Abstract
Pregnancy anamnesis is a crucial part of child and adolescent psychiatry diagnostics. In previous works, the reliability of retrospective maternal self-report on perinatal characteristics was heterogeneous. This prospective longitudinal study aimed to evaluate women’s recall of prenatal events in a within-subject design. A sample of 241 women gave a self-report on prenatal alcohol, smoking, partnership quality, pregnancy satisfaction, and obstetric complications during the 3rd trimester (t0), childhood (t1, 6–10 y), and adolescence (t2, 12–14 y). The intra-individual agreement was examined. The t0–t1–(t2) agreement was poor to substantial; this was highest for smoking and worst for obstetric complications, followed by alcohol (Fleiss’ κ = 0.719 to −0.051). There were significant t0–t1–(t2) differences for all pregnancy variables (p < 0.017), except for 3rd trimester satisfaction (p = 0.256). For alcohol (t0 25.8%, t1 17.4%, t2 41.0%) and smoking (t0 11.9%, t1 16.4%, t2 22.6%), the highest self-reported rates were found during adolescence. During childhood, fewer obstetric complications (t0 84.9%, t1 42.2%) and worse partnerships were reported (t0 M = 8.86, t1 M = 7.89). Thought to be due to social stigmata and memory effects, pregnancy self-reports cannot be precisely reproduced. Creating a respectful and trusting atmosphere is essential for mothers to give honest self-reports that are in the best interest of their children.
Keywords:
prenatal; pregnancy; self-report; alcohol; smoking; partnership; pregnancy satisfaction; risk perceptions; retrospective 1. Introduction
Both external and internal stressors influence multiple psychiatric disorders during the prenatal period. Emotional (e.g., anxiety, depression) as well as behavioural disorders (e.g., attention-deficit/hyperactivity disorder [ADHD], conduct disorders, or attachment disorders) are known to be linked to prenatal adversity [1,2,3]. Maternal psychosocial burden, mental illnesses or obstetric complications, notably prenatal toxin exposure, impair child neurodevelopment via fetal programming.
Several studies report the effects of maternal alcohol consumption and smoking during pregnancy on intrauterine development, as these toxins cross the blood-placental barrier [4,5,6]. Worldwide, almost every 10th child is estimated to suffer from prenatal alcohol exposure (PAE), with data peaking in Europe (reaching 25%), despite explicit information and awareness programs as well as high-standard medical care [7]. Severe impairments in a combination of cognitive, sensorimotor, neurophysiological, neurological and psychiatric functioning, as a consequence of maternal drinking, are described as Fetal Alcohol Spectrum Disorder (FASD). Facial stigmata, including microcephaly, hypertelorism, a low nasal bridge, thin upper lip, and smooth philtrum might also be diagnosed in the most severe form (Fetal Alcohol Syndrome, FAS), but are often non-existent in subclinical manifestations [8,9]. Therefore, FASD is often missed and presumably underdiagnosed [10]. A delayed diagnosis limits the therapeutic possibilities and increases the risk of poor clinical outcomes. Hence, early interventions are essential for the best possible quality of life for PAE-affected patients [11]. PAE is also a proven risk factor for ADHD development in childhood and adolescence. However, neurobiological etiology is suspected to differ between PAE-linked ADHD and non-PAE ADHD symptoms [12,13].
The global prevalence of smoking during pregnancy is between 14 to 38%, having fortunately decreased since the 1990’s in developed countries. However, the rate of smoking cessation during pregnancy is reported as 27–47%, indicating that between one in four and one in two fetuses of a previously smoking woman are still exposed to tobacco in utero. Furthermore, the literature indicates an increasing prevalence in developing countries [14]. Smoking and stand-alone nicotine consumption (e.g., via e-cigarettes) may induce embryotic physical abnormalities such as reduced head size or body length, limb reduction, and orofacial clefts. Further effects are cardiovascular risk profiles, obstructive respiratory diseases, impaired immune system responses, and an increased risk of the fetus being preterm or stillborn [15,16]. Besides somatic effects, in utero nicotine and smoking exposure is likely to cause neurocognitive (executive or sensory) functioning and behavioural problems such as ADHD or conduct disorders. The risk of nicotine addiction during the offspring’s adulthood is also increased [17]. The impetus of maternal smoking on other mental diseases such as anxiety or depression is discussed heterogeneously [14,18]. Nevertheless, a history of toxin contact of any kind is hence more likely among psychiatric patients.
Obstetric complications, e.g., bleeding, nausea, gestational diabetes mellitus, preeclampsia or infections, not only impact the infant’s somatic outcome, but also take effect indirectly. Prenatal psychosocial factors such as mothers’ stress level, mood, and partnership are proven to influence the offspring’s psychological and neurological development, cognitive ability, and fearfulness [19,20]. As mechanisms, altered cortisol release and glucocorticoid receptor sensitivity via DNA-methylation as well as changes in neuropeptides, neurotransmitters (e.g., glutamate), and impairment of the hypothalamic-pituitary-adrenal-axis have been identified [21,22,23]. Furthermore, epigenetic variations of the oxytocin receptor genotype are discussed to influence the infant’s susceptibility to stress [24]. Changes in the cortical neural structures responsible for cognitive-emotional, sensory, and socio-emotional functioning were identified via diffusion-weighted MRI scans [25]. The literature indicates an increased risk for mental illnesses in the offspring, such as anxiety, depression, and externalising disorders, as consequences of maternal psychosocial stress during pregnancy [26].
