Short- and Long-Term Outcomes of Preeclampsia in Offspring: Review of the Literature
Abstract
1. Introduction
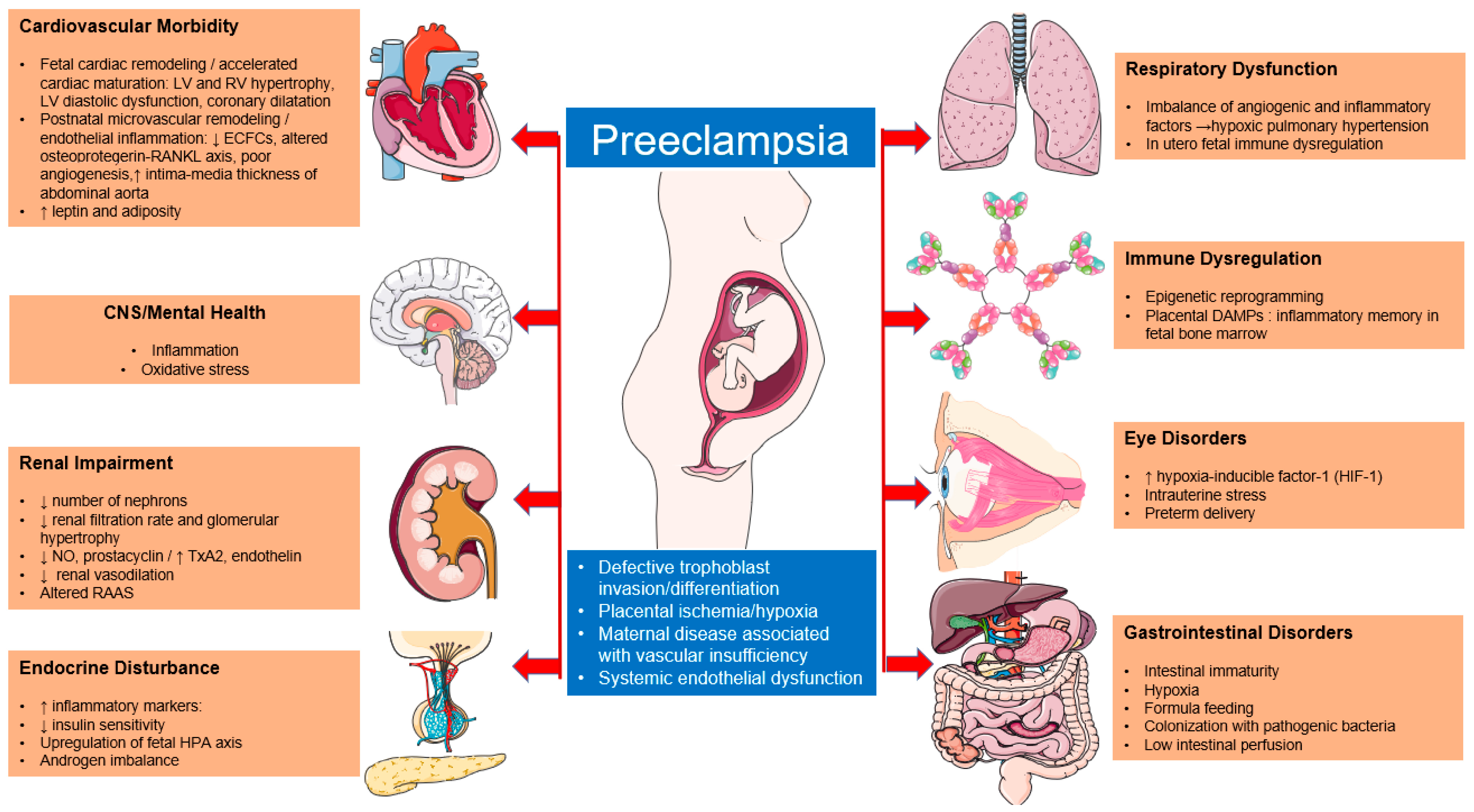
Pathophysiology of Preeclampsia
2. Outcome in Offspring
2.1. Neurodevelopment
2.1.1. Autism Spectrum Disorder (ASD)
2.1.2. Attention-Deficit/Hyperactivity Disorder (ADHD)
2.1.3. Intellectual Disability (ID) and Cognitive Function
2.1.4. Cerebral Palsy (CP)
2.1.5. Psychiatric Disruptions
2.2. Eye Disorders
2.3. Immune System and Susceptibility to Infections
2.4. Gastrointestinal Diseases
2.4.1. Neonatal Age
2.4.2. Childhood
2.5. Cardiovascular System
2.5.1. Neonatal Age– Early Childhood
2.5.2. Adolescence–Early Adulthood
2.6. Renal System
2.7. Endocrine System
2.8. Respiratory System
2.8.1. Neonatal Life
2.8.2. Childhood
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frost, A.L.; Suriano, K.; Aye, C.Y.L.; Leeson, P.; Lewandowski, A.J. The Immediate and Long-Term Impact of Preeclampsia on Offspring Vascular and Cardiac Physiology in the Preterm Infant. Front. Pediatr. 2021, 1, 625726. [Google Scholar] [CrossRef]
- Turbeville, H.R.; Sasser, J.M. REVIEW Sex and Gender in Renal Health and Function. Am. J. Physiol. Renal. Physiol. 2020, 318, 1315–1326. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynecol. Obstet. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy: Diagnosis and Management NICE Guideline. 2019. Available online: www.nice.org.uk/guidance/ng133 (accessed on 1 February 2023).
