Children with FASD—Evolving Patterns of Developmental Problems and Intervention Costs in Ages 0 through 16 in Finland
Abstract
1. Introduction
1.1. Classification of Fetal Alcohol Spectrum Disorders (FASD) and Its Subtypes
1.2. Diagnostic Criteria for FASD
1.3. The Prevalence of FASD
1.4. Health, Societal and Economic Significance of FASD
1.5. Motivation for the Present Study
1.6. The Aims and Type of the Study
- What were the most common diagnoses and problems in everyday life for those diagnosed with FASD within four age groups: infancy, preschool age, primary school age, and adolescence?
- What were the typical interventions in different age groups?
- What were the estimated additional costs associated with problems and interventions in different age groups?
2. Data
2.1. Study Population Definition
- -
- P04.3 (Fetus and newborn affected by maternal use of alcohol);
- -
- Q86.0 (Fetal alcohol syndrome or Partial fetal alcohol syndrome);
- -
- F83 (Mixed specific developmental disorders) or F81.3 (Mixed disorder of scholastic skills) or F90 (ADHD) and had FASD or ARND listed as a written sub-diagnosis, or maternal alcohol use during pregnancy was listed as the etiology for developmental disorder.
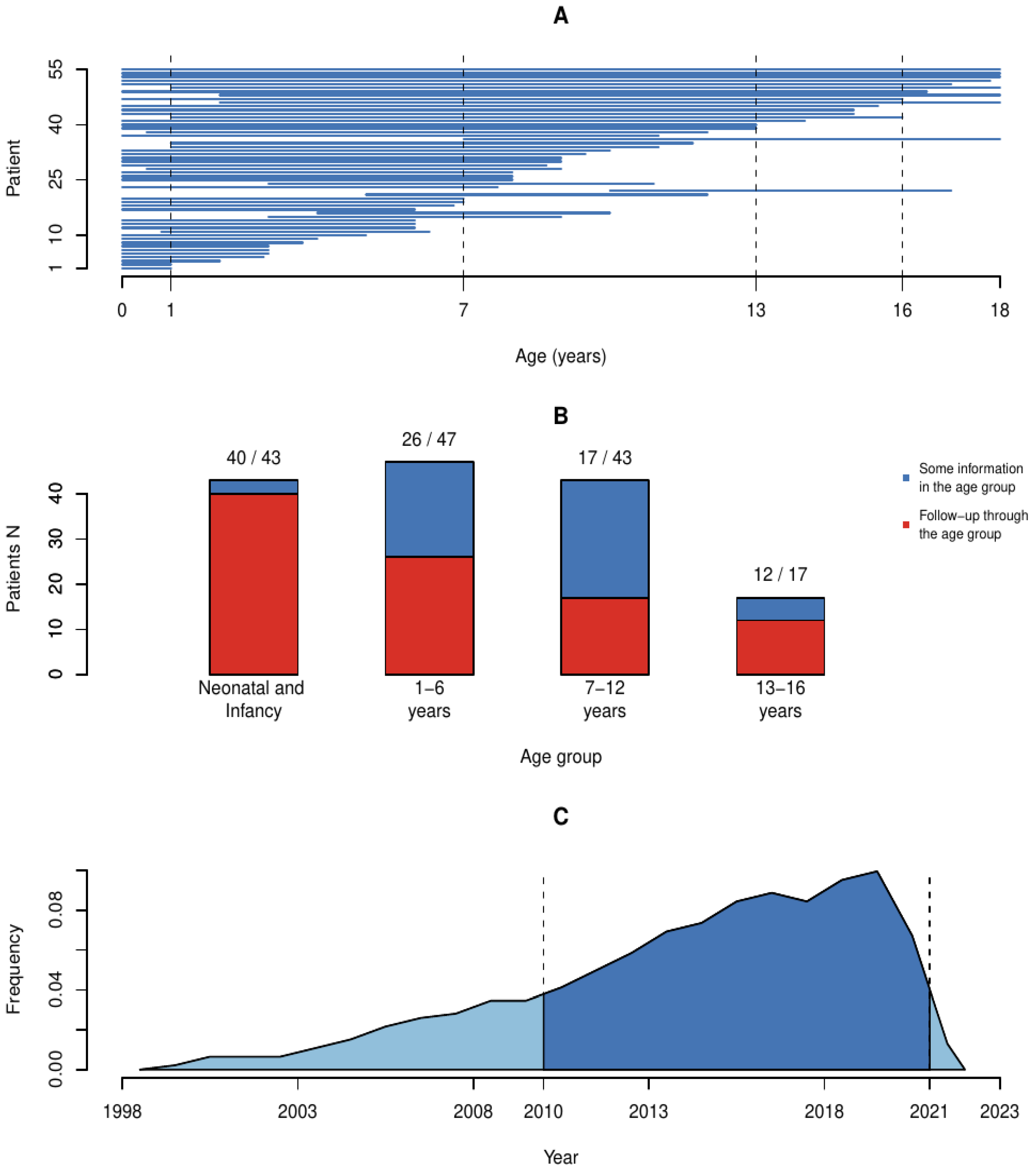
2.2. Data Coverage
3. Methods
3.1. Data Collection
3.1.1. Birth Information
3.1.2. Diagnosis-Related Information and Medications
3.1.3. Developmental and Rehabilitation Information
3.1.4. Educational Information
3.1.5. Family Information
3.1.6. Forming of Age Groups
3.2. Cost Estimation Methods
3.2.1. Selection Process of Cost Estimation Factors
3.2.2. Index Correction and Discounting
3.2.3. Estimated Cost Types
- (1)
- Hospital costs, including both outpatient clinic visits and inpatient ward stays;
- (2)
- Out-of-home placement costs, both in foster families and in institutional placements (not including hospital stays, which are under hospital costs);
- (3)
- Disability allowance costs, which is money paid by the Social Insurance Institution when a child has a chronic illness or a disability and has greater than normal need for care and attention;
- (4)
- Rehabilitation costs including physiotherapy, speech therapy, occupational therapy, neuropsychological rehabilitation and psychiatric therapies;
- (5)
- Special needs education costs (average schooling costs were subtracted);
- (6)
- Long-term medication costs for regularly used prescription medication (not including asthma and allergy medications, which are common also in general population).
