COVID-19 and Related Vaccinations in Children: Pathogenic Aspects of Oral Lesions
Abstract
1. Introduction
2. Materials and Methods
3. Results
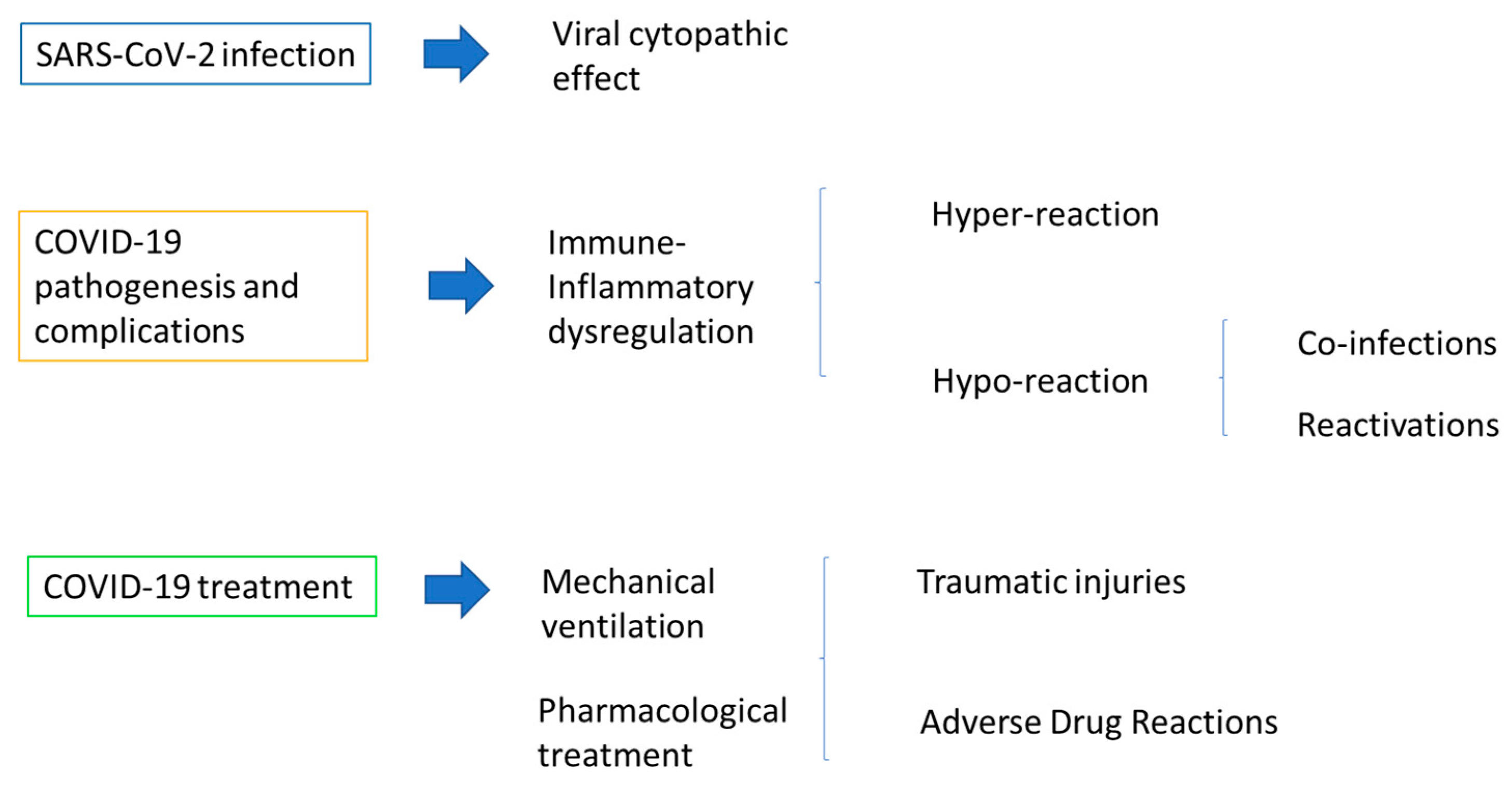
3.1. SARS-CoV-2 Infection in Pediatric Subjects
3.1.1. Oral Lesions in Pediatric SARS-CoV-2-Positive Subjects
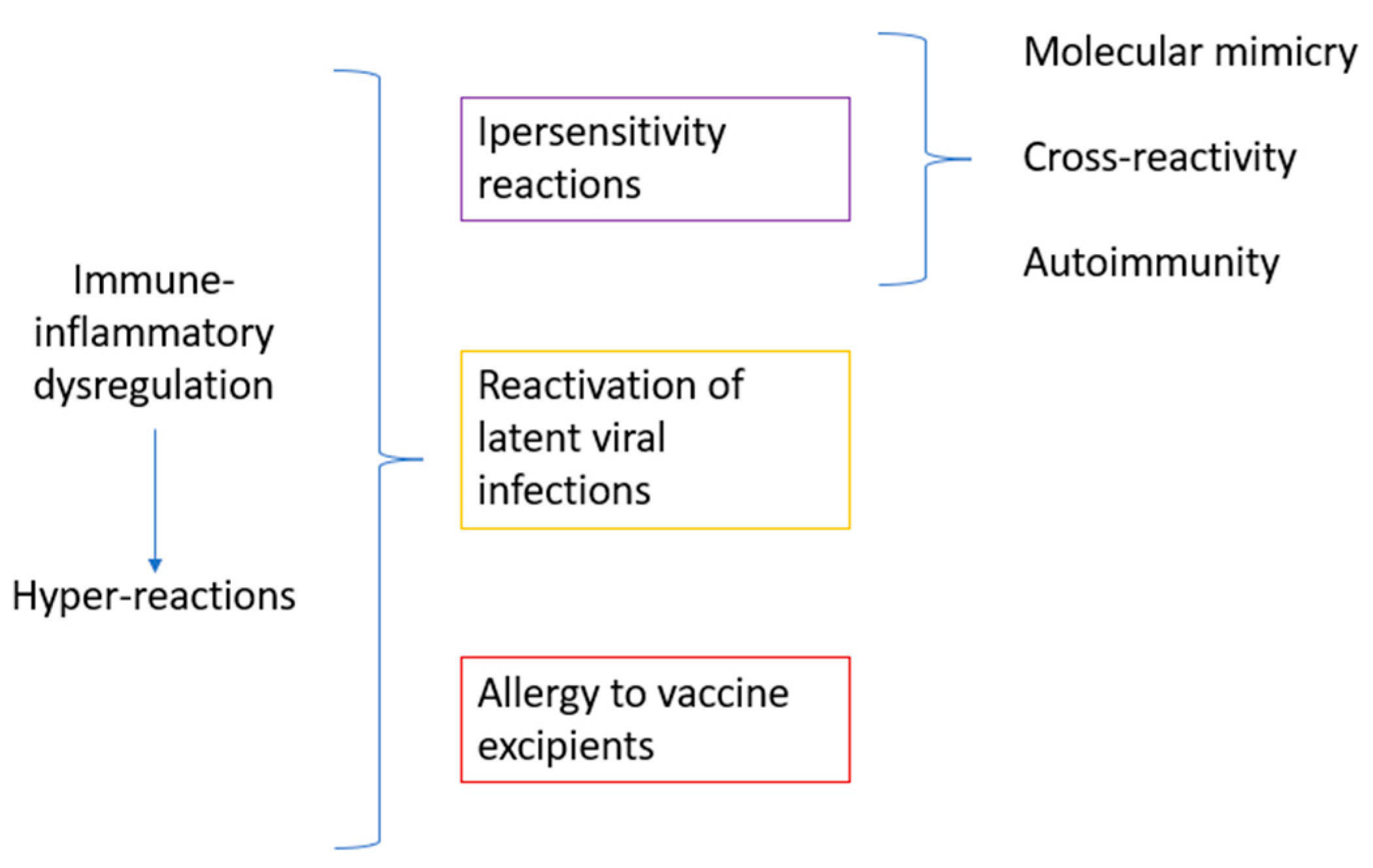
3.1.2. Possible Pathogenic Aspects of Oral Lesions in Pediatric SARS-CoV-2-Positive Subjects
3.2. COVID-19 Vaccination in Pediatric Subjects
3.2.1. Oral Lesions in Pediatric Subjects following COVID-19 Vaccination
3.2.2. Possible Pathogenic Aspects for Oral Lesions following COVID-19 Vaccination in Pediatric Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Spirito, F.; Iandolo, A.; Amato, A.; Caggiano, M.; Raimondo, A.; Lembo, S.; Martina, S. Prevalence, Features and Degree of Association of Oral Lesions in COVID-19: A Systematic Review of Systematic Reviews. Int. J. Environ. Res. Public Health 2022, 19, 7486. [Google Scholar] [CrossRef]