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P.; Longo, D.L. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Wojczakowski, W.; Kimber-Trojnar, Ż.; Dziwisz, F.; Słodzińska, M.; Słodziński, H.; Leszczyńska-Gorzelak, B. Preeclampsia and cardiovascular risk for offspring. J. Clin. Med. 2021, 10, 3154. [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Giannakou, K.; Evangelou, E.; Papatheodorou, S.I. Genetic and non-genetic risk factors for pre-eclampsia: Umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet. Gynecol. 2018, 51, 720–730. [Google Scholar] [CrossRef]
- Pinheiro, T.V.; Brunetto, S.; Ramos, J.G.L.; Bernardi, J.R.; Goldani, M.Z. Hypertensive disorders during pregnancy and health outcomes in the offspring: A systematic review. J. Dev. Orig. Health Dis. 2016, 7, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Ahmad, S.; Mi, T.; Abbasi, S.; Xia, L.; Day, M.-C.; Ramin, S.M.; Ahmed, A.; Kellems, R.E.; Xia, Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension 2008, 51, 1010–1019. [Google Scholar]
- Lu, H.Q.; Hu, R. Lasting effects of intrauterine exposure to preeclampsia on offspring and the underlying mechanism. AJP Rep. 2019, 9, E275–E291. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P.; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, Evaluation, and Management of the Hypertensive Disorders of Pregnancy: Executive Summary. J. Obstet. Gynaecol. Can. 2014, 36, 416–438. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia—Pathophysiology and Clinical Presentations. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.Q.; Zhang, L. Gestational Hypoxia and Developmental Plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef]
- Aarnoudse-Moens, C.S.H.; Weisglas-Kuperus, N.; van Goudoever, J.B.; Oosterlaan, J. Meta-Analysis of Neurobehavioral Outcomes in Very Preterm and/or Very Low Birth Weight Children. Pediatrics 2009, 124, 717–728. [Google Scholar] [CrossRef]
- Das Banerjee, T.; Middleton, F.; Faraone, S.V. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007, 96, 1269–1274. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Ersch, J.; Adams, M.; Bernet, V.; Bucher, H.U.; Latal, B. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. 2010, 99, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, W.; Yu, V.Y. Severe pre-eclampsia and infants of very low birth weight. Arch. Dis. Child. 1987, 62, 712–716. [Google Scholar] [CrossRef]
- Maher, G.M.; O’Keeffe, G.W.; Kearney, P.M.; Dinan, T.G.; Mattsson, M.; Khashan, A.S. Association of Hypertensive Disorders of Pregnancy With Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 809–819. [Google Scholar] [CrossRef]
- Kay, V.R.; Rätsep, M.T.; Figueiró-Filho, E.A.; Croy, B.A. Preeclampsia may influence offspring neuroanatomy and cognitive function: A role for placental growth factor †. Biol. Reprod. 2019, 101, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Luo, Z.C.; Nuyt, A.M.; Kaufman, J.S.; Naimi, A.I.; Platt, R.W.; Fraser, W.D. Secular Trends in Preeclampsia Incidence and Outcomes in a Large Canada Database: A Longitudinal Study Over 24 Years. Can. J. Cardiol. 2016, 32, e15–e987. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.; McCarthy, C.M.; O’Keeffe, G.W. Preeclampsia and Neurodevelopmental Outcomes: Potential Pathogenic Roles for Inflammation and Oxidative Stress? Mol. Neurobiol. 2021, 58, 2734–2756. [Google Scholar] [CrossRef]
- Figueiró-Filho, E.A.; Mak, L.E.; Reynolds, J.N.; Stroman, P.W.; Smith, G.N.; Forkert, N.D.; Paolozza, A.; Rätsep, M.T.; Croy, B.A. Neurological function in children born to preeclamptic and hypertensive mothers—A systematic review. Pregnancy Hypertens. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Kajantie, E.; Eriksson, J.G.; Osmond, C.; Thornburg, K.; Barker, D.J.P. Pre-Eclampsia Is Associated With Increased Risk of Stroke in the Adult Offspring. Stroke 2009, 40, 1176–1180. [Google Scholar] [CrossRef]
- Brand, J.S.; Lawlor, D.A.; Larsson, H.; Montgomery, S. Association Between Hypertensive Disorders of Pregnancy and Neurodevelopmental Outcomes Among Offspring. JAMA Pediatr. 2021, 175, 577. [Google Scholar] [CrossRef] [PubMed]
- DSM-5(ASD.Guidelines). 2013. Available online: https://www.cdc.gov/ncbddd/autism/hcp-dsm.html (accessed on 8 March 2023).