Based on the increasing prevalence of various psychiatric disorders among minors and growing parental stress levels, not least because of the COVID-19 pandemic, sufficient and impactful diagnostics in child and adolescent psychiatry are essential to identify pathological mechanisms and provide effective therapy solutions [27,28]. Current medical practice guidelines demand a sufficient pregnancy and birth anamnesis, particularly with parents or further caregivers, or both, when assessing mental disorders among minors [29,30,31]. The International Classification of Diseases (ICD-10) describes disorders originating in the perinatal period in chapters P90–96 [32]. The Diagnostic Classification of Mental Health and Developmental Disorders of Infancy and Early Childhood (DC: 0–5) includes ‘prenatal conditions and exposures’ (e.g., to medications, alcohol, other substances or teratogens) on axis III (physical health conditions and considerations) and ‘pregnancy-related stressors’ to be diagnosed on axis IV (psychosocial stressors) [33]. ICD-11 redefines these categories under Chapter 19: ‘certain conditions originating in the perinatal period’, specified as ‘Fetus or newborn affected by maternal factors or by complications of pregnancy, labour or delivery’ and exposure to ‘noxious influences transmitted via placenta or breast milk’ [34].
Thus, for child and adolescent psychiatry clinicians, pregnancy anamnesis is a crucial tool for diagnosis. Exposure-specific biomarkers for detecting maternal prenatal substance use are current subjects of research but are still limited in their validity for clinical usage. Furthermore, these biomarkers are limited to prenatal utilization and are not available during later child and adolescent psychiatric assessments [14,35]. Therefore, third-party interviews (e.g., with parents) most commonly in face-to-face or questionnaire format, or both, are a viable and easy-to-perform diagnostic method. Pre- and perinatal stress is often assessed by various instruments, for instance using the ‘Prenatal Distress Questionnaire’, ‘Pregnancy Experience Scale’ or ‘Prenatal Social Environment Inventory’ [36,37]. They are expected to provide substantial information on patients’ symptom development and possible psychosocial and biological influencing factors. However, this method has several limitations, pointed out by mothers differing in (repeated) interviews about their gravidity in previous works. Suspected reasons for under- or over-reporting include recall biases, e.g., because of long time gaps or maternal psychiatric disorders that may influence memory and rumination biases. Furthermore, the concealment of substance use or maternal psychological stress is possible due to social stigmata, social desirability or conscience issues, where cultural and ethnic influences may play a role as well [38,39,40,41]. Hence, the potential development of adolescent psychiatric disorders in (initially) subclinical children is a considerable risk.
Study aims: The aim of this longitudinal prospective cohort study, with three points of data collection, was to validate women’s subjective self-reports regarding hard-risk pregnancy factors, such as substance use (alcohol, smoking). In addition, self-reports of emotional soft-risk pregnancy characteristics, such as psychosocial parameters, were evaluated. To date, their assessment is not generally standardized, and therefore, pregnancy risks are difficult to detect, resulting in a low number of studies addressing this concern. Current literature addresses maternal recall validity predominantly via single- or two-point data collection and comparison to mostly objective birth data collection in the perinatal examination, postnatal visits or national databases [19,38]. We intended to review the gap in evaluating subjective parental interviews as an integral element of anamnesis. We also aimed to detect potential changes in mothers’ self-report during their children’s development and discuss possible age-specific contributing factors.
2. Materials and Methods
2.1. Study Design and Sample Characteristics
This study is based on data from the Franconian Cognition and Emotion Studies (FRANCES) [12,42], a follow-up cohort study of the prospective longitudinal Franconian Maternal Health Evaluation Studies (FRAMES) [43]. Women in their 3rd trimester, with a minimum age of 18 years and 30 weeks of gestation time, were recruited at the Department of Obstetrics and Gynecology (n = 1100) from 2005 to 2007 (t0). The 2nd (t1) and 3rd (t2) assessments were conducted at the Department of Child and Adolescent Mental Health. Between 2012 and 2015, when children attended primary school (age 6 to 10 years), a subsample with n = 618 (56.2%) of these women was contacted again for re-participation in the FRANCES I follow-up (t1). This FRANCES I cohort was contacted again from 2019 to 2021, during the children’s early adolescence (age 12 to 14 years), for a second data acquisition (FRANCES II, t2) [9]. Out of n = 618 contacted FRAMES-women, n = 245 (with n = 248 children) participated at t1 (39.6%; age of children: M = 7.74, SD = 0.74, range 6.00–9.90). All families who attended t1 were contacted again; 76% (n = 186 mothers with n = 188 adolescents) of the FRANCES I sample re-participated in FRANCES II (age of children: M = 13.3, SD = 0.34, range 12.8–14.5) (see Figure 1). When comparing t2 participating families with t2 non-participating families, no differences in marital status (χ2(1) = 0.35, p = 0.552), family income (χ2(4) = 3.94, p = 0.414) or maternal total psychopathology (t(234) = −0.93, p = 0.353) at t1 were found. However, higher-educated mothers were more willing to re-participate (χ2(1) = 7.60, p = 0.006).
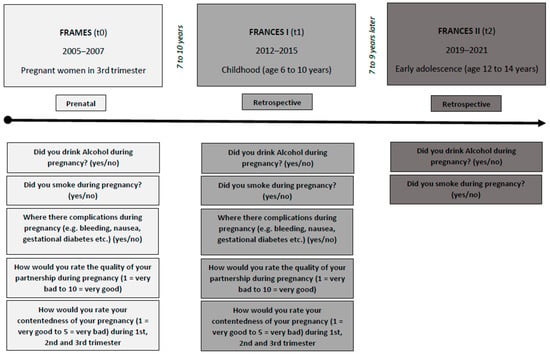
Figure 1.
The study design and questioning of FRAMES (t0), FRANCES I (t1) and FRANCES II (t2).