- Hoste, L.; van Paemel, R.; Haerynck, F. Multisystem Inflammatory Syndrome in Children Related to COVID-19: A Systematic Review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Di Spirito, F.; Caggiano, M.; Di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; Martina, S.; Amato, A. Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Appl. Sci. 2022, 12, 8995. [Google Scholar] [CrossRef]
- Egido-Moreno, S.; Valls-Roca-Umbert, J.; Jané-Salas, E.; López-López, J.; Estrugo-Devesa, A. COVID-19 and Oral Lesions, Short Communication and Review. J. Clin. Exp. Dent. 2021, 13, e287–e294. [Google Scholar] [CrossRef]
- Khazeei Tabari, M.A.; Najary, S.; Khadivi, G.; Yousefi, M.J.; Samieefar, N.; Abdollahimajd, F. Oral Lesions after COVID-19 Vaccination: Immune Mechanisms and Clinical Approach. Infect. Med. 2022, 1, 171–179. [Google Scholar] [CrossRef]
- di Spirito, F.; Amato, A.; di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; lo Giudice, R.; Amato, M. Oral Lesions Following Anti-SARS-CoV-2 Vaccination: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10228. [Google Scholar] [CrossRef] [PubMed]
- Fathy, R.A.; McMahon, D.E.; Lee, C.; Chamberlin, G.C.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; et al. Varicella-zoster and Herpes Simplex Virus Reactivation Post-COVID-19 Vaccination: A Review of 40 Cases in an International Dermatology Registry. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e6–e9. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker European Centre for Disease Prevention and Control (Europa.Eu). Available online: https://www.EcDc.Europa.Eu/En/Publications-Data/Covid-19-Vaccine-Tracker (accessed on 2 September 2022).
- Children and COVID-19 Vaccination Trends (Aap.Org). Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/ (accessed on 12 August 2022).