3.3. Cost Estimations
3.3.1. Hospitalizations
3.3.2. Out-of-Home Placements
3.3.3. Disability Allowance
3.3.4. Rehabilitation
3.3.5. Special Needs Educational Support
- Entry in specialty school for disabled with extended compulsory education and individualized syllabus; very high adult/student ratio; at this support level, taxi transfers and morning and afternoon care are included in total cost;
- Extended compulsory education in a small special needs class and with individualized syllabus, but situated in regular school; high adult/student ratio;
- Special needs education and special support in small class without extended compulsory education; general or modified syllabus; higher than average adult/student ratio;
- Special support with inclusion and integration to mainstream classroom;
- Intensified support in mainstream classroom.
3.3.6. Medication
3.3.7. Calculation Methods and Assumptions Used in Estimations
4. Results
4.1. Infancy, Age under 1 Year (Diagnoses and Interventions n = 43, Complete Follow-Up Time n = 38)
4.1.1. Diagnoses
4.1.2. Interventions
4.1.3. Costs
4.2. Preschool Age (1–6 Years, Diagnoses and Interventions n = 45, Complete Follow-Up Time n = 26)
4.2.1. Diagnoses
4.2.2. Interventions
4.2.3. Costs
4.3. Primary School Years (7–12 Years, Diagnoses n = 37, Complete Follow-Up Time n = 17)
4.3.1. Diagnoses
4.3.2. Interventions
4.3.3. Costs
4.4. Adolescence (13–16 Years, Diagnoses n = 15, Complete Follow-Up Time n = 12)
4.4.1. Diagnoses
4.4.2. Interventions
4.4.3. Costs
4.5. Results of the Whole Childhood (0–16 Years)
4.5.1. Etiological Examinations Performed
4.5.2. Non-Age-Specific Somatic Findings
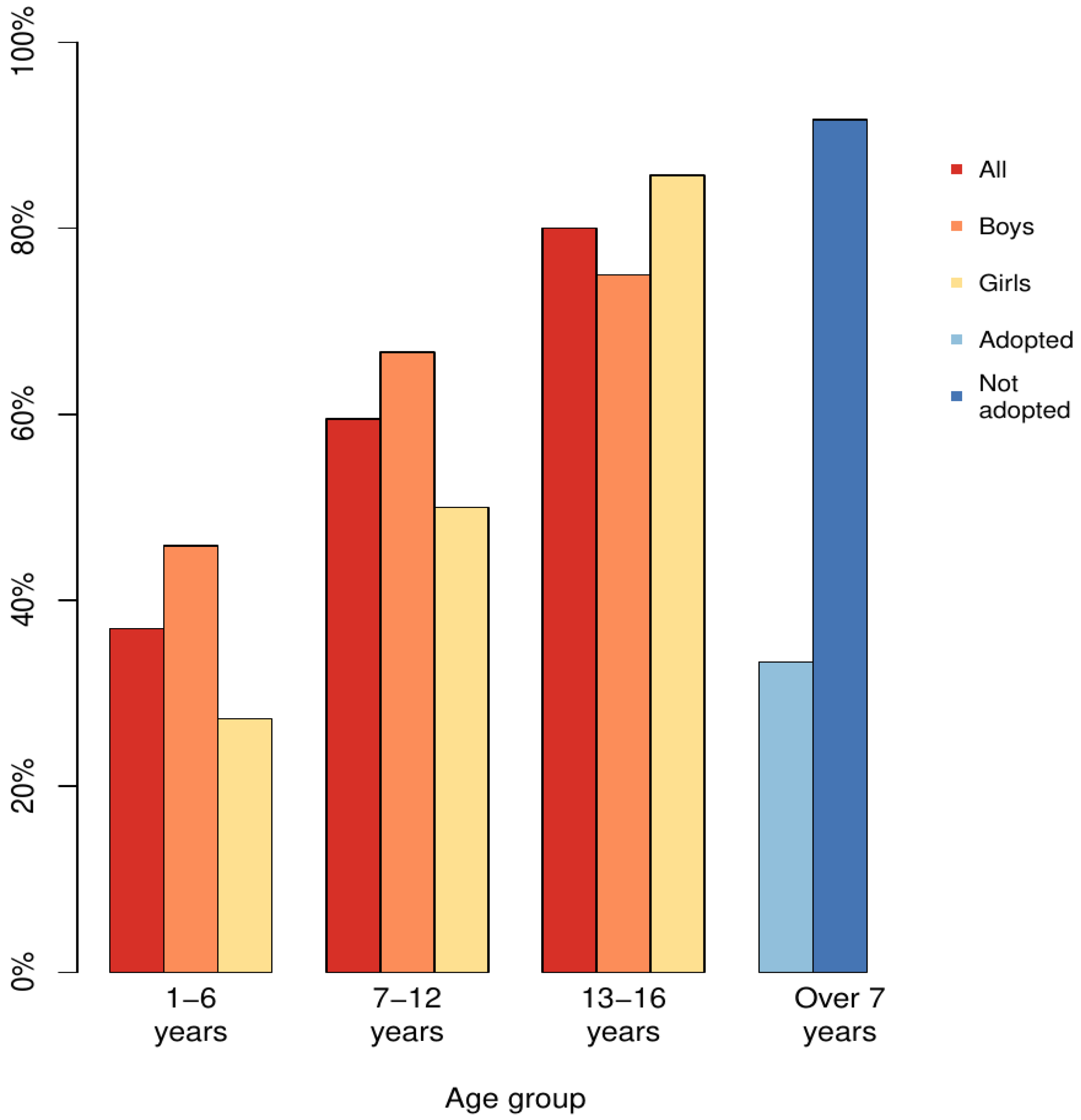
4.5.3. Adopted Compared to Those Living in Birth Families or Foster Care System
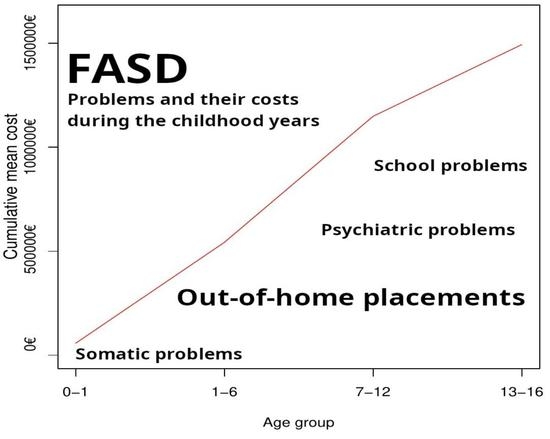
4.5.4. Cumulative Additional Costs for the Childhood (0–16 Years)
5. Discussion
5.1. Strengths of the Study
5.2. Limitations of the Study
5.2.1. Limitations Related to Data and Study Population
5.2.2. Limitation Related to Cost Estimations
5.2.3. Limitations Related to Transferability to Other Countries
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. All Used Prices and Their Sources before Index Correction
| Price Category | Price (EUR) | Code/Unit | Notes | Source |
|---|---|---|---|---|
| DRG price | ICD-10 codes or category | Päijät-Häme DRG price list (1) | |
| Concussion/mild traumatic brain injury, child | 1315 | S06 | (1) | |
| Seizure or headache, child | 2079 | G40–G44 | (1) | |
| Operation for strabismus, short stay | 2660 | H50 | (1) | |
| Other ophthalmological disease | 2789 | H51–H54, Q13–Q14 | (1) | |
| Tonsillectomy/Adenoidectomy | 2596 | J35–J36 | (1) | |
| Otitis media, complicated, or tympanostomy, child | 2302 | H65–H66 | (1) | |
| Pneumonia or pleuritis, child | 3805 | J09–J18 | (1) | |
| Obstructive bronchitis/bronchiolitis or acute asthma, child | 2918 | J20–J22, J40–J47 | (1) | |
| Other ear–nose–throat disease, child | 2168 | Q32 | (1) | |
| Congenital heart defect, child | 6024 | Q20–Q28 | (1) | |
| Operation for hernia, child | 6367 | K40–K46 | (1) | |
| Appendectomy, uncomplicated | 4223 | K35–K37 | (1) | |
| Juvenile diabetes, young patient | 4465 | E10 | (1) | |
| Urinary tract infection/disease, short stay, no significant operation | 1085 | N10, N30, N39 | (1) | |
| Testicular operation for non-malignant cause, child | 4143 | Q53, N44 | (1) | |
| Newborn, birthweight <1000 g | 146,652 | P07.0 | (1) | |
| Newborn, birthweight 1000–1499 g | 57,785 | P07.1 | (1) | |
| Newborn, birthweight 1500–2499 g, problems in multiple organ systems | 41,760 | P07.3 | Several other diagnoses, long NICU stay | (1) |
| Newborn, birthweight 1500–2499 g, no problems in multiple organ systems | 17,058 | P07.3 | Short NICU stay, no severe complications | (1) |
| Newborn, birthweight 2500 g or more, problems in multiple organ systems | 21,179 | long NICU stay, no P07 | Severe congenital problems or long morphine treatment for neonatal abstinence syndrome | (1) |
| Newborn, birthweight 2500 g or more, other significant problem | 7632 | Short <7 days of NICU stay, no P07 | For example, transient respiratory problems, need for IV glucose or IV antibiotics for an infection | (1) |