For present data analyses, after the exclusion of two twin pairs, valid t1 data of n = 241 and valid t2 data of n = 183 mother-child dyads were available. It was first gravidity for 99 women (41.1%) of the cohort, second for 75 (31.1%) and third or higher for 77 (27.8%). A total of 190 mothers (78.8%) were nullipara. A total of 228 mothers (94.6%) had German nationality, and 13 (5.4%) had another (including dual) citizenship. At t0, 73 mothers (30.8%) rated above the cut-off for possible depression on the Edinburgh Postnatal Depression Scale (EPDS) [44], and the cohort’s mean t0-rating was below the cut-off (M = 6.86, SD = 4.90). In the follow-ups, 59 (25.4%) mothers at t1 and 20 (10.9%) at t2 self-reported depression. The demographic sample characteristics are summarized in Table 1 and the study design is shown in Figure 1.

Table 1.
The sample characteristics (mothers, fathers, children) and over-time differences.
2.2. Prenatal and Retrospective Self-Report
Alcohol consumption (t0, t1, t2): At t0, women were asked in a structured interview (face-to-face format) about their drinking and smoking behaviour during pregnancy. The question ‘Did you drink alcohol during your current pregnancy?’ could be answered with the response alternatives: ‘No, I generally do not drink’, ‘No, I did not drink during pregnancy’, ‘Yes, I rarely drank during pregnancy’, ‘Yes, I drank 1 glass /day during pregnancy’ and ‘Yes, I drank more than 1 glass /day during pregnancy’. For t1 and t2, women answered standardized questions (paper-and-pencil format) with the options: ‘No, I did not drink alcohol during my pregnancy’ or ‘Yes, I did drink alcohol during my pregnancy’, followed by a quantification via glasses per day/week/month/trimester. For analysis, data were dichotomized: Women declaring no alcohol consumption during pregnancy (0 = ‘I did not drink alcohol during pregnancy’) vs. women declaring alcohol consumption during pregnancy (1 = ‘I drank alcohol during pregnancy’).
Smoking behaviour (t0, t1, t2): In the 3rd-trimester interview and t1/t2 questionnaires, women chose between the following options: ‘No, I am a non-smoker’, ‘Yes, I smoked before pregnancy’, ‘I have stopped smoking since I knew I was pregnant’ and ‘Yes, I smoke(d) during pregnancy’. Where applicable, participants were asked to quantify the number of cigarettes smoked per day. The data were also split into a nominal scale: Women stating no nicotine consumption (0 = ‘No, I am a non-smoker’ and ‘Yes, I smoked before pregnancy’) versus women stating nicotine consumption (1 = ‘Yes, I smoked during pregnancy’, ‘I have stopped smoking since I knew I was pregnant’).
Obstetric complications (t0, t1): Mothers provided information on the dichotomous categories: ‘There were complications during my pregnancy’ or ‘There were no complications during my pregnancy’, including further differentiation where necessary (e.g., bleeding, nausea, gestational diabetes, among others).
Partnership quality and pregnancy satisfaction (t0, t1): For an estimation of prenatal partnership quality and pregnancy satisfaction during the 1st, 2nd, and 3rd trimesters, Likert-Scale ratings were given by the mothers (partnership: 1 = very bad to 10 = excellent; pregnancy satisfaction analogous to German school grades 1 = very good to 5 = very bad).
2.3. Statistical Analyses
Data were analyzed using IBM® SPSS® Statistics (Version 24.0). The level of analysis significance was defined as p < 0.05 (two-tailed). Normal (Gaussian) distribution was evaluated via the Kolmogorov-Smirnov test. In the first step, intraindividual agreement was determined by Fleiss’ (3 points of data collection) or Cohen’s (2 points of data collection) Kappa (κ) and interpreted as <0.00 (poor), 0.00–0.20 (slight), 0.21–0.40 (fair), 0.40–0.60 (moderate), 0.61–0.80 (substantial), and 0.81–1.00 (almost perfect) for categorical data. For interval-scaled continuous variables, the intra-individual agreement was calculated using Spearman’s correlations (rs), with |rs| ≥ 0.10 considered low, |rs| ≥ 0.30 moderate, and |rs| ≥ 0.50 strong or high relations.
Additionally, group comparisons for repeated or dependent measures were performed by Cochran’s Q Test with post hoc Dunn Test (alcohol consumption and smoking behavior: dichotomous at t0, t1, t2), McNemar Test (obstetric complications: dichotomous at t0, t1), Wilcoxon signed-rank test with effect size measure r, interpreted as 0.1–0.3 (weak), 0.3–0.5 (moderate) and >0.5 (strong) (partnership quality and pregnancy satisfaction: continuous at t0, t1). An overview of the data scale levels and the corresponding statistical tests are shown in Table 2.

Table 2.
The data arrangement and statistic methods.
3. Results
3.1. Alcohol Consumption
Figure 2A shows the t0–t1–t2 self-report data. Fleiss’ κ was slight (κ = 0.203) for mothers’ t0–t1–t2 reports on alcohol consumption. Cochran’s Q showed significant differences for positive t0–t1–t2 answers (‘Yes, I did drink alcohol during my pregnancy’) (p < 0.001), for which the post hoc analysis yielded a significantly higher number of positive recalls at t2 (n = 73; [41.0%]) compared to t1 (n = 31; [17.4%]; p < 0.01) and t0 (n = 46 [25.8%]; p = 0.001). No significant differences were shown between t0 and t1 (p = 0.143) (see Table 3).
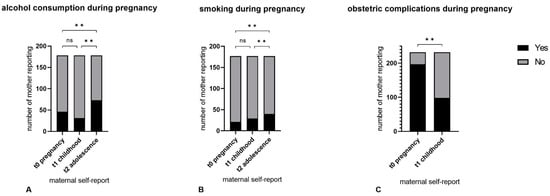
Figure 2.