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where Do We Stand? Arch. Med. Res. 2022, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakra, N.; Blumberg, D.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef]
- Neto, R.P.; Mazzo, F.A.T.; de Almeida Vieira, F.; de Souza Bueno, G.; Previdi, J.V.C.; da Silva, L.R.; da Silva, N.K.B.; Jorizzo, J.L.; Cerci, F.B. COVID-19 Cutaneous Manifestations in Children and Adolescents: A Systematic Review. Rev. Paul. Pediatr. 2022, 40, e2021134. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.B.; Araujo, N.S.; Silva, J.C.; Xavier, F.C.A. Oral Manifestations of Multisystemic Inflammatory Syndrome in Children (MIS-C) and Kawasaki Disease Associated to COVID-19: A Systematic Review. Spec. Care Dent. 2022, 42, 266–280. [Google Scholar] [CrossRef]
- Shah, S.; Akhade, K.; Ganguly, S.; Nanda, R.; Mohapatra, E.; Goel, A. Cutaneous Manifestations Associated with COVID-19 in Children: A Systematic Review. J. Fam. Med. Prim. Care 2021, 10, 93. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Skaare, D. COVID-19 Med Nedsatt Lukte-Og Smakssans Som Eneste Symptom. Tidsskr. Den. Nor. Legeforening 2020, 140, 683–685. [Google Scholar] [CrossRef]
- Patel, N.A. Pediatric COVID-19: Systematic Review of the Literature. Am. J. Otolaryngol. 2020, 41, 102573. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.; Brodsky, N.N.; Sumida, T.S.; Comi, M.; Asashima, H.; Hoehn, K.B.; Li, N.; Liu, Y.; Shah, A.; Ravindra, N.G.; et al. Immune Dysregulation and Autoreactivity Correlate with Disease Severity in SARS-CoV-2-Associated Multisystem Inflammatory Syndrome in Children. Immunity 2021, 54, 1083–1095.e7. [Google Scholar] [CrossRef]
- Menni, S. Dermatite Atopica: Patologie Orali. In La Scuola Dell’atopia; Springer Milan: Milano, Italy, 2007; pp. 165–170. [Google Scholar]
- UNICEF Data: Monitoring the Situation of Children and Women. Available online: Https://Data.Unicef.Org/Resources/Covid-19-Confirmed-Cases-and-Deaths-Dashboard/ (accessed on 1 January 2023).
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pelella, S.; Argentino, S.; Sisalli, L.; Sbordone, L. Oral Manifestations and the Role of the Oral Healthcare Workers in COVID-19. Oral Dis. 2022, 28, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Brandini, D.A.; Takamiya, A.S.; Thakkar, P.; Schaller, S.; Rahat, R.; Naqvi, A.R. COVID-19 and Oral Diseases: Crosstalk, Synergy or Association? Rev. Med. Virol. 2021, 31, e2226. [Google Scholar] [CrossRef]
- Erbaş, G.S.; Botsali, A.; Erden, N.; Arı, C.; Taşkın, B.; Alper, S.; Vural, S. COVID-19-related Oral Mucosa Lesions among Confirmed SARS-CoV-2 Patients: A Systematic Review. Int. J. Dermatol. 2022, 61, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Orilisi, G.; Mascitti, M.; Togni, L.; Monterubbianesi, R.; Tosco, V.; Vitiello, F.; Santarelli, A.; Putignano, A.; Orsini, G. Oral Manifestations of COVID-19 in Hospitalized Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12511. [Google Scholar] [CrossRef] [PubMed]
- Martín Carreras-Presas, C.; Amaro Sánchez, J.; López-Sánchez, A.F.; Jané-Salas, E.; Somacarrera Pérez, M.L. Oral Vesiculobullous Lesions Associated with SARS-CoV-2 Infection. Oral Dis. 2021, 27, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Haddad, G.; Martin, P.; Martin, J. Cutaneous Manifestations of COVID-19 in Children: Literature Review. Residência Pediátrica 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Dondi, A.; Sperti, G.; Gori, D.; Guaraldi, F.; Montalti, M.; Parini, L.; Piraccini, B.M.; Lanari, M.; Neri, I. Epidemiology and Clinical Evolution of Non-Multisystem Inflammatory Syndrome (MIS-C) Dermatological Lesions in Pediatric Patients Affected by SARS-CoV-2 Infection: A Systematic Review of the Literature. Eur. J. Pediatr. 2022, 181, 3577–3593. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Woolley, J. Necrotizing Periodontal Disease: Oral Manifestation of COVID-19. Oral Dis. 2021, 27, 768–769. [Google Scholar] [CrossRef] [PubMed]
- di Spirito, F.; Contaldo, M.; Amato, A.; di Palo, M.P.; Pantaleo, G.; Amato, M. COVID-19 Vaccine and Oral Lesions: Putative Pathogenic Mechanisms. Oral Dis. 2022, 28 (Suppl. S2), 2639–2640. [Google Scholar] [CrossRef] [PubMed]
