A Pediatric Case of COLQ-Related Congenital Myasthenic Syndrome with Marked Fatigue
Abstract
1. Introduction
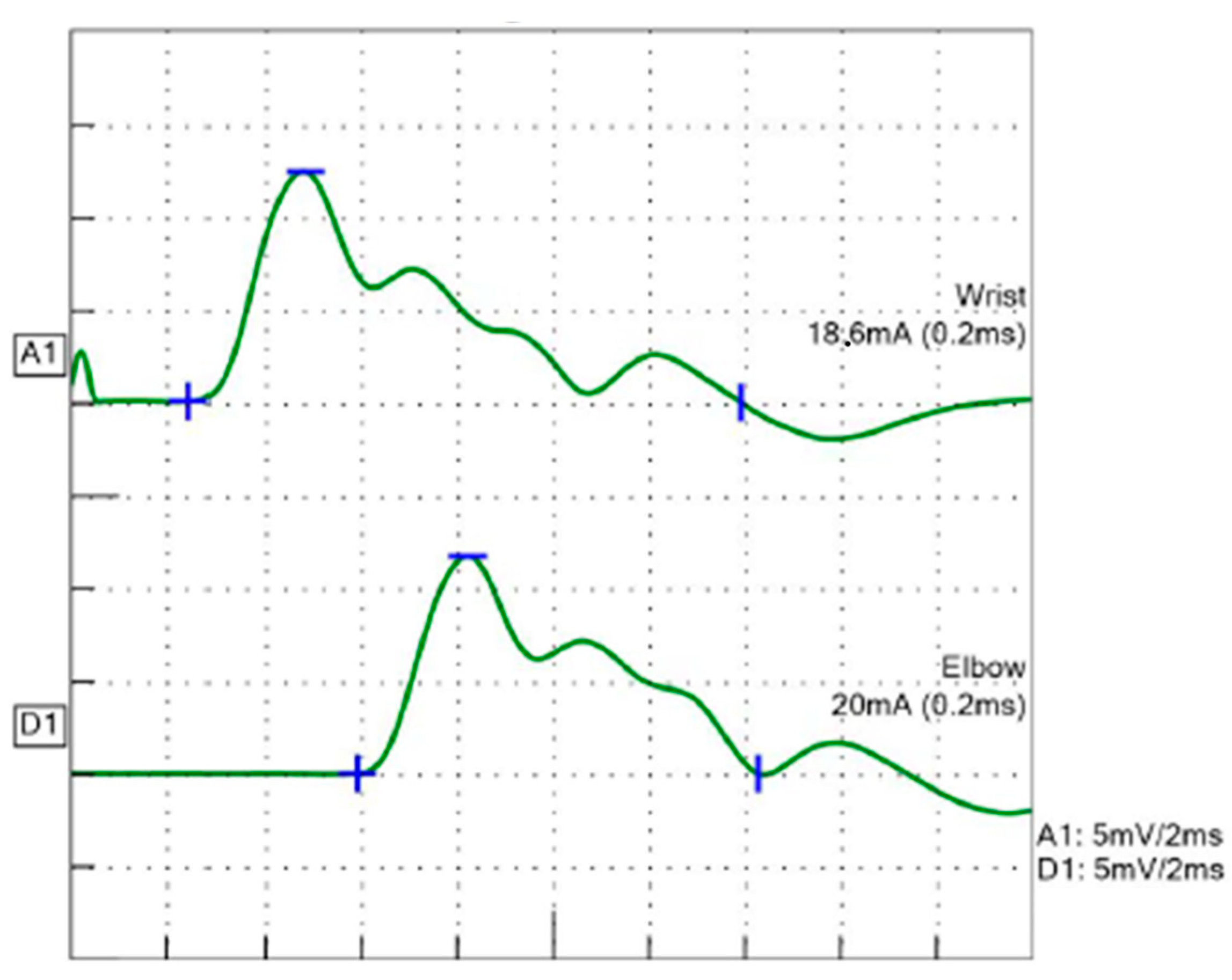
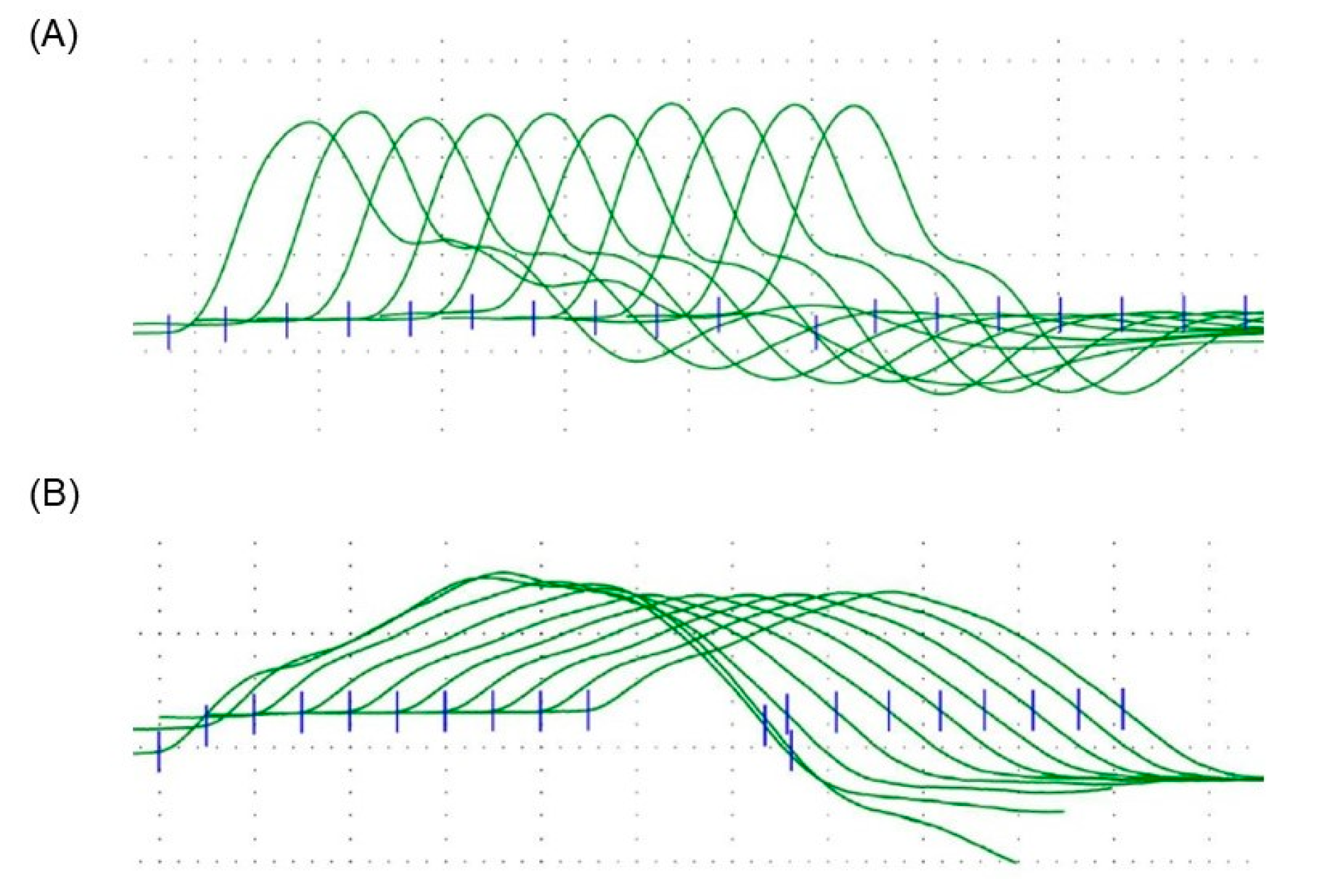