| Intellectual disability | 2628 | F70–F79 | (1) | |
| Neuropsychiatric disorder | 4834 | F84–F90 | (1) | |
| Other psychiatric, emotional or behavioral disorder in childhood | 5261 | F91–F99 | (1) | |
| Mood or anxiety disorder | 3123 | F30–F48 | (1) | |
| Intellectual disability | 2628 | F70–F79 | (1) | |
| Traumatic injury (other than concussion), child | 1017 | S-(except S06) and T-codes | (1) | |
| Poisoning or toxic effect of a substance | 1439 | X44, Y91 | (1) | |
| Neurologic disease or disorder, without significant complications | 1090 | R62, Q86, F80, F81, F82, Q02 | (1) | |
| Pregnancy related complication | 1274 | O24 | (1) | |
| Eating disorder | 8424 | F50 | (1) | |
| Gastrointestinal disease, short stay without significant operation | 754 | A08, A09, K5 | (1) | |
| Other central nervous system disorder, uncomplicated | 2250 | F83 | (1) | |
| Endocrine disorder, short treatment, without significant operation | 911 | E34, E46, E23 | (1) | |
| Other or unspecified issue, short treatment without significant operation | 722 | UNSPECIFIED | Other diagnoses not listed here | (1) |
| Day in adolescent psychiatric ward | 681 | price/day | (1) | |
| Brain MRI with anesthesia | 750 | MRI | (1) | |
| Sleep–wake EEG registration | 300 | EEG | (1) | |
| Molecular karyotype/chromosomal microarray | 800 | Mksyn | (1) | |
| DRGHUS Esophagus atresia | 5851 | Q39 | Operated in HUS | (2) |
| DRGHUS Cleft lip | 6327 | Q36 | Operated in HUS | (2) |
| Price for a typical rehabilitation period | Price per unit and number of units | Primary care (1). Kela/SII (3, 4) | |
| Psychiatric rehabilitation/psychotherapy | 19,014 | If psychotherapy | (1) | |
| Physical therapy, in primary health care | 1965 | EUR 131/45 min × 15 | If only between 1 and 12 months | (1) |
| Physical therapy, via Kela | 2400 | EUR 60/45 min × 40 | If physical therapy, 1–6 years | (3, 4) |
| Speech therapy, in primary health care | 6360 | EUR 212/45 min × 30 | If no diagnosis F80 or F83 1–6 years | (1) |
| Speech therapy, via Kela | 9600 | EUR 120/45 min × 80 | If F80 or F83 or F7 1–6 years, if 7+ years, additional 4800 is added | (3, 4) |
| Occupational therapy, in primary health care | 5640 | EUR 141/45 min × 40 | If no diagnosis F82 or F83 | (1) |
| Music therapy | 4600 | EUR 115/45 min × 40 | (1) | |
| Neuropsychological rehabilitation | 9280 | EUR 116/45 min × 80 | (3, 4) | |
| Theraplay | 3360 | EUR 168/60 min × 20 | (1) | |
| Price | Time | Estimation and what is included | Finnish National Agency for Education: Prices and Funding (5), Statistics (6, 7, 8, 9) |
| Special school for severely disabled (category 1) | 28,021 | year | Includes taxi transfers, morning, evening and holiday day care, and structural costs associated with special school | (5) |
| Extra year of kindergarten as a part of extended compulsory education | 9221 | year | Added once to those in categories 1 and 2 | (6) |
| Extended compulsory education for disabled (category 2) | 18,584 | year | EUR 28,021–9437 (basic price) | (7) |
| Special needs education special support in smaller class, regular school (category 3) | 13,645 | year | One teaching price subtracted from the price in category 2 | (6, 7) |
| Special support inclusion/integration to mainstream classroom (category 4) | 4939 | year | Only teaching costs are doubled | (6, 7) |
| Intensified support in mainstream classroom (category 5) | 2564 | year | Cost for flexible teaching arrangements | (6, 7) |
| Extra school year (repeating a grade) | 9437 | year | Basic price for a schoolyear | (6) |
| Care for a special needs student in the afternoons after school day | 1568 | year | Grades 2–3 if diagnosed with F83/F81.3 or F90, and grades 2–9 if diagnosed with F70–F79, in categories 2–5 (included in the price in category 1) | (8) |
| Taxi transfer to school and back home | 1303 | year | For those diagnosed with F70–F79 and F83/F81.3 and F90 in categories 2–5 (included in the price in category 1) | (9) |
| Price/year | Price/month | Kela/SII (10) | |
| Disability allowance, basic | 1145 | 95.42 | A total of 0.5 year if long-term problems during infancy, after 3 years for others with developmental (F70–F84) diagnosis and medication or 1 therapy or psychiatric diagnosis | (10) |