- Farinazzo, E.; Dianzani, C.; Zalaudek, I.; Conforti, C.; Grabbe, S.; Goldust, M. Synthesis of the Data on COVID-19 Skin Manifestations: Underlying Mechanisms and Potential Outcomes. Clin. Cosmet. Investig. Dermatol. 2021, 14, 991–997. [Google Scholar] [CrossRef]
- Algaadi, S.A. Herpes Zoster and COVID-19 Infection: A Coincidence or a Causal Relationship? Infection 2022, 50, 289–293. [Google Scholar] [CrossRef]
- Saxena, S.; Kumar, S. Understanding the Mechanism of Commonly Occurring COVID-19-Associated Oral Lesions. J. Oral Maxillofac. Pathol. 2021, 25, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Farid, H.; Khan, M.; Jamal, S.; Ghafoor, R. Oral Manifestations of COVID-19-A Literature Review. Rev. Med. Virol. 2022, 32, e2248. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Barile, G.; Brienza, N.; Capodiferro, S.; Vestito, M.C.; Crudele, L.; Procacci, V.; Ingravallo, G.; Maiorano, E.; et al. COVID-19 Symptomatic Patients with Oral Lesions: Clinical and Histopathological Study on 123 Cases of the University Hospital Policlinic of Bari with a Purpose of a New Classification. J. Clin. Med. 2021, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.D.; Souza, L.L.; de Carvalho, M.G.F.; Pontes, H.A.R.; Mosqueda-Taylor, A.; Hernandez-Guerrero, J.C.; do Nascimento Medeiros, S.D.; de Oliveira Sales, A.; Alves, F.A.; Lopes Pinto, C.A.; et al. Oral Manifestations of Coronavirus Disease 2019 (COVID-19). Am. J. Surg. Pathol. 2022, 46, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Amato, A. Oral-Systemic Health and Disorders: Latest Advances on Oral–Gut–Lung Microbiome Axis. Appl. Sci. 2022, 12, 8213. [Google Scholar] [CrossRef]
- Sharma, P.; Malik, S.; Wadhwan, V.; Gotur Palakshappa, S.; Singh, R. Prevalence of oral manifestations in COVID-19: A systematic review. Reviews in medical virology 2022, 32, e2345. [Google Scholar] [CrossRef] [PubMed]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef]
- Di Spirito, F. Oral-Systemic Health and Disorders: Latest Prospects on Oral Antisepsis. Appl. Sci. 2022, 12, 8185. [Google Scholar] [CrossRef]
- Boccia, G.; Di Spirito, F.; D’Ambrosio, F.; De Caro, F.; Pecora, D.; Giorgio, R.; Fortino, L.; Longanella, W.; Franci, G.; Santella, B.; et al. Microbial Air Contamination in a Dental Setting Environment and Ultrasonic Scaling in Periodontally Healthy Subjects: An Observational Study. Int. J. Environ. Res. Public Health 2023, 20, 2710. [Google Scholar] [CrossRef]
- Nambiar, M.; Varma, S.R.; Jaber, M.; Sreelatha, S.V.; Thomas, B.; Nair, A.S. Mycotic Infections–Mucormycosis and Oral Candidiasis Associated with COVID-19: A Significant and Challenging Association. J. Oral Microbiol. 2021, 13, 1967699. [Google Scholar] [CrossRef]
- di Spirito, F.; Amato, A.; di Palo, M.P.; Ferraro, G.A.; Baroni, A.; Serpico, R.; Contaldo, M. COVID-19 Related Information on Pediatric Dental Care Including the Use of Teledentistry: A Narrative Review. Children 2022, 9, 1942. [Google Scholar] [CrossRef]
- Rajendra Santosh, A.B.; Muddana, K.; Bakki, S.R. Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef]
- Petruzzi, M.; Galleggiante, S.; Messina, S.; della Vella, F. Oral Erythema Multiforme after Pfizer-BioNTech COVID-19 Vaccination: A Report of Four Cases. BMC Oral Health 2022, 22, 90. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.D.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, F. Oral Mycobiome and COVID-19. Microorganisms 2023, 11, 982. [Google Scholar] [CrossRef]
- Pisano, M.; Romano, A.; Di Palo, M.P.; Baroni, A.; Serpico, R.; Contaldo, M. Oral Candidiasis in Adult and Pediatric Patients with COVID-19. Biomedicines 2023, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Sharawat, I.; Natarajan, V.; Bhakat, R.; Panda, P.; Dawman, L. COVID-19 Treatment in Children: A Systematic Review and Meta-Analysis. J. Fam. Med. Prim. Care 2021, 10, 3292. [Google Scholar] [CrossRef]
- Bennardo, L.; Nisticò, S.P.; Dastoli, S.; Provenzano, E.; Napolitano, M.; Silvestri, M.; Passante, M.; Patruno, C. Erythema Multiforme and COVID-19: What Do We Know? Medicina 2021, 57, 828. [Google Scholar] [CrossRef]
- Jouhar, L.; Yahya, M.; Elsiddiq, S. Toxic Epidermal Necrolysis Associated with COVID-19 Infection: A Case Report. Clin. Case Rep. 2022, 10, e05565. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (europa.eu) COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html (accessed on 1 January 2023).