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Dachew, B.A.; Mamun, A.; Maravilla, J.C.; Alati, R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: Meta-analysis. Br. J. Psychiatry 2018, 212, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, perinatal, and postnatal factors associated with autism. Medicine 2017, 96, e6696. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.T.; Chang, Q.X.; Wang, Q.Q.; Zhang, J.; Xia, L.-X.; Zhong, N.; Yu, Y.-H.; Zhong, M.; Huang, Q.-T. Association between hypertensive disorders of pregnancy and risk of autism in offspring: A systematic review and meta-analysis of observational studies. Oncotarget 2018, 9, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; McDermott, S.; Bao, H.; Hardin, J.; Gregg, A. Pre-eclampsia, birth weight, and autism spectrum disorders. J. Autism. Dev. Disord. 2010, 40, 548–554. [Google Scholar] [CrossRef]
- Sun, B.Z.; Moster, D.; Harmon, Q.E.; Wilcox, A.J. Association of Preeclampsia in Term Births With Neurodevelopmental Disorders in Offspring. JAMA Psychiatry 2020, 77, 823–829. [Google Scholar] [CrossRef]
- Wallace, A.E.; Anderson, G.M.; Dubrow, R. Obstetric and parental psychiatric variables as potential predictors of autism severity. J. Autism. Dev. Disord. 2008, 38, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Halmøy, A.; Klungsøyr, K.; Skjærven, R.; Haavik, J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2012, 71, 474–481. [Google Scholar] [CrossRef]
- Silva, D.; Colvin, L.; Hagemann, E.; Bower, C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014, 133, e14–e22. [Google Scholar] [CrossRef]
- Tuovinen, S.; Räikkönen, K.; Kajantie, E.; Pesonen, A.-K.; Heinonen, K.; Osmond, C.; Barker, D.J.P.; Eriksson, J.G. Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: The Helsinki Birth Cohort Study. BJOG 2010, 117, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Heikura, U.; Hartikainen, A.L.; Nordström, T.; Pouta, A.; Taanila, A.; Järvelin, M.R. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr. Perinat. Epidemiol. 2013, 27, 188–198. [Google Scholar] [CrossRef]
- Many, A.; Fattal, A.; Leitner, Y.; Kupferminc, M.J.; Harel, S.; Jaffa, A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens. Pregnancy 2003, 22, 25–29. [Google Scholar] [CrossRef]
- van Wassenaer, A.G.; Westera, J.; van Schie, P.E.M.; Houtzager, B.A.; Cranendonk, A.; de Groot, L.; Ganzevoort, W.; Wolf, H.; de Vries, J.I. Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2011, 204, e1–e510. [Google Scholar] [CrossRef]
- Blair, E.; Watson, L. Epidemiology of cerebral palsy. Semin. Fetal Neonatal. Med. 2006, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Trønnes, H.; Wilcox, A.J.; Lie, R.T.; Markestad, T.; Moster, D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: A national cohort study. Dev. Med. Child Neurol. 2014, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Badagionis, M.; Sergentanis, T.N.; Pervanidou, P.; Kalampokas, E.; Vlahos, N.; Eleftheriades, M. Preeclampsia and Cerebral Palsy in Offspring. Children 2022, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Oddy, W.H.; Whitehouse, A.J.O.; Pennell, C.E.; Kendall, G.E.; McLean, N.J.; Jacoby, P.; Zubrick, S.R.; Stanley, F.J.; Newnham, J.P. Hypertensive diseases of pregnancy predict parent-reported difficult temperament in infancy. J. Dev. Behav. Pediatr. 2013, 34, 174–180. [Google Scholar] [CrossRef]
- Gujrati, V.R.; Shanker, K.; Vrat, S.; Chandravati Parmar, S.S. Novel appearance of placental nuclear monoamine oxidase: Biochemical and histochemical evidence for hyperserotonomic state in preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 1996, 175, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; St-Louis, J. Reactivities to serotonin and histamine in umbilical and placental vessels during the third trimester after normotensive pregnancies and pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 1999, 180, 650–659. [Google Scholar] [CrossRef]
- Eide, M.G.; Moster, D.; Irgens, L.M.; Reichborn-Kjennerud, T.; Stoltenberg, C.; Skjærven, R.; Susser, E.; Abel, K. Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol. Med. 2013, 43, 2057–2066. [Google Scholar] [CrossRef]
- Smyth, A.M.; Lawrie, S.M. The neuroimmunology of schizophrenia. Clin. Psychopharmacol. Neurosci. 2013, 11, 107–117. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Kedar Sade, E.; Wainstock, T.; Tsumi, E.; Sheiner, E. Prenatal Exposure to Preeclampsia and Long-Term Ophthalmic Morbidity of the Offspring. J. Clin. Med. 2020, 9, 1271. [Google Scholar] [CrossRef]