The t0–t1–t2 self-report data on alcohol (A), smoking (B) and obstetric complications (C) during pregnancy. ** p < 0.01, ns: not significant.

Table 3.
The statistics for maternal alcohol consumption, smoking, and complications during pregnancy (dichotomous items).
3.2. Smoking Behaviour
Figure 2B shows the t0–t1–t2 self-report data. Maternal self-reports on prenatal smoking behaviour yielded substantial t0–t1–t2 agreement (Fleiss’ κ = 0.719; p < 0.01). Cochran’s Q showed significant t0–t1–t2 differences (p < 0.01). Post hoc analysis revealed a significantly higher number of positive recalls at t2 (n = 40; [22.6%]) compared to t1 (n = 29; [16.4%]; p = 0.01) and t0 (n = 21; [11.9%]; p < 0.001). No significant differences were found between t0 and t1 (p = 0.98) (see Table 3).
3.3. Complications during Pregnancy
Cohen’s κ showed poor strength of t0–t1 agreement (κ = −0.051). Significantly fewer mothers reported pregnancy or birth complications at t1 (n = 98 [42.2%]) compared to t0 (n = 197; [84.9%]; p < 0.001) (see Figure 2, Table 3).
Figure 3 shows the t0–t1 self-report data. The t0–t1 agreement of mother-rated partnership quality during pregnancy was moderate (Spearman’s t0–t1 correlation rs = 0.371, pr < 0.01). Wilcoxon signed-rank test yielded a significantly lower estimation of partnership quality at t1 (M = 7.89 ± 2.05) than at t0 (M = 8.86, SD = 1.19; p < 0.001) at a nearly strong effect size (r = 0.49) (see Table 4).
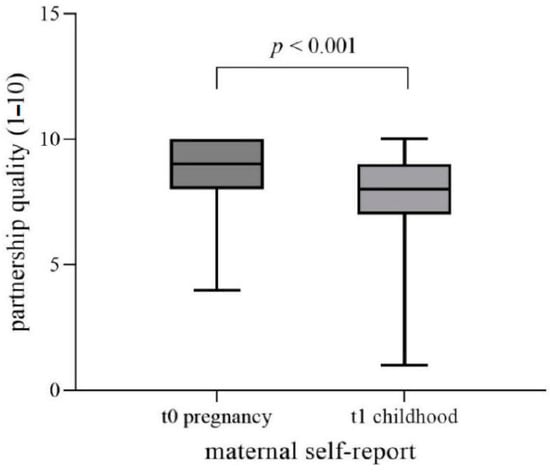
Figure 3.
The t0–t1 self-report data for partnership quality during pregnancy. Rating of partnership quality via Likert-Scale: 1 = very bad to 10 = excellent.

Table 4.
The statistics for mother ratings of quality of partnership and subjective satisfaction with pregnancy (continuous items).
3.4. Subjective Satisfaction with Pregnancy
Figure 4 shows the t0–t1 self-report data. For all trimesters, the t0–t1 agreement of mother-rated pregnancy satisfaction was moderate (Spearman’s t0–t1 correlation for 1st trimester: rs = 0.544; pr < 0.01; 2nd trimester: rs = 0.525; pr < 0.01; 3rd trimester: rs = 0.467; p < 0.01). Wilcoxon signed-rank test yielded significant differences in pregnancy satisfaction reports for 1st and 2nd trimesters (however, not for 3rd trimester, p = 0.256): for the 1st trimester, subjective pregnancy satisfaction was reported higher (resulting in lower values) at t1 (M = 2.28, SD = 1.16) compared to t0 (M = 2.50, SD = 1.20) (p = 0.003); for the 2nd trimester, vice versa (t1: M = 2.06 ± 1.03; t0: M = 1.91 ± 0.93; p = 0.017) (see Table 4).
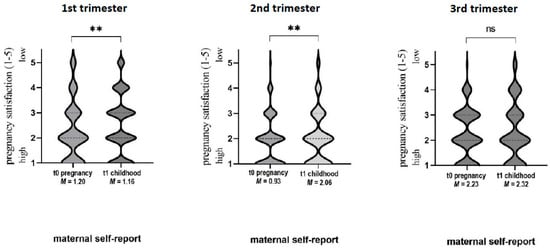
Figure 4.
The t0–t1 self-report data for pregnancy satisfaction. Rating of pregnancy satisfaction via Linkert-Scale analogous to German school grades 1 = very good to 5 = very bad: ** p < 0.01, ns: not significant, M: mean.
4. Discussion
This study examined the retrospective reliability of women’s self-report on pregnancy events in a longitudinal within-subject design study. We intended to detect potential changes in the mothers’ self-reports from pregnancy over childhood and into adolescence to give clinicians a valid basis for self-report interpretation in child and adolescent mental health anamnesis. For that purpose, we tried to differentiate more reliable from less reliable domains of maternal retrospective pregnancy anamnesis. We compared current and retrospective maternal self-reports on prenatal substance use, obstetric complications, and psychosocial well-being at three measurement points (pregnancy, childhood, and adolescence).