| Disability allowance, middle | 2671 | 222.60 | Ages 3–16 if diabetes or developmental diagnosis (F70–F84) and at least two therapies or psychiatric care and medication or therapy | (10) |
| Disability allowance, highest | 5071 | 422.58 | If needs round-the-clock care for severe disability or diabetes; 0–3 years | (10) |
| Price/year (nominal prices) | Price/month (nominal prices) | Mean price per 0–17-year-old person placed out of home in the Hospital catchment area | Finnish Institute on health and welfare and Statistics of Finland (11, 12) and Kuusikko statistics (13) |
| Out-of-home placement 2006 | 47,890 | 3990 | (11, 12) | |
| Out-of-home placement 2007 | 46,670 | 3890 | (11, 12) | |
| Out-of-home placement 2008 | 56,160 | 4680 | (11, 12) | |
| Out-of-home placement 2009 | 57,940 | 4830 | (11, 12) | |
| Out-of-home placement 2010 | 58,040 | 4840 | (11, 12) | |
| Out-of-home placement 2011 | 62,260 | 5190 | (11, 12) | |
| Out-of-home placement 2012 | 64,710 | 5390 | (11, 12) | |
| Out-of-home placement 2013 | 59,090 | 4924 | (11, 12) | |
| Out-of-home placement 2014 | 60,990 | 5080 | (11, 12) | |
| Out-of-home placement 2015 | 72,090 | 6010 | (11, 12) | |
| Out-of-home placement 2016 | 78,700 | 6560 | (11, 12) | |
| Out-of-home placement 2017 | 73,900 | 6160 | (11, 12) | |
| Out-of-home placement 2018 | 74,530 | 6211 | (11, 12) | |
| Out-of-home placement 2019 | 66,900 | 5580 | (11, 12) | |
| Out-of-home placement 2020 | 72,810 | 6070 | (11, 12) | |
| Expensive special institutional placement, when length is known | Each year’s nominal price multiplied by 1.5 | Each month’s nominal price multiplied by 1.5 | Estimated as having 1.5 times the mean price, using prices from other Finnish cities | (13) |
| Price | unit | All costs including required health checks and laboratory tests, diagnostic cost not included (included in DRG price of a diagnosis) | All pharmacies have same prices, Yliopiston apteekki used as source (14), Unit costs of health and social care in Finland (15), Medication statistics (16) |
| ADHD medication (methylphenidate, typical dosage 40 mg) | 600 | year | Medication price for 12 months and health check by school nurse | (14, 15, 16) |
| Risperidone (typical dosage 1 mg/day) | 500 | year | Medication price for 12 months, safety laboratory follow-up tests, two health checks by nurse, doctor phone control | (14, 15, 16) |
| Other antipsychotic behavioral medications (aripipratzole, quetiapine) | 200 | year | Medication price for 12 months, nurse’s check and doctor phone control | (14, 15, 16) |
| SSRI (fluoxetine used typically in children and adolescents) | 200 | year | Medication price for 12 months, nurse’s check and doctor phone control | (14, 15, 16) |
| Valproate medication | 1000 | year | Doctor’s appointment once a year and nurse’s check once a year, laboratory checks occasionally after first treatment year, occasional EEG-registrations included, not including hospitalizations in case of seizures | (14, 15, 16) |
| Insulin | 2900 | year | Not including complications or hospitalizations at diagnosis or later due to complications | Price of diabetes in Finland, (17). |
| Growth hormone | 12,000 | year | Including health checks, laboratory safety follow-up tests and bone age X-rays. | (14, 15, 16) |
References
- Hoyme, H.E.; May, P.A.; Kalberg, W.O.; Kodituwakku, P.; Gossage, J.P.; Trujillo, P.M.; Robinson, L.K. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics 2005, 115, 39–47. [Google Scholar] [CrossRef]
- Koponen, A.; Kalland, M.; Autti-Rämö, I. Caregiving environment and socio-emotional development of foster-placed FASD-children. Child. Youth Serv. Rev. 2009, 31, 1049–1056. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.S.; May, P.A. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- May, P.A.; Hasken, J.M.; Baete, A.; Russo, J.; Elliott, A.J.; Kalberg, W.O.; Hoyme, H.E. Fetal alcohol spectrum disorders in a Midwestern city: Child characteristics, maternal risk traits, and prevalence. Alcohol. Clin. Exp. Res. 2020, 44, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, S.J.A.; Bledsoe, J.M.; Brooks, A.; Davies, J.K.; Jirikowic, T.; Olson, E.; Thorne, J.C. Comparison of the 4-digit code, Canadian 2015, Australian 2016 and Hoyme 2016 fetal alcohol spectrum disorder diagnostic guidelines. Adv. Pediatr. Res. 