- Kanasaki, K.; Kalluri, R. The biology of preeclampsia. Kidney Int. 2009, 76, 831–837. [Google Scholar] [CrossRef]
- Tal, R. The Role of Hypoxia and Hypoxia-Inducible Factor-1Alpha in Preeclampsia Pathogenesis. Biol Reprod. 2012, 87, 134. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Feldman, B.; Avivi, C.; Harats, D. Effects of Hypoxia-Inducible Factor-1α Overexpression in Pregnant Mice. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Nohr, E.A.; Bech, B.H.; Vestergaard, M.; Catov, J.M.; Olsen, J. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am. J. Obstet. Gynecol. 2009, 201, e1–e269. [Google Scholar] [CrossRef]
- Nahum Sacks, K.; Friger, M.; Shoham-Vardi, I.; Spiegel, E.; Sergienko, R.; Landau, D.; Sheiner, E. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. 2018, 13, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.; Senterman, M.K.; Hakim, A.M. Preeclampsia Is a Biomarker for Vascular Disease in Both Mother and Child: The Need for a Medical Alert System. Int. J. Pediatr. 2013, 2013, 953150. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. New Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.P.; Weng, C.; Wilkes, J.; Greene, T.; Hartnett, M.E. Association of Maternal Preeclampsia With Infant Risk of Premature Birth and Retinopathy of Prematurity. JAMA Ophthalmol. 2017, 135, 947. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Markham, K.; Moorehead, P.; Cordero, L.; Nankervis, C.A.; Giannone, P.J. Maternal Preeclampsia and Neonatal Outcomes. J. Pregnancy 2011, 2011, 214365. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N. Why much of the pathophysiology of preeclampsia-eclampsia must be of an autoimmune nature. Am J Obstet Gynecol. 2007, 196, e1–e7. [Google Scholar] [CrossRef]
- Lodge-Tulloch, N.A.; Toews, A.J.; Atallah, A.; Cotechini, T.; Girard, S.; Graham, C.H. Cross-Generational Impact of Innate Immune Memory Following Pregnancy Complications. Cells 2022, 11, 3935. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Olsen, J.; Agerbo, E.; Yuan, W.; Wu, C.; Sen Li, J. Maternal preeclampsia and childhood asthma in the offspring. Pediatr. Allergy Immunol. 2015, 26, 181–185. [Google Scholar] [CrossRef]
- Kristensen, K.; Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunology 2016, 137, 587–590. [Google Scholar] [CrossRef]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Rorman, E.; Freud, A.; Wainstock, T.; Sheiner, E. Maternal preeclampsia and long-term infectious morbidity in the offspring—A population based cohort analysis. Pregnancy Hypertens. 2020, 21, 30–34. [Google Scholar] [CrossRef]
- Harrison, R.K.; Palatnik, A. The association between preeclampsia and ICD diagnosis of neonatal sepsis. J. Perinatol. 2021, 41, 460–467. [Google Scholar] [CrossRef]
- Miller, J.E.; Hammond, G.C.; Strunk, T.; Moore, H.C.; Leonard, H.; Carter, K.W.; Bhutta, Z.; Stanley, F.; de Klerk, N.; Burgner, D.P. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: A population-based, data-linkage study from Western Australia. Lancet Infect Dis. 2016, 16, 952–961. [Google Scholar] [CrossRef]
- Wainstock, T.; Walfisch, A.; Shoham-Vardi, I.; Segal, I.; Sergienko, R.; Landau, D.; Sheiner, E. Term Elective Cesarean Delivery and Offspring Infectious Morbidity. Pediatr. Infect. Dis. J. 2019, 38, 176–180. [Google Scholar] [CrossRef]
- Bashiri, A.; Zmora, E.; Sheiner, E.; Hershkovitz, R.; Shoham-Vardi, I.; Mazor, M. Maternal Hypertensive Disorders Are an Independent Risk Factor for the Development of Necrotizing Enterocolitis in Very Low Birth Weight Infants. Fetal Diagn. Ther. 2003, 18, 404–407. [Google Scholar] [CrossRef]
- Kirsten, G.; van Zyl, N.; Smith, M.; Odendaal, H. Necrotizing Enterocolitis in Infants Born to Women with Severe Early Preeclampsia and Absent End-Diastolic Umbilical Artery Doppler Flow Velocity Waveforms. Am. J. Perinatol. 1999, 16, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, M.; Ozkan, H.; Koksal, N. Maternal preeclampsia is associated with increased risk of necrotizing enterocolitis in preterm infants. Early Hum. Dev. 2012, 88, 893–898. [Google Scholar] [CrossRef]
- Leybovitz-Haleluya, N.; Wainstock, T.; Sheiner, E. Maternal preeclampsia and the risk of pediatric gastrointestinal diseases of the offspring: A population-based cohort study. Pregnancy Hypertens. 2019, 17, 144–147. [Google Scholar] [CrossRef]