Substance use: The maternal self-report on prenatal alcohol consumption fluctuated. We demonstrated an over-time decrease (childhood) followed by a nearly doubled increase (adolescence), with the highest rates in adolescence. The intra-individual agreement was only slight. This corresponds with earlier findings, where retrospective maternal ratings of alcohol use showed low agreement with birth data: Ramos et al. reported κ = 0.23 within seven and a half years [45]. Hannigan et al. showed significantly higher reports of drinking during adolescence (age 14 y) than at birth, with an over tenfold increase (p < 0.001) in consumed alcohol, matching with, and even surpassing, our results [46]. Similar findings were demonstrated by Jacobson et al. in 2002, where mothers reported higher alcohol consumption at t1 (M = 0.88 oz/d) than before birth (M = 0.23 oz/d; p < 0.001) but with a higher agreement (r = 0.60, p < 0.001) than our data. It is worth noting that they applied a shorter t0–t1 gap (13 months after birth) [41]. The TRAILS Study revealed a low Cohen’s κ for recollection agreement (κ = 0.03–0.11), similar to our results, and concluded over-reporting [38] as here the national birth databank was taken as the gold standard (‘true’ data), and so under-reporting at birth was assumed. Even further studies support the under-reporting of alcohol consumption during pregnancy [47,48]. If the probability of obtaining reliable self-reports on prenatal alcohol consumption increases with child age, our data suggest a substance use rate of >40% in a German general population cohort.
The literature on prenatal smoking, however, as demonstrated by our data, shows more consistent maternal self-reporting. We were able to replicate substantial retrospective agreements in this regard, published by TRAILS (κ = 0.77, with balanced rates of over- and under-reporting around 5–6%) [38], Pickett et al. (κ = 0.75) [39] and Ramos et al. (κ = 0.65) [45]. It has to be noted that these studies used a longitudinal study design with only two points of data collection, whereas our study design included three (t0–t1–t2). Furthermore, our statistical tests confirm the agreement between t0 and t1 (6 to 9 years after pregnancy report) regarding smoking during pregnancy but indicate significant differences compared to questioning after a longer period (12 to 14 years after pregnancy report, t2). Similar to alcohol consumption, maternal report rates raised from pregnancy to adolescence, as our data revealed a doubling of positive smoking reports from offspring’s birth to adolescence. This is supported by Czeizel et al., who postulated low reliability for retrospective self-reports on prenatal smoking in a cohort of children with suspected nicotine-related congenital defects [48]. Similar to the self-reports on prenatal drinking, women might under-report tobacco use at birth or during childhood and primarily come out with ‘true’ statements later on. According to cultural norms [38,39,40,41], cognitive avoidance behaviour—during pregnancy due to social stigmata, during childhood due to fear of negative consequences for child development—might contribute to the mother’s negation of prenatal alcohol consumption [42]. Recall biases certainly play a role but cannot explain the systematically higher reported rates in adolescence. More precise retrospective self-reports on prenatal smoking behaviour might be explained by less social stigma or less public knowledge of child developmental consequences, or both.
For the clinical setting, our results confirm the highest reliability of maternal retrospective anamnesis on prenatal substance use during the offspring’s adolescence. At this age, youths often present in the clinical psychiatric setting with symptoms of depression, anxiety, eating disorders or substance use. At primary school age, patients predominantly show symptoms of ADHD, conduct disorders, emotional disorders or autism spectrum disorders [49]. Particularly in ADHD and conduct disorders, the literature stated the predisposing risk of prenatal alcohol and smoking exposure [12,13,14,18]. Thus, clinicians are troubled because the maternal retrospective self-report on substance use during pregnancy seems less reliable during this illness-specific age period. Impaired diagnostic procedures might result in delayed diagnosis and therapy. Diagnostic awareness and sufficient methods are required to gather this information, particularly during pathognomonic periods. Third-party interviews, e.g., fathers or relatives, are further diagnostic sources. In our study, no data from third-party evaluations were included, which also poses a limitation. First and foremost, creating a respectful and trusting atmosphere is essential to enable the mother herself to give reliable self-reports, free of shame and in the best interest of the child.
Obstetric complications: Our data showed a significantly smaller number of obstetric complications reported at t1 compared to the pre-birth assessment. The number of reported complications halved from pregnancy to childhood. This reinforces the findings by Dietz et al., who reported poor retrospective maternal agreement, particularly on placenta praevia and urinary tract infections [50]. They hypothesize that women missing medical knowledge, unspecific questioning, and recall bias as possible explanations. Ramos et al. demonstrated a heterogeneous retrospective agreement for obstetric complications self-reports, strongly depending on differentiated types of medical problems [45]. As our questioning was widely generalized, obstetric complications were less likely to be retrospectively assessed. Recall bias remains a notable limitation in our study as in earlier works.
Psychosocial prenatal risks: Partnership quality and personal satisfaction during pregnancy are social and psychological dimensions of maternal stress [19]. Partner support is suggested to have an important influence on maternal satisfaction, therefore influencing fetal outcomes and child health [51,52]. Multiple factors influence the rating of these complex domains, making standardized assessment difficult. Our data indicate a higher estimation of partnership quality during pregnancy compared to the offspring’s childhood. Is the first or the second rating the gold standard in this context? During pregnancy, the joyful anticipation of having a baby might strengthen the feeling of togetherness. Later, when children grow up, parenting could be seen as more differentiated. Additionally, postnatal partnership conflicts might result in biased retrospective ratings. In clinical child and adolescent psychiatric anamnesis, it thus proves crucial to retrospectively consider mothers’ partnership and support as protection or risk factors during pregnancy, as stated in the literature [2].
Quality of life and satisfaction during pregnancy have become relevant parameters in clinical treatment, however, measurements are not widely standardized [53,54]. Analysis of our data on subjective pregnancy satisfaction showed heterogeneous trimester-depending results. Retrospectively, there were upgrading tendencies for the 1st trimester and downgrading tendencies for the 2nd trimester. For the 3rd trimester, the agreement was good and without significant differences. One explanation for the trimester-depending results could be that bio-psycho-social dimensions of stress influence general satisfaction differently at specific periods. Most typical 1st-trimester complications are nausea and vomiting [55]. Especially a general, unspecific malaise during the 1st trimester seems difficult to remember in retrospect. In contrast, subjective pregnancy satisfaction during the 3rd trimester might be easier for mothers to reproduce. For clinicians addressing child and adolescent mental health, our data show that the retrospective ratings of maternal satisfaction during the 3rd trimester of pregnancy were the most reliable to work with.