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Autti-Rämö, I.; Fagerlund, A.; Ervalahti, N.; Loimu, L.; Korkman, M.; Hoyme, H.E. Fetal alcohol spectrum disorders in Finland: Clinical delineation of 77 older children and adolescents. Am. J. Med. Genet. Part A 2006, 140, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Shield, K.; Mihic, A.; Chudley, A.E.; Mukherjee, R.A.; Rehm, J. Comorbidity of fetal alcohol spectrum disorder: A systematic review and meta-analysis. Lancet 2016, 387, 978–987. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Bookstein, F.L.; Barr, H.M.; Sampson, P.D.; O’malley, K.; Young, J.K. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J. Dev. Behav. Pediatr. 2004, 25, 228–238. [Google Scholar] [CrossRef]
- McLachlan, K.; Flannigan, K.; Temple, V.; Unsworth, K.; Cook, J.L. Difficulties in daily living experienced by adolescents, transition-aged youth, and adults with fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2020, 44, 1609–1624. [Google Scholar] [CrossRef]
- Popova, S.; Stade, B.; Bekmuradov, D.; Lange, S.; Rehm, J. What do we know about the economic impact of fetal alcohol spectrum disorder? A systematic literature review. Alcohol Alcohol. 2011, 46, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ericson, L.; Magnusson, L.; Hovstadius, B. Societal costs of fetal alcohol syndrome in Sweden. Eur. J. Health Econ. 2016, 18, 575–585. [Google Scholar] [CrossRef]
- Frappier, J.; Tremblay, G.; Charny, M.; Cloutier, L.M. Costing bias in economic evaluations. J. Med. Econ. 2015, 18, 596–599. Available online: https://www.tandfonline.com/doi/full/10.3111/13696998.2015.1033423 (accessed on 10 March 2023). [CrossRef]
- Official Statistics of Finland (OSF). Price Index of Public Expenditure, Local Government Finances by Function Area, Old Annual Data, 1975–2022; Statistics Finland: Helsinki, Finland, 2023; ISSN 2954-1514. Available online: https://pxdata.stat.fi/PxWeb/pxweb/en/StatFin/StatFin__jmhi/statfin_jmhi_pxt_11me.px/ (accessed on 12 April 2023).
- Official Statistics of Finland (OSF). Consumer Price Indices, Overall Index, Yearly Data, 1972–2022; Statistics Finland’s open statistical databases; Statistics Finland: Helsinki, Finland, 2023; ISSN 1799-0254. Available online: https://pxdata.stat.fi/PxWeb/pxweb/en/StatFin/StatFin__khi/statfin_khi_pxt_11xt.px/ (accessed on 12 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 January 2023).
- Päijät-Hämeen Hyvinvointiyhtymä: Palveluhinnasto 2021; Price List for Services. 2021. Available online: https://paijat-sote.fi/wp-content/uploads/2021/05/palveluhinnasto_2021.pdf (accessed on 10 October 2022).
- Sotkanet. Sotkanet.fi Statistics and Indicator Bank. The Finnish Institute for Health and Welfare 2005–2022. Institutional and Family Care in Child Welfare, Total Operating Expenditure, Placements Outside the Home for Those Aged 0–17, as % of Total Population of Same Age. Päijät-Häme Hospital Distict. 2022. Available online: https://sotkanet.fi/sotkanet/en/taulukko/?indicator=szaytfbzBQA=®ion=s7YsAQA=&year=sy5zsk7S0zUEAA==&gender=t&abs=f&color=f&buildVersion=3.1.1&buildTimestamp=202211091024 (accessed on 20 January 2023).
- Official Statistics of Finland (OSF). Population Structure; Statistics Finland’s Open Statistical Databases; Statistics Finland: Helsinki, Finland, 2022; ISSN 1797-5395. Population on Dec 31 by Area [Päijät-Häme Hospital District], Age [0–17] and Year [2010–2021]; Available online: https://pxdata.stat.fi/PxWeb/pxweb/en/StatFin/StatFin__vaerak/statfin_vaerak_pxt_11re.px/ (accessed on 20 January 2023).
- Kuusikko-Työryhmä. Kuuden Suurimman Kaupungin Lastensuojelun Palvelut ja Kustannukset Vuonna 2021. [Child Care Services and Costs in the Six Largest Cities in 2021]; Lastensuojelun Kuusikko-Työryhmän Julkaisusarja; Kuusikko-Työryhmä: Vantaa, Finland, 2022; Available online: https://www.hel.fi/hel2/tietokeskus/julkaisut/pdf/22_06_01_Kuusikko_Lastensuojelu_2021.pdf (accessed on 20 January 2023).
- Kela. Alle 16-Vuotiaan Vammaistuki 15.12.2021 [Disability Allowance for Those under 16 Years]. 2021. Available online: https://www.kela.fi/etti/Alle16-vuotiaanvammaistuki.pdf?version=1664542286228 (accessed on 20 January 2023).