- Yeung, E.H.; Robledo, C.; Boghossian, N.; Zhang, C.; Mendola, P. Developmental Origins of Cardiovascular Disease. Reprod. Perinat. Epidemiol. 2014, 1, 9–16. [Google Scholar] [CrossRef] [PubMed]
- de la Calzada, D.G.; García, L.O.; Remírez, J.M.; Lázaro, A.; Cajal, M.D. Study of early detection of cardiovascular risk factors in children born small (SGA) and review of literature. Pediatr. Endocrinol. Rev. 2009, 3, 343–349. [Google Scholar]
- Thornburg, K.L.; Drake, R.; Valent, A.M. Maternal Hypertension Affects Heart Growth in Offspring. J. Am. Heart Assoc. 2020, 9, e016538. [Google Scholar] [CrossRef] [PubMed]
- Balli, S.; Kibar, A.E.; Ece, I.; Oflaz, M.B.; Yilmaz, O. Assessment of Fetal Cardiac Function in Mild Preeclampsia. Pediatr. Cardiol. 2013, 34, 1674–1679. [Google Scholar] [CrossRef]
- Jonker, S.S.; Louey, S. Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment. J. Endocrinol. 2016, 228, R1–R18. [Google Scholar] [CrossRef] [PubMed]
- Aye, C.Y.; Lewandowski, A.J.; Lamata, P.; Upton, R.; Davis, E.; Ohuma, E.; Kenworthy, Y.; Boardman, H.; Frost, A.; Adwani, S.; et al. Prenatal and Postnatal Cardiac Development in Offspring of Hypertensive Pregnancies. J. Am. Heart Assoc. 2020, 9, e014586. [Google Scholar] [CrossRef]
- Lin, I.C.; Hsu, T.Y.; Tain, Y.L.; Tsai, C.-C.; Huang, H.-C.; Lai, Y.-J.; Chou, M.-H.; Huang, C.-F.; Yu, H.-R.; Huang, L.-T. Coronary Dilatation and Endothelial Inflammation in Neonates Born to Mothers with Preeclampsia. J. Pediatr. 2021, 228, 58–65.e3. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Stiefel, P.; Lin, R.Z.; Praena-Fernández, J.M.; Dominguez-Simeon, M.J.; Villar, J.; Moreno-Luna, R.; Melero-Martin, J.M. Decreased Level of Cord Blood Circulating Endothelial Colony-Forming Cells in Preeclampsia Preeclampsia. Hypertension 2014, 64, 165–171. [Google Scholar]
- Yu, G.Z.; Aye, C.Y.L.; Lewandowski, A.J.; Davis, E.F.; Khoo, C.P.; Newton, L.; Yang, C.T.; Haj Zen, A.A.; Simpson, L.J.; O’Brien, K.; et al. Association of maternal antiangiogenic profile at birth with early postnatal loss of microvascular density in offspring of hypertensive pregnancies. Hypertension 2016, 68, 749–759. [Google Scholar]
- Oikonomou, N.; Papadopoulou, C.; Fouzas, S.; Kritikou, D.; Chrysis, D.; Sinopidis, X.; Dimitriou, G.; Karatza, A.A. Osteoprotegerin and RANKL serum concentrations in neonates of mothers with early-onset pre-eclampsia: Comparison with neonates of normotensive mothers. Early Hum. Dev. 2019, 135, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chourdakis, E.; Fouzas, S.; Papadopoulou, C.; Oikonomou, N.; Hahalis, G.; Dimitriou, G.; Karatza, A.A. Effect of Early-Onset Preeclampsia on Offspring’s Blood Pressure during the First Month of Life. J. Pediatr. 2020, 220, 21–26.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ren, Y.; Yan, Y.; Chu, C.; Gui, Y.; Li, X. Fetal tissue Doppler imaging in pregnancies complicated with preeclampsia with or without intrauterine growth restriction. National High Technology Research and Development Program (863 program) (054119512), the Key Project of Clinical Disciplines of Chinese Ministry of Health (2007-353) and National Science and Technology Key Program of the Eleventh Five-year Plan of China. Sci. Technol. Comm. Shanghai Munic. Prenatal Diagnosis 2012, 32, 1021–1028. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Bostan, Ö.; Köksal, N.; Semizel, E.; Özkan, H.; Çakir, S. Early left ventricular diastolic dysfunction in premature infants born to preeclamptic mothers. J. Perinat. Med. 2011, 39, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fugelseth, D.; Ramstad, H.B.; Kvehaugen, A.S.; Nestaas, E.; Støylen, A.; Staff, A.C. Myocardial function in offspring 5-8years after pregnancy complicated by preeclampsia. Early Hum. Dev. 2011, 87, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Boyd, H.A.; Basit, S.; Behrens, I.; Leirgul, E.; Bundgaard, H.; Wohlfahrt, J.; Melbye, M.; Øyen, N. Association Between Fetal Congenital Heart Defects and Maternal Risk of Hypertensive Disorders of Pregnancy in the Same Pregnancy and Across Pregnancies. Circulation 2017, 136, 39–48. [Google Scholar] [CrossRef]
- Musa, N.L.; Hjortdal, V.; Zheleva, B.; Murni, I.K.; Sano, S.; Schwartz, S.; Staveski, S.L. The global burden of paediatric heart disease. Cardiol. Young 2017, 27, S3–S8. [Google Scholar] [CrossRef]