5. Conclusions
Due to social stigmata and memory effects, subjective pregnancy self-reports cannot be precisely reproduced in retrospection. Nevertheless, there is an intra-individual relevant pregnancy-childhood (-adolescence) correlation for all presented pregnancy risks. That is why the maternal retrospective information is not random and is valuable for anamnesis in child and adolescent psychiatry. However, the child and adolescent health specialist should keep in mind that especially pregnancy complications are retrospectively under-reported, fluctuating substance use is reported, with increasing—and perhaps more realistic—amounts from childhood to adolescence, the partnership could be devalued in retrospect, with 3rd-trimester satisfaction being the most reliable marker. Clinicians should be aware of potentially biased maternal information, particularly regarding prenatal substance use in the treatment of children at elementary school age. Despite all inconsistencies, the mother is the most important source of information for intrauterine stress in child and adolescent psychiatric anamnesis. Other child caregivers—who have already been present during pregnancy—can be additionally consulted. Apart from details, creating a respectful and trusting atmosphere is essential to enable the mother to give reliable self-reports, free of shame and in the best interest of the child.
Author Contributions
Study conception: A.E., P.A.F., M.W.B., O.K., G.H.M. and J.K.; Study design: A.E., P.A.F., M.W.B., O.K., G.H.M. and J.K.; Data acquisition: J.G. and A.E.; Data analysis and interpretation: S.M. and A.E.; Manuscript preparation: S.M., A.E. and J.G.; Revision of Manuscript: all authors; Approving the submitted version: all authors. The study is part of the IMAC-Mind research project. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Federal Ministry of Education and Research, Germany (01GL1745B, subproject TP1) and by the Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding programme “Open Access Publication Funding”.
Institutional Review Board Statement
The study was approved by the Local Ethics Committee (Ethikkommission des Universitätsklinikums Erlangen) (3374, 14.06.2005; 4596, 17.01.2012 and 353_18B, 09.10.2018) and was conducted in accordance with the Declaration of Helsinki. All mothers gave informed consent, all children informed assent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
With the public-funded research project IMAC-Mind: Improving Mental Health and Reducing Addiction in Childhood and Adolescence through Mindfulness: Mechanisms, Prevention and Treatment (01GL1745B, subproject TP1), the Federal Ministry of Education and Research contributes to improving the prevention and treatment of children and adolescents with substance use disorders and associated mental disorders. Project coordination was realized by the German Center of Addiction Research in Childhood and Adolescence at the University Medical Center Hamburg-Eppendorf. The consortium comprises seven projects in Germany. Principal Investigators are Rainer Thomasius (Coordinator, University Medical Center Hamburg-Eppendorf), Nicolas Arnaud (Coordinator, University Medical Center Hamburg-Eppendorf), Tobias Banaschewski, Herta Flor (Central Institute of Mental Health, Mannheim), Frauke Nees (Central Institute of Mental Health, Mannheim, and University Medical Center Schleswig Holstein, Kiel University), Johannes Kornhuber (Friedrich-Alexander-Universität Erlangen-Nürnberg), Michael Klein (Catholic University of Applied Sciences, Cologne), Olaf Reis (University Medicine of Rostock), Tanja Legenbauer (Ruhr-University Bochum), and Antonia Zapf (University Medical Center Hamburg-Eppendorf). For more information, please visit our homepage www.IMAC-Mind.de. We acknowledge financial support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding programme “Open Access Publication Funding”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rakers, F.; Rupprecht, S.; Dreiling, M.; Bergmeier, C.; Witte, O.W.; Schwab, M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2020, 117, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, I. Prä- und perinatale Aspekte kinder- und jugendpsychiatrischer Störungen. Hebamme 2011, 24, 98–103. [Google Scholar] [CrossRef]
- Graham, Y.P.; Heim, C.; Goodman, S.H.; Miller, A.H.; Nemeroff, C.B. The effects of neonatal stress on brain development: Implications for psychopathology. Dev. Psychopathol. 1999, 11, 545–565. [Google Scholar] [CrossRef] [PubMed]
- Glover, V.; O’Donnell, K.J.; O’Connor, T.G.; Fisher, J. Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—A global perspective. Dev. Psychopathol. 2018, 30, 843–854. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Meaney, M.J. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. Am. J. Psychiatry 2017, 174, 319–328. [Google Scholar] [CrossRef]
- Amgalan, A.; Andescavage, N.; Limperopoulos, C. Prenatal origins of neuropsychiatric diseases. Acta Paediatr. 2021, 110, 1741–1749. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Mattson, S.N.; Bernes, G.A.; Doyle, L.R. Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated With Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1046–1062. [Google Scholar] [CrossRef]
- Maschke, J.; Roetner, J.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; Lenz, B.; Kornhuber, J.; Eichler, A.; et al. Prenatal Alcohol Exposure and the Facial Phenotype in Adolescents: A Study Based on Meconium Ethyl Glucuronide. Brain Sci. 2021, 11, 154. [Google Scholar] [CrossRef]