- Kela. Valitut Palveluntuottajat Vakuutuspiirien Järjestämissä Vaativan Lääkinnällisen Kuntoutuksen Terapiahankinnoissa 2018/Eteläinen Vakuutuspiiri. [SII’s Procurement Decisions of the Southern INSURANCE District Year 2018]. 2021. Available online: https://www.kela.fi/valitut-palveluntuottajat_etelainen (accessed on 15 June 2022).
- Kela. Rehabilitation Services Arranged by Kela: Number of Clients and Total Expenditure. 2022. Available online: https://raportit.kela.fi/ibi_apps/WFServlet?IBIF_ex=NIT099AL&YKIELI=E (accessed on 30 March 2023).
- Opetushallitus. Opetus-ja Kulttuuritoimen Rahoitus-Yksikköhintojen ja Rahoituksen Määräytyminen Vuonna 2020. [Finnish National Agency for Education. Determination of Teaching and Cultural Activity Funding Unit Prices and Funding in 2020]. Oppaat ja Käsikirjat 2020:5. 2020. Available online: https://www.oph.fi/sites/default/files/documents/opetus_ja_kulttuuritoimen_rahoitus_2020.pdf (accessed on 20 January 2023).
- Opetushallitus. Kustannustilastot 2020. [Finnish National Agency for Education.Statistics of Expenses 2020]. 2020. Available online: https://vos.oph.fi/rap/kust/v20/raportit.html (accessed on 20 January 2023).
- Basnet, S.; Onyeka, I.N.; Tiihonen, J.; Föhr, J.; Kauhanen, J. Characteristics of drug-abusing females with and without children seeking treatment in Helsinki, Finland. Scand. J. Public Health 2015, 43, 221–228. [Google Scholar] [CrossRef]
- Reid, N.; Dawe, S.; Shelton, D.; Harnett, P.; Warner, J.; Armstrong, E.; O’Callaghan, F. Systematic review of fetal alcohol spectrum disorder interventions across the life span. Alcohol. Clin. Exp. Res. 2015, 39, 2283–2295. [Google Scholar] [CrossRef]
- Hannigan, J.H.; Armant, D.R. Alcohol in pregnancy and neonatal outcome. In Seminars in Neonatology; WB Saunders: Philadelphia, PA, USA, 2000; Volume 5, pp. 243–254. [Google Scholar]
- Burd, L.; Deal, E.; Rios, R.; Adickes, E.; Wynne, J.; Klug, M.G. Congenital heart defects and fetal alcohol spectrum disorders. Congenit. Heart Dis. 2007, 2, 250–255. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gyllencreutz, E.; Aring, E.; Landgren, V.; Svensson, L.; Landgren, M.; Grönlund, M.A. Ophthalmologic findings in fetal alcohol spectrum disorders–a cohort study from childhood to adulthood. Am. J. Ophthalmol. 2020, 214, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Pakzad, R.; Heydarian, S.; Yekta, A.; Aghamirsalim, M.; Shokrollahzadeh, F.; Khabazkhoob, M. Global and regional prevalence of strabismus: A comprehensive systematic review and meta-analysis. Strabismus 2019, 27, 54–65. [Google Scholar] [CrossRef]
- Strömland, K.; Pinazo-Durán, M.D. Ophthalmic involvement in the fetal alcohol syndrome: Clinical and animal model studies. Alcohol Alcohol. 2002, 37, 2–8. [Google Scholar] [CrossRef]
- Aring, E.; Gyllencreutz, E.; Landgren, V.; Svensson, L.; Landgren, M.; Grönlund, M.A. The FASD Eye Code: A complementary diagnostic tool in fetal alcohol spectrum disorders. BMJ Open Ophthalmol. 2021, 6, e000852. [Google Scholar] [CrossRef]
- Lehikoinen, A.; Sorri, I.; Voutilainen, R.; Heinonen, S. Optical coherence tomography shows decreased thickness of retinal nerve fibre layer among foetal alcohol exposed young adults in a case–control study. Acta Ophthalmol. 2021, 99, e1243–e1244. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.; Davison, P.A.; O’Dwyer, V. School performance and undetected and untreated visual problems in schoolchildren in Ireland; a population-based cross-sectional study. Ir. Educ. Stud. 2022, 41, 367–388. [Google Scholar] [CrossRef]
- Church, M.W.; Eldis, F.; Blakley, B.W.; Bawle, E.V. Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 1997, 21, 227–237. [Google Scholar] [CrossRef]
- Sarkola, T.; Gissler, M.; Kahila, H.; Autti-Rämö, I.; Halmesmäki, E. Early healthcare utilization and welfare interventions among children of mothers with alcohol and substance abuse: A retrospective cohort study. Acta Paediatr. 2011, 100, 1379–1385. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Shin, J.J.; Schwartz, S.R.; Coggins, R.; Gagnon, L.; Hackell, J.M.; Corrigan, M.D. Clinical practice guideline: Otitis media with effusion (update). Otolaryngol.-Head Neck Surg. 2016, 154, S1–S41. [Google Scholar] [CrossRef]
- Long, X.; Little, G.; Beaulieu, C.; Lebel, C. Sensorimotor network alterations in children and youth with prenatal alcohol exposure. Hum. Brain Mapp. 2018, 39, 2258–2268. [Google Scholar] [CrossRef]