- Kanata, M.; Liazou, E.; Chainoglou, A.; Kotsis, V.; Stabouli, S. Clinical outcomes of hypertensive disorders in pregnancy in the offspring during perinatal period, childhood, and adolescence. J. Hum. Hypertens. 2021, 35, 1063–1073. [Google Scholar] [CrossRef]
- Hoodbhoy, Z.; Mohammed, N.; Nathani, K.R.; Sattar, S.; Chowdhury, D.; Maskatia, S.; Tierney, S.; Hasan, B.; Das, J.K. The Impact of Maternal Preeclampsia and Hyperglycemia on the Cardiovascular Health of the Offspring: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2021, 40, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Jayet, P.Y.; Rimoldi, S.F.; Stuber, T.; Salmòn, C.S.; Hutter, D.; Rexhaj, E.; Thalmann, S.; Schwab, M.; Turini, P.; Sartori-Cucchia, C.; et al. Pulmonary and Systemic Vascular Dysfunction in Young Offspring of Mothers With Preeclampsia. Circulation 2010, 122, 488–494. [Google Scholar] [CrossRef]
- Davis, E.F.; Lazdam, M.; Lewandowski, A.J.; Worton, S.A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A.R.; McCormick, K.; Sargent, I.; et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012, 129, e1552–e1561. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.F.; Lewandowski, A.J.; Aye, C.; Williamson, W.; Boardman, H.; Huang, R.C.; Mori, T.A.; Newnham, J.; Beilin, L.J.; Leeson, P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: Insights from a 20-year prospective follow-up birth cohort. BMJ Open 2015, 5, e008136. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-W.; Oh, M.-J.; Park, K.-V.; Han, S.-W.; Kim, H.-S.; Sohn, I.-S.; Kwon, H.-S.; Cho, G.-J.; Hwang, H.-S. Risk of Early Childhood Obesity in Offspring of Women with Preeclampsia: A Population-Based Study. J. Clin. Med. 2021, 10, 3758. [Google Scholar] [CrossRef]
- Davidesko, S.; Nahum Sacks, K.; Friger, M.; Haim, A.; Sheiner, E. Prenatal exposure to preeclampsia as a risk factor for long-term endocrine morbidity of the offspring. Hypertens. Pregnancy. 2021, 40, 21–28. [Google Scholar] [CrossRef]
- Patel, L.; Cavazzoni, E.; Whatmore, A.; Carney, S.; Wales, J.K.; Clayton, P.E.; Gibson, A.T. The Contributions of Plasma IGF-I, IGFBP-3 and Leptin to Growth in Extremely Premature Infants During the First Two Years. Pediatr. Res. 2007, 61, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Timpka, S.; Macdonald-Wallis, C.; Hughes, A.D.; Chaturvedi, N.; Franks, P.W.; Lawlor, D.A.; Fraser, A. Hypertensive Disorders of Pregnancy and Offspring Cardiac Structure and Function in Adolescence. J. Am. Heart Assoc. 2016, 5, e003906. [Google Scholar] [CrossRef]
- Lazdam, M.; de la Horra, A.; Pitcher, A.; Mannie, Z.; Diesch, J.; Trevitt, C.; Kylintireas, I.; Contractor, H.; Singhal, A.; Lucas, A.; et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: Is endothelial dysfunction the underlying vascular mechanism? Hypertension 2010, 56, 159–165. [Google Scholar]
- Lazdam, M.; de la Horra, A.; Diesch, J.; Kenworthy, Y.; Davis, E.; Lewandowski, A.J.; Szmigielski, C.; Shore, A.; Mackillop, L.; Kharbanda, R.; et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension 2012, 60, 1338–1345. [Google Scholar]
- Huang, C.; Li, J.; Qin, G.; Liew, Z.; Hu, J.; László, K.D.; Tao, F.; Obel, C.; Olsen, J.; Yu, Y. Maternal hypertensive disorder of pregnancy and offspring early-onset cardiovascular disease in childhood, adolescence, and young adulthood: A national population-based cohort study. PLoS Med. 2021, 18, e1003805. [Google Scholar] [CrossRef]
- Andraweera, P.H.; Lassi, Z.S. Cardiovascular Risk Factors in Offspring of Preeclamptic Pregnancies—Systematic Review and Meta-Analysis. J. Pediatr. 2019, 208, 104–113.e6. [Google Scholar] [CrossRef] [PubMed]
- Libby, G.; Murphy, D.J.; McEwan, N.F.; Greene, S.A.; Forsyth, J.S.; Chien, P.W.; Morris, A.D. DARTS/MEMO Collaboration. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007, 50, 523–530. [Google Scholar] [CrossRef]
- Bi, S.; Zhang, L.; Huang, L.; Li, Y.; Liang, Y.; Huang, M.; Huang, B.; Liang, J.; Gu, S.; Chen, J.; et al. Long-term effects of preeclampsia on metabolic and biochemical outcomes in offspring: What can be expected from a meta-analysis? Obes. Rev. 2022, 23, e13411. [Google Scholar] [CrossRef] [PubMed]
- Keller, G.; Zimmer, G.; Mall, G.; Ritz, E.; Amann, K. Nephron Number in Patients with Primary Hypertension. Volume 348. 2003. Available online: www.nejm.org (accessed on 10 January 2023).