- Moder, J.E.; Ordenewitz, L.K.; Schlüter, J.A.; Weinmann, T.; Altebäumer, P.; Jung, J.; Heinen, F.; Landgraf, M.N. Fetal alcohol spectrum disorders-diagnosis, prognosis, and prevention. Bundesgesundheitsblatt Gesundh. Gesundh. 2021, 64, 747–754. [Google Scholar] [CrossRef]
- Kalberg, W.O.; May, P.A.; Buckley, D.; Hasken, J.M.; Marais, A.S.; De Vries, M.M.; Bezuidenhout, H.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; et al. Early-Life Predictors of Fetal Alcohol Spectrum Disorders. Pediatrics 2019, 144, e20182141. [Google Scholar] [CrossRef] [PubMed]
- Eichler, A.; Hudler, L.; Grunitz, J.; Grimm, J.; Raabe, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; et al. Effects of prenatal alcohol consumption on cognitive development and ADHD-related behaviour in primary-school age: A multilevel study based on meconium ethyl glucuronide. J. Child Psychol. Psychiatry 2018, 59, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Burden, M.J.; Jacobson, J.L.; Westerlund, A.; Lundahl, L.H.; Morrison, A.; Dodge, N.C.; Klorman, R.; Nelson, C.A.; Avison, M.J.; Jacobson, S.W. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2010, 34, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Tiesler, C.M.; Heinrich, J. Prenatal nicotine exposure and child behavioural problems. Eur. Child Adolesc. Psychiatry 2014, 23, 913–929. [Google Scholar] [CrossRef] [PubMed]
- McGrath-Morrow, S.A.; Gorzkowski, J.; Groner, J.A.; Rule, A.M.; Wilson, K.; Tanski, S.E.; Collaco, J.M.; Klein, J.D. The Effects of Nicotine on Development. Pediatrics 2020, 145, e20191346. [Google Scholar] [CrossRef]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef]
- Buka, S.L.; Shenassa, E.D.; Niaura, R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: A 30-year prospective study. Am. J. Psychiatry 2003, 160, 1978–1984. [Google Scholar] [CrossRef]
- Dong, T.; Hu, W.; Zhou, X.; Lin, H.; Lan, L.; Hang, B.; Lv, W.; Geng, Q.; Xia, Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod. Toxicol. 2018, 76, 63–70. [Google Scholar] [CrossRef]
- Bergman, K.; Sarkar, P.; O’Connor, T.G.; Modi, N.; Glover, V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1454–1463. [Google Scholar] [CrossRef]
- Loomans, E.M.; van Dijk, A.E.; Vrijkotte, T.G.; van Eijsden, M.; Stronks, K.; Gemke, R.J.; Van den Bergh, B.R. Psychosocial stress during pregnancy is related to adverse birth outcomes: Results from a large multi-ethnic community-based birth cohort. Eur. J. Public Health 2013, 23, 485–491. [Google Scholar] [CrossRef]
- Talge, N.M.; Neal, C.; Glover, V.; the Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J. Child Psychol. Psychiatry 2007, 48, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Raikkonen, K.; King, S.; et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef] [PubMed]
- Lautarescu, A.; Craig, M.C.; Glover, V. Prenatal stress: Effects on fetal and child brain development. Int. Rev. Neurobiol. 2020, 150, 17–40. [Google Scholar] [CrossRef]
- Kajanoja, J.; Nolvi, S.; Kantojarvi, K.; Karlsson, L.; Paunio, T.; Karlsson, H. Oxytocin receptor genotype moderates the association between maternal prenatal stress and infant early self-regulation. Psychoneuroendocrinology 2022, 138, 105669. [Google Scholar] [CrossRef] [PubMed]
- Rifkin-Graboi, A.; Meaney, M.J.; Chen, H.; Bai, J.; Hameed, W.B.; Tint, M.T.; Broekman, B.F.; Chong, Y.S.; Gluckman, P.D.; Fortier, M.V.; et al. Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 313–321.e2. [Google Scholar] [CrossRef] [PubMed]
- DeSocio, J.E. Epigenetics, maternal prenatal psychosocial stress, and infant mental health. Arch. Psychiatr. Nurs. 2018, 32, 901–906. [Google Scholar] [CrossRef]
- Sacco, R.; Camilleri, N.; Eberhardt, J.; Umla-Runge, K.; Newbury-Birch, D. A systematic review and meta-analysis on the prevalence of mental disorders among children and adolescents in Europe. Eur. Child Adolesc. Psychiatry 2022, 1–18. [Google Scholar] [CrossRef]
- Adams, E.L.; Smith, D.; Caccavale, L.J.; Bean, M.K. Parents Are Stressed! Patterns of Parent Stress Across COVID-19. Front. Psychiatry 2021, 12, 626456. [Google Scholar] [CrossRef]
- Kölch, M.; Fegert, J.M. Chronische Tic-Störungen und Tourette-Syndrom. In Klinikmanual Kinder- und Jugendpsychiatrie und -Psychotherapie; Kölch, M., Rassenhofer, M., Fegert, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 169–181. [Google Scholar]
- Plener, P.L.; Fegert, J.M. Störungen des Sozialverhaltens. In Klinikmanual Kinder- und Jugendpsychiatrie und -Psychotherapie; Kölch, M., Rassenhofer, M., Fegert, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 25–38. [Google Scholar]
- Thomas, J.M.; Benham, A.L.; Gean, M.; Luby, J.; Minde, K.; Turner, S.; Wright, H.H. Practice parameters for the psychiatric assessment of infants and toddlers (0–36 months). American Academy of Child and Adolescent Psychiatry. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 21S–36S. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Poustka, F. Multiaxiales Klassifikationsschema für Psychische Störungen des Kindes- und Jugendalters nach ICD-10: Mit einem Synoptischen Vergleich von ICD-10 und DSM-5; Hogrefe: Göttingen, Germany, 2017. [Google Scholar]
- Zero to Three. In DC: 0-5: Diagnostic Classification of Mental Health and Developmental Disorders of Infancy and Early Childhood; Zero to Three: Washington, DC, USA, 2016.
- WHO. International Classification of Diseases, Eleventh Revision (ICD-11). Available online: https://icd.who.int/browse11 (accessed on 7 February 2023).