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F. Epidemiology, Pathogenesis, Clinical Presentations, Diagnosis and Treatment of COVID-19: A Review of Current Evidence. Expert. Rev. Clin. Pharm. 2021, 14, 601–621. [Google Scholar] [CrossRef]
- Caggiano, M.; Amato, M.; Di Spirito, F.; Galdi, M.; Sisalli, L. MRNA COVID-19 Vaccine and Oral Lichen Planus: A Case Report. Oral Dis. 2022, 28, 2624–2626. [Google Scholar] [CrossRef] [PubMed]
- Štefan, M.; Dlouhý, P.; Bezdíčková, L. Vaccination against COVID-19. Klin. Mikrobiol. Infekc. Lek. 2021, 27, 49–60. [Google Scholar]
- Di Spirito, F.; Pantaleo, G.; Di Palo, M.P.; Amato, A.; Raimondo, A.; Amato, M. Oral Human Papillomavirus Benign Lesions and HPV-Related Cancer in Healthy Children: A Systematic Review. Cancers 2023, 15, 1096. [Google Scholar] [CrossRef] [PubMed]
- Seirafianpour, F.; Pourriyahi, H.; Gholizadeh Mesgarha, M.; Pour Mohammad, A.; Shaka, Z.; Goodarzi, A. A Systematic Review on Mucocutaneous Presentations after COVID-19 Vaccination and Expert Recommendations about Vaccination of Important Immune-mediated Dermatologic Disorders. Dermatol. Ther. 2022, 35, e15461. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Boms, S.; Susok, L.; Dickel, H.; Finis, C.; Abu Rached, N.; Barras, M.; Stücker, M.; Kasakovski, D. Cutaneous Findings Following COVID-19 Vaccination: Review of World Literature and Own Experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous Reactions after SARS-CoV-2 Vaccination: A Cross-Sectional Spanish Nationwide Study of 405 Cases. Br. J. Dermatol. 2022, 186, 142–152. [Google Scholar] [CrossRef]
- Avallone, G.; Quaglino, P.; Cavallo, F.; Roccuzzo, G.; Ribero, S.; Zalaudek, I.; Conforti, C. SARS-CoV-2 Vaccine-related Cutaneous Manifestations: A Systematic Review. Int. J. Dermatol. 2022, 61, 1187–1204. [Google Scholar] [CrossRef] [PubMed]
- Bogs, T.; Saleh, N.; Yavuz, S.T.; Fazeli, W.; Ganschow, R.; Schreiner, F. Aseptic Meningitis, Mucocutaneous Lesions and Arthritis after COVID-19 Vaccination in a 15-Year-Old Boy. Vaccines 2022, 10, 325. [Google Scholar] [CrossRef]
- Cirillo, N. Reported Orofacial Adverse Effects of COVID-19 Vaccines: The Knowns and the Unknowns. J. Oral Pathol. Med. 2021, 50, 424–427. [Google Scholar] [CrossRef]
- Riad, A. Oral Side Effects of COVID-19 Vaccine. Br. Dent. J. 2021, 230, 59. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Jurado, C.A.; Abu-Fanas, S.H.; Jaber, M.A. Health and Well-Being through COVID-19 Vaccination: Physical, Oral, and Psychological Effects. Int. J. Environ. Res. Public Health 2023, 20, 3117. [Google Scholar] [CrossRef]