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Ali, A.; Hadlich, F.; Abbas, M.W.; Iqbal, M.A.; Tesfaye, D.; Bouma, G.J.; Winger, Q.A.; Ponsuksili, S. MicroRNA-mRNA Networks in Pregnancy Complications: A Comprehensive Downstream Analysis of Potential Biomarkers. Int. J. Mol. Sci. 2021, 22, 2313. [Google Scholar] [CrossRef]
- Gathiram, P.; Moodley, J. The Role of the Renin-Angiotensin-Aldosterone System in Preeclampsia: A Review. Curr. Hypertens. Rep. 2020, 22, 89. [Google Scholar] [CrossRef]
- Danser, A.H.J.; Verdonk, K.; Saleh, L.; Stephanie Lankhorst, J.E. Ilse Smilde, Manon M. van Ingen, Ingrid M. Garrelds, Edith C.H. Friesema, Henk Russcher, Anton H. van den Meiracker, Willy Visser and A.H. Jan Danse.From the Division of Vascular Medicine and Pharmacology, Department of Internal. Hypertension 2015, 65, 1316–1323. [Google Scholar]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 77. [Google Scholar] [CrossRef]
- Yu, Y.C.; Jiang, Y.; Yang, M.M.; He, S.N.; Xi, X.; Xu, Y.T.; Hu, W.S.; Luo, Q. Hypermethylation of delta-like homolog1/maternally expressed gene 3 loci in human umbilical veins: Insights into offspring vascular dysfunction born after preeclampsia. J. Hypertens. 2019, 37, 581–589. [Google Scholar] [CrossRef]
- outsikou, T.; Mastorakos, G.; Kyriakakou, M.; Margeli, A.; Hassiakos, D.; Papassotiriou, I.; Kanaka-Gantenbein, C.; Malamitsi-Puchner, A. Circulating Levels of Inflammatory Markers in Intrauterine Growth Restriction. Mediat. Inflamm. 2010, 2010, 790605. [Google Scholar] [CrossRef]
- Bhuiyan, A.R.; Srinivasan, S.R.; Chen, W.; Azevedo, M.J.; Berenson, G.S. Influence of low birth weight on C-reactive protein in asymptomatic younger adults: The bogalusa heart study. BMC Res. Notes 2011, 4, 71–75. [Google Scholar] [CrossRef] [PubMed]
- 116. Alsnes, I.V.; Janszky, I.; Åsvold, B.O.; Økland, I.; Forman, M.R.; Vatten, L.J. Maternal Preeclampsia and Androgens in the Offspring around Puberty: A Follow-Up Study. PLoS ONE 2016, 11, e0167714. [Google Scholar] [CrossRef]
- Ogland, B.; Nilsen, S.T.; Forman, M.R.; Vatten, L.J. Pubertal development in daughters of women with pre-eclampsia. Arch. Dis. Child. 2011, 96, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Idkowiak, J.; Lavery, G.G.; Dhir, V.; Barrett, T.G.; Stewart, P.M.; Krone, N.; Arlt, W. Premature adrenarche: Novel lessons from early onset androgen excess. Eur. J. Endocrinol. 2011, 165, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Lykke, L.; Lunddorf, H.; Brix, N.; Ernst, A.; Arendt, L.H.; Støvring, H.; Clemmensen, P.J.; Olsen, J.; Ramlau-Hansen, C.H. Hypertensive disorders in pregnancy and timing of pubertal development in daughters and sons STUDY QUESTION: Do maternal hypertensive disorders affect pubertal development in daughters and sons? Hum. Reprod. 2020, 35, 2124–2133. [Google Scholar] [CrossRef]
- Vatten, L. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet. Gynecol. 2003, 101, 529–533. [Google Scholar] [CrossRef] [PubMed]
- D’aloisio, A.A.; Deroo, L.A.; Baird, D.D.; Weinberg, C.R.; Sandler, D.P. Prenatal and infant exposures and age at menarche. Epidemiology 2013, 24, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Salonen Ros, H.; Lichtenstein, P.; Ekbom, A.; Cnattingius, S. Tall or Short? Twenty Years after Preeclampsia Exposure In Utero: Comparisons of Final Height, Body Mass Index, Waist-to-Hip Ratio, and Age at Menarche among Women, Exposed and Unexposed to Preeclampsia during Fetal Life. Pediatr Res. 2001, 49, 763–769. [Google Scholar] [CrossRef]
- Henley, D.; Brown, S.; Pennell, C.; Lye, S.; Torpy, D.J. Evidence for central hypercortisolism and elevated blood pressure in adolescent offspring of mothers with pre-eclampsia. Clin. Endocrinol. 2016, 85, 583–589. [Google Scholar] [CrossRef]
- Yeung, K.R.; Sunderland, N.; Lind, J.M.; Heffernan, S.; Pears, S.; Xu, B.; Hennessy, A.; Makris, A. Increased salt sensitivity in offspring of pregnancies complicated by experimental preeclampsia. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1302–1308. [Google Scholar] [CrossRef]