- Jirikowic, T.; Olson, H.C.; Kartin, D. Sensory processing, school performance, and adaptive behavior of young school-age children with fetal alcohol spectrum disorders. Phys. Occup. Ther. Pediatr. 2008, 28, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.C.; Conry, J.L.; Conry, R.F. Clinical profile and prevalence of fetal alcohol syndrome in an isolated community in British Columbia. CMAJ Can. Med. Assoc. J. 1987, 137, 203–207. [Google Scholar]
- Streissguth, A.P. A long-term perspective of FAS. Alcohol Health Res. World 1994, 18, 74. [Google Scholar]
- Mukherjee, R.A.S.; Cook, P.A.; Norgate, S.H.; Price, A.D. Neurodevelopmental outcomes in individuals with fetal alcohol spectrum disorder (FASD) with and without exposure to neglect: Clinical cohort data from a national FASD diagnostic clinic. Alcohol 2019, 76, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Autti-Rämö, I.; Autti, T.; Korkman, M.; Kettunen, S.; Salonen, O.; Valanne, L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev. Med. Child Neurol. 2002, 44, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Moore, E.M.; Lane, B.; Pohl, K.M.; Riley, E.P.; Pfefferbaum, A. Graded cerebellar lobular volume deficits in adolescents and young adults with fetal alcohol spectrum disorders (FASD). Cereb. Cortex 2020, 30, 4729–4746. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Burd, L.; Rehm, J. Cost attributable to fetal alcohol spectrum disorder in the Canadian correctional system. Int. J. Law Psychiatry 2015, 41, 76–81. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Finland: Country Health Profile 2021, State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Varabyova, Y.; Müller, J.M. The efficiency of health care production in OECD countries: A systematic review and meta-analysis of cross-country comparisons. Health Policy 2016, 120, 252–263. [Google Scholar] [CrossRef]


| FAS, n = 32 | PFAS, n = 17 | ARND, n = 6 | All, n = 55 | |
|---|---|---|---|---|
| Sex (male), n (%) | 20 (62.5%) | 8 (47.1%) | 3 (50%) | 31 (56.4%) |
| Tobacco exposure, n (%) | 20 (62.5%) | 10 (58.8%) | 4 (66.7%) | 34 (61.8%) |
| Known drug * exposure, n (%) | 7 (21.9%) | 7 (41.2%) | 1 (16.7%) | 15 (27.3%) |
| Gestation at birth mean (SD) | 38.0 weeks (3.1) | 39.5 weeks (1.8) | 37.5 weeks (4.9) | 38.4 weeks (3.1) |
| Birthweight mean (SD) | 2459 g (677) | 3138 g (376) | 3041 g (1023) | 2735 g (720) |
| Birth length mean (SD) | 45.0 cm (3.5) | 48.3 cm (1.4) | 47.3 cm (5.4) | 46.3 cm (3.6) |
| Birth head circumference mean (SD) | 32.0 cm (2.0) | 34.5 cm (1.5) | 32.0 cm (3.2) | 32.8 cm (2.3) |
| Born abroad, ** n (%) | 5 (15.6%) | 3 (17.6%) | 0 (%) | 8 (14.5%) |
| Adopted, n (%) | 6 (18.8%) | 2 (11.8%) | 0 (%) | 8 (14.5%) |
| Placed out of home, n (%) | 27 (84.4%) | 12 (70.6%) | 5 (83.3%) | 44 (80%) |
| Age at first out-of-home placement mean (SD) | 1.5 years (2.9) | 2.3 years (3.9) | 2.1 years (2.3) | 1.8 years (3.1) |
| Multiple (>3) placements or institutional placement, n (%) | 7 (21.9%) | 6 (35.3%) | 1 (16.7%) | 14 (25.5%) |
| Age at FASD diagnosis mean (SD) | 5.1 years (5.2) | 6.1 years (3.8) | 6.8 years (4.4) | 5.5 years (4.7) |
| Age at data collection mean (SD) | 11.6 years (5.8) 2–24 years | 11.1 years (6.4) 3–28 years | 9.0 years (3.9) 3.7–15 years | 11.2 years (5.8) 2–28 years |
| Age < 6 years, n (%) | 5 (15.6%) | 3 (17.6%) | 1 (16.7%) | 9 (16.4%) |
| Age 18 years or older, n (%) | 6 (18.8%) | 3 (17.6%) | 0 (0%) | 9 (16.4%) |
| <1 Year n = 43 | 1–6 Years n = 45 | 7–12 Years n = 37 | 13–16 Years n = 15 | 0–16 Years * n = 55 | |
|---|---|---|---|---|---|
| Most common diagnosis, n (%) | P04 Fetus and newborn affected by noxious influences | F82 Developmental disorder of motor function | F90 Hyperkinetic disorders (ADHD) | F90 Hyperkinetic disorders (ADHD) | F90 Hyperkinetic disorders (ADHD) |
| 23 (53.5%) | 18 (40%) | 25 (67.6%) | 10 (66.7%) | 27 (49.1%) | |
| Second most common diagnosis, n (%) | P07 Short gestation and low birth weight | F80 Developmental disorders of speech and language | F81 Developmental disorders of scholastic skills | F70 Mild intellectual disabilities | P04 Fetus and newborn affected by noxious influences |
| 15 (34.9%) | 15 (33%) | 12 (32.4%) | 8 (53.3%) | 23 (41.8%) | |
| Third most common diagnosis, n (%) | R62 Lack of expected normal development | F83 Mixed developmental disorders | F70 Mild intellectual disabilities | F81 Developmental disorders of scholastic skills | F82 Developmental disorder of motor function |
| 12 (27.9%) | 14 (31.1%) | 8 (21.6%) | 4 (26.7%) | 18 (32.7%) | |
| Fourth most common diagnosis, n (%) | P05 Slow fetal growth | H50 Other strabismus | F94 Disorders of social functioning | F32 Depressive episode | F81 Developmental disorders of scholastic skills |
| 11 (28.9%) | 12 (26.7%) | 5 (13.5%) | 3 (20%) | 16 (29.1%) | |
