagon, N., Iraqi, R., Blumer, S., & Fadela, S. (2023). The Prevalence of Developmental Enamel Defects in Israeli Children and Its Association with Perinatal Conditions: A Cross-Sectional Study. Children, 10(5), 903. https://doi.org/10.3390/children10050903