- Tagliaferro, T.; Jain, D.; Vanbuskirk, S.; Bancalari, E.; Claure, N. Maternal preeclampsia and respiratory outcomes in extremely premature infants. Pediatr. Res. 2019, 85, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Matyas, M.; Hasmasanu, M.; Silaghi, C.N.; Samasca, G.; Lupan, I.; Orsolya, K.; Zaharie, G. Early Preeclampsia Effect on Preterm Newborns Outcome. J. Clin. Med. 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.; Chaput, K.; Alshaikh, B.; Yusuf, K. Preeclampsia and the Risk of Bronchopulmonary Dysplasia in Preterm Infants Less Than 32 Weeks’ Gestation. Am. J. Perinatol. 2017, 34, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.H.; Yang, H.I.; Chou, H.C.; Chen, C.Y.; Hsieh, W.S. Association of Maternal preeclampsia with neonatal Respiratory Distress Syndrome in Very-Low-Birth-Weight infants. Sci. Rep. 2019, 9, 13212. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Shin, S.H.; Kim, S.H.; Kim, Y.J.; Cho, H.; Kim, E.K.; Kim, H.S. The Association of Pregnancy-induced Hypertension with Bronchopulmonary Dysplasia—A Retrospective Study Based on the Korean Neonatal Network database. Sci. Rep. 2020, 10, 5600. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, H.; Cetinkaya, M.; Koksal, N. Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia. J. Matern.-Fetal Neonatal Med. 2012, 25, 2681–2685. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Carey, V.J.; Mcelrath, T.F.; Qiu, W.; Hollis, B.W.; O’Connor, G.T.; Zeiger, R.S.; Bacharier, L.; Litonjua, A.A.; Weiss, S.T. Impact of Preeclampsia on the Relationship between Maternal Asthma and Offspring Asthma An Observation from the VDAART Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 32–42. [Google Scholar] [CrossRef]
- Sacks, K.N.; Friger, M.; Shoham-Vardi, I.; Sergienko, R.; Landau, D.; Sheiner, E. In utero exposure to pre-eclampsia as an independent risk factor for long-term respiratory disease. Pediatr. Pulmonol. 2020, 55, 723–728. [Google Scholar] [CrossRef] [PubMed]
| Definition | |
|---|---|
| Chronic Hypertension | Systolic BP ≥ 140 mmHg or/and diastolic BP ≥ 90 mmHg on at least two occasions before 20 weeks of pregnancy or hypertension first diagnosed during pregnancy that persists for ≥12 weeks postpartum |
| Chronic Hypertension with superimposed preeclampsia | Development of new-onset proteinuria, other maternal organ dysfunction, or evidence of uteroplacental dysfunction among pregnant women with chronic hypertension |
| Gestational Hypertension —Nonsevere | Systolic BP of 140–159 mmHg or/and diastolic BP of 90–109 mmHg on at least two readings 4 h apart after 20 weeks of gestation in a previously normotensive individual |
| Gestational Hypertension—Severe | Systolic BP ≥ 160 mmHg or/and diastolic BP ≥ 110 mmHg on at least two occasions within a short interval (minutes) after 20 weeks of gestation in a previously normotensive individual |
| Preeclampsia | Gestational hypertension (systolic BP ≥ 140 mmHg or/and diastolic BP ≥ 90 mmHg at ≥20 weeks of gestation) accompanied by one or more of the following new-onset conditions at ≥20 weeks of gestation:
|
| Preeclampsia with severe features |
|
| Eclampsia | In a patient with PE, a novel onset of tonic clonic, focal, or multifocal seizures in the absence of other causative conditions |
| HELLP syndrome | Hemolysis, elevated concentrations of liver enzymes, and low-platelet syndrome including:
|
| System | Short-Term Outcomes | Long-Term Outcomes |
|---|---|---|
| Neurodevelopmental/CNS * |
|
|
| Cardiovascular |
|
|
| Renal |
|
|
| Endocrine |
|
|
| Respiratory |
|
|
| Immune |
|
|
| Gastrointestinal |
|
|
| Eyes |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koulouraki, S.; Paschos, V.; Pervanidou, P.; Christopoulos, P.; Gerede, A.; Eleftheriades, M. Short- and Long-Term Outcomes of Preeclampsia in Offspring: Review of the Literature. Children 2023, 10, 826. https://doi.org/10.3390/children10050826
Koulouraki S, Paschos V, Pervanidou P, Christopoulos P, Gerede A, Eleftheriades M. Short- and Long-Term Outcomes of Preeclampsia in Offspring: Review of the Literature. Children. 2023; 10(5):826. https://doi.org/10.3390/children10050826
Chicago/Turabian StyleKoulouraki, Sevasti, Vasileios Paschos, Panagiota Pervanidou, Panagiotis Christopoulos, Angeliki Gerede, and Makarios Eleftheriades. 2023. "Short- and Long-Term Outcomes of Preeclampsia in Offspring: Review of the Literature" Children 10, no. 5: 826. https://doi.org/10.3390/children10050826
APA StyleKoulouraki, S., Paschos, V., Pervanidou, P., Christopoulos, P., Gerede, A., & Eleftheriades, M. (2023). Short- and Long-Term Outcomes of Preeclampsia in Offspring: Review of the Literature. Children, 10(5), 826. https://doi.org/10.3390/children10050826