- Colonna, C.; Restano, L.; Monzani, N.A.; Zussino, M.; Ponziani, A.; Cambiaghi, S.; Cavalli, R. Rare and Common Manifestations of COVID-19 in Children. JEADV Clin. Pract. 2022, 1, 21–30. [Google Scholar] [CrossRef]
- Sharda, P.; Mohta, A.; Ghiya, B.C.; Mehta, R.D. Development of Oral Lichen Planus after COVID-19 Vaccination—A Rare Case Report. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Gogl, M.; Berndt, R.; Troeltzsch, M. Oral Lichen Planus Following the Administration of Vector-based COVID-19 Vaccine (Ad26.COV2.S). Oral Dis. 2022, 28, 2595–2596. [Google Scholar] [CrossRef]
- Babazadeh, A.; Miladi, R.; Barary, M.; Shirvani, M.; Ebrahimpour, S.; Aryanian, Z.; Mohseni Afshar, Z. COVID-19 Vaccine-related New-onset Lichen Planus. Clin. Case Rep. 2022, 10, e05323. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Sirka, C.S.; Mishra, S.; Viswan, P. COVID-19 Vaccine-induced Stevens–Johnson Syndrome. Clin. Exp. Dermatol. 2021, 46, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Borg, L.; Mercieca, L.; Mintoff, D.; Micallef, D.; Pisani, D.; Betts, A.; Scerri, L. Pfizer-BioNTech SARS-CoV-2 MRNA Vaccine-associated Erythema Multiforme. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e22–e24. [Google Scholar] [CrossRef] [PubMed]
- Shikha; Gupta, S.; Mahajan, A.; Ambika; Garg, R.; Ghosh, S. Childhood Oral Lichen Planus: A Case Series with Review of Literature. Eur. Arch. Paediatr. Dent. 2022, 23, 341–353. [Google Scholar] [CrossRef]
- Cascone, M.; Celentano, A.; Adamo, D.; Leuci, S.; Ruoppo, E.; Mignogna, M.D. Oral Lichen Planus in Childhood: A Case Series. Int. J. Dermatol. 2017, 56, 641–652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Spirito, F.; D’Ambrosio, F.; Di Palo, M.P.; Giordano, F.; Coppola, N.; Contaldo, M. COVID-19 and Related Vaccinations in Children: Pathogenic Aspects of Oral Lesions. Children 2023, 10, 809. https://doi.org/10.3390/children10050809
Di Spirito F, D’Ambrosio F, Di Palo MP, Giordano F, Coppola N, Contaldo M. COVID-19 and Related Vaccinations in Children: Pathogenic Aspects of Oral Lesions. Children. 2023; 10(5):809. https://doi.org/10.3390/children10050809
Chicago/Turabian StyleDi Spirito, Federica, Francesco D’Ambrosio, Maria Pia Di Palo, Francesco Giordano, Nicoletta Coppola, and Maria Contaldo. 2023. "COVID-19 and Related Vaccinations in Children: Pathogenic Aspects of Oral Lesions" Children 10, no. 5: 809. https://doi.org/10.3390/children10050809
APA StyleDi Spirito, F., D’Ambrosio, F., Di Palo, M. P., Giordano, F., Coppola, N., & Contaldo, M. (2023). COVID-19 and Related Vaccinations in Children: Pathogenic Aspects of Oral Lesions. Children, 10(5), 809. https://doi.org/10.3390/children10050809