- Avila-Tang, E.; Al-Delaimy, W.K.; Ashley, D.L.; Benowitz, N.; Bernert, J.T.; Kim, S.; Samet, J.M.; Hecht, S.S. Assessing secondhand smoke using biological markers. Tob. Control 2013, 22, 164–171. [Google Scholar] [CrossRef]
- Nast, I.; Bolten, M.; Meinlschmidt, G.; Hellhammer, D.H. How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatr. Perinat. Epidemiol. 2013, 27, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.P.; Colton, J.; Priest, S.; Reilly, N.; Hadzi-Pavlovic, D. The antenatal risk questionnaire (ANRQ): Acceptability and use for psychosocial risk assessment in the maternity setting. Women Birth 2013, 26, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.; de Meer, G.; Verhulst, F.C.; Ormel, J.; Reijneveld, S.A. Limited validity of parental recall on pregnancy, birth, and early childhood at child age 10 years. J. Clin. Epidemiol. 2010, 63, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Pickett, K.E.; Kasza, K.; Biesecker, G.; Wright, R.J.; Wakschlag, L.S. Women who remember, women who do not: A methodological study of maternal recall of smoking in pregnancy. Nicotine Tob. Res. 2009, 11, 1166–1174. [Google Scholar] [CrossRef]
- Newport, D.J.; Brennan, P.A.; Green, P.; Ilardi, D.; Whitfield, T.H.; Morris, N.; Knight, B.T.; Stowe, Z.N. Maternal depression and medication exposure during pregnancy: Comparison of maternal retrospective recall to prospective documentation. BJOG 2008, 115, 681–688. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Chiodo, L.M.; Sokol, R.J.; Jacobson, J.L. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics 2002, 109, 815–825. [Google Scholar] [CrossRef]
- Eichler, A.; Grunitz, J.; Grimm, J.; Walz, L.; Raabe, E.; Goecke, T.W.; Beckmann, M.W.; Kratz, O.; Heinrich, H.; Moll, G.H.; et al. Did you drink alcohol during pregnancy? Inaccuracy and discontinuity of women’s self-reports: On the way to establish meconium ethyl glucuronide (EtG) as a biomarker for alcohol consumption during pregnancy. Alcohol 2016, 54, 39–44. [Google Scholar] [CrossRef]
- Reulbach, U.; Bleich, S.; Knorr, J.; Burger, P.; Fasching, P.A.; Kornhuber, J.; Beckmann, M.W.; Goecke, T.W. Pre-, peri- and postpartal depression. Fortschr. Neurol. Psychiatr. 2009, 77, 708–713. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Ramos, A.M.; Marceau, K.; Neiderhiser, J.M.; De Araujo-Greecher, M.; Natsuaki, M.N.; Leve, L.D. Maternal Consistency in Recalling Prenatal Experiences at 6 Months and 8 Years Postnatal. J. Dev. Behav. Pediatr. 2020, 41, 698–705. [Google Scholar] [CrossRef]
- Hannigan, J.H.; Chiodo, L.M.; Sokol, R.J.; Janisse, J.; Ager, J.W.; Greenwald, M.K.; Delaney-Black, V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 2010, 44, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Rice, F.; Lewis, A.; Harold, G.; van den Bree, M.; Boivin, J.; Hay, D.F.; Owen, M.J.; Thapar, A. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: The influence of maternal and child characteristics. Early Hum. Dev. 2007, 83, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Petik, D.; Puho, E. Smoking and alcohol drinking during pregnancy. The reliability of retrospective maternal self-reported information. Cent. Eur. J. Public Health 2004, 12, 179–183. [Google Scholar] [PubMed]
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; de Pablo, G.S.; Shin, J.I.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatr. 2022, 27, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Dietz, P.; Bombard, J.; Mulready-Ward, C.; Gauthier, J.; Sackoff, J.; Brozicevic, P.; Gambatese, M.; Nyland-Funke, M.; England, L.; Harrison, L.; et al. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern. Child Health J. 2014, 18, 2489–2498. [Google Scholar] [CrossRef]
- Castelar-Rios, M.J.; De Los Santos-Roig, M.; Robles-Ortega, H.; Diaz-Lopez, M.A.; Maldonado-Lozano, J.; Bellido-Gonzalez, M. Moderating Effect of Changes in Perceived Social Support during Pregnancy on the Emotional Health of Mothers and Fathers and on Baby’s Anthropometric Parameters at Birth. Children 2022, 9, 648. [Google Scholar] [CrossRef]
- Cheng, E.R.; Rifas-Shiman, S.L.; Perkins, M.E.; Rich-Edwards, J.W.; Gillman, M.W.; Wright, R.; Taveras, E.M. The Influence of Antenatal Partner Support on Pregnancy Outcomes. J. Womens Health 2016, 25, 672–679. [Google Scholar] [CrossRef]
- Morin, M.; Vayssiere, C.; Claris, O.; Irague, F.; Mallah, S.; Molinier, L.; Matillon, Y. Evaluation of the quality of life of pregnant women from 2005 to 2015. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 115–130. [Google Scholar] [CrossRef]
- Lagadec, N.; Steinecker, M.; Kapassi, A.; Magnier, A.M.; Chastang, J.; Robert, S.; Gaouaou, N.; Ibanez, G. Factors influencing the quality of life of pregnant women: A systematic review. BMC Pregnancy Childbirth 2018, 18, 455. [Google Scholar] [CrossRef]
- Hasan, R.; Baird, D.D.; Herring, A.H.; Olshan, A.F.; Jonsson Funk, M.L.; Hartmann, K.E. Patterns and predictors of vaginal bleeding in the first trimester of pregnancy. Ann. Epidemiol. 2010, 20, 524–531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).