| Fifth most common diagnosis, n (%) | H66/H65 Otitis media | H66/H65 Otitis media | F83 Mixed developmental disorders | F94 Disorders of social functioning | H65/H66 Otitis media |
| 10 (23.3%) | 10 (22.2%) | 5 (13.5%) | 2 (13.3%) | 16 (29.1%) | |
| Physiotherapy, n (%) | 29 (72.5%) | 6 (13.3%) | 0 | 0 | 31 (56.4%) |
| Speech therapy, n (%) | - | 25 (55.6%) | 0 (0%) | 2 (13.3%) | 26 (47.3%) |
| Occupational therapy, n (%) | - | 39 (86.7%) | 2 (5.4%) | 1 (6.7%) | 40 (72.7%) |
| Psychosocial therapy, n (%) | - | 2 (4.4%) (Theraplay) | 2 (5.4%) | 1 (6.7%) | 5 (9.1%) |
| Neuropsychologic rehabilitation n (%) | . | - | 2 (5.4%) | 0 | 2 (3.6%) |
| First out-of-home placement, n (%) | 23 (53.5%) | 17 (37.8%) | 3 (8.1%) | 1 (6.7%) | 44 (80%) (all with any placements) |
| Psychostimulant medication, n (%) | - | 3(6.7%) | 24 (64.9%) | 5 (33.3%) | 27 (49.1%) |
| Risperidone medication, n (%) | - | 1 (2.2%) | 4 (10.8%) | 3 (20%) | 6 (10.9%) |
| Other psychiatric medication, n (%) | - | - | 3 (8.1%) | 4 (26.7%) | 6 (10.9%) |
| Valproate for epilepsy, n (%) | 0 | 1 (2.2%) | 2 (5.4%) | 0 | 2 (3.6%) |
| Growth hormone, n (%) | - | 1 (2.2%) | 1 (2.7%) | 1 (6.7%) | 3 (5.5%) |
| Insulin, n (%) | 0 | 1 (2.2%) | 1 (2.7%) | 1 (6.7%) | 1 (1.8%) |
| <1 Year | 1–6 Years | 7–12 Years | 13–16 Years |
|---|---|---|---|---|
| Follow-up time in years | 39.2 | 232.0 | 148.5 | 42.5 |
| Diagnosis-related costs, mean (median, IQR) | EUR 21,677 (8963, 22,772) | EUR 2094 (2010, 1747) | EUR 2991 (1971, 1455) | EUR 5567 (4278, 5409) |
| Out-of-home placement costs, mean (median, IQR) | EUR 35,552 (0, 72,952) | EUR 46,276 (58,564, 73,292) | EUR 48,914 (67,394, 75,360) | EUR 64,877 (79,029, 32,361) |
| Disability allowance costs, mean (median, IQR) | EUR 811 (612, 420) | EUR 1154 (1395, 856) | EUR 2402 (2831, 1456) | EUR 942 (1204, 1214) |
| Rehabilitation costs, mean (median, IQR) | EUR 1576 (2063, 1629) | EUR 2814 (3209, 2696) | EUR 849 (0, 0) | EUR 511 (0, 403) |
| Special support costs for school, mean (median, IQR) | EUR 0 (0, 0) | EUR 470 (0, 1584) | EUR 21,248 (17,504, 8144) | EUR 20,613 (17,690, 10,328) |
| Long-term medication costs, mean (median, IQR) | EUR 0 (0, 0) | EUR 175 (0, 0) | EUR 769 (640, 822) | EUR 1452 (850, 1285) |
| Total mean annual costs (median, IQR) | EUR 57,957 (68,344, 84,633) | EUR 51,899 (70,580, 73,767) | EUR 73,912 (81,450, 48,216) | EUR 82,394 (101,666, 88,444) |
| <1 year | 1–6 years | 7–12 years | 13–16 years |
| Number of patients | 38 | 26 | 17 | 12 |
| Diagnosis-related costs, mean (median, IQR) | EUR 21,246 (8956, 17,655) | EUR 2179 (2053, 1512) | EUR 1533 (1321, 1103) | EUR 5912 (4794, 6199) |
| Out-of-home placement costs, mean (median, IQR) | EUR 32,527 (0, 73,616) | 43,566 EUR (63,626, 76,131) | EUR 54,971 (72,178, 76,991) | EUR 68,218 (84,022, 28,144) |
| Disability allowance costs, mean (median, IQR) | EUR 725 (612, 13) | EUR 981 (945, 808) | EUR 2298 (2833, 1626) | EUR 890 (1204, 1218) |
| Rehabilitation costs, mean (median, IQR) | EUR 1497 (2063, 2099) | EUR 1985 (1465, 2408) | EUR 392 (0, 0) | EUR 444 (0, 0) |
| Special support costs for school, mean (median, IQR) | EUR 0 (0, 0) | EUR 499 (0, 1600) | EUR 15,601 (16,549, 3783) | EUR 20,285 (17,647, 7454) |
| Long-term medication costs, mean (median, IQR) | EUR 0 (0, 0) | EUR 139 (0, 0) | EUR 758 (637, 749) | EUR 1680 (865, 1144) |
| Total mean annual costs (median, IQR) | EUR 55,530 (50,781, 81,269) | EUR 49,349 (71,990, 75,332) | EUR 75,283 (94,486, 74,802) | EUR 91,241 (104,484, 69,363) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jolma, L.M.; Koivu-Jolma, M.; Sarajuuri, A.; Torkki, P.; Autti-Rämö, I.; Sätilä, H. Children with FASD—Evolving Patterns of Developmental Problems and Intervention Costs in Ages 0 through 16 in Finland. Children 2023, 10, 788. https://doi.org/10.3390/children10050788
Jolma LM, Koivu-Jolma M, Sarajuuri A, Torkki P, Autti-Rämö I, Sätilä H. Children with FASD—Evolving Patterns of Developmental Problems and Intervention Costs in Ages 0 through 16 in Finland. Children. 2023; 10(5):788. https://doi.org/10.3390/children10050788
Chicago/Turabian StyleJolma, Laura Mirjami, Mikko Koivu-Jolma, Anne Sarajuuri, Paulus Torkki, Ilona Autti-Rämö, and Heli Sätilä. 2023. "Children with FASD—Evolving Patterns of Developmental Problems and Intervention Costs in Ages 0 through 16 in Finland" Children 10, no. 5: 788. https://doi.org/10.3390/children10050788
APA StyleJolma, L. M., Koivu-Jolma, M., Sarajuuri, A., Torkki, P., Autti-Rämö, I., & Sätilä, H. (2023). Children with FASD—Evolving Patterns of Developmental Problems and Intervention Costs in Ages 0 through 16 in Finland. Children, 10(5), 788. https://doi.org/10.3390/children10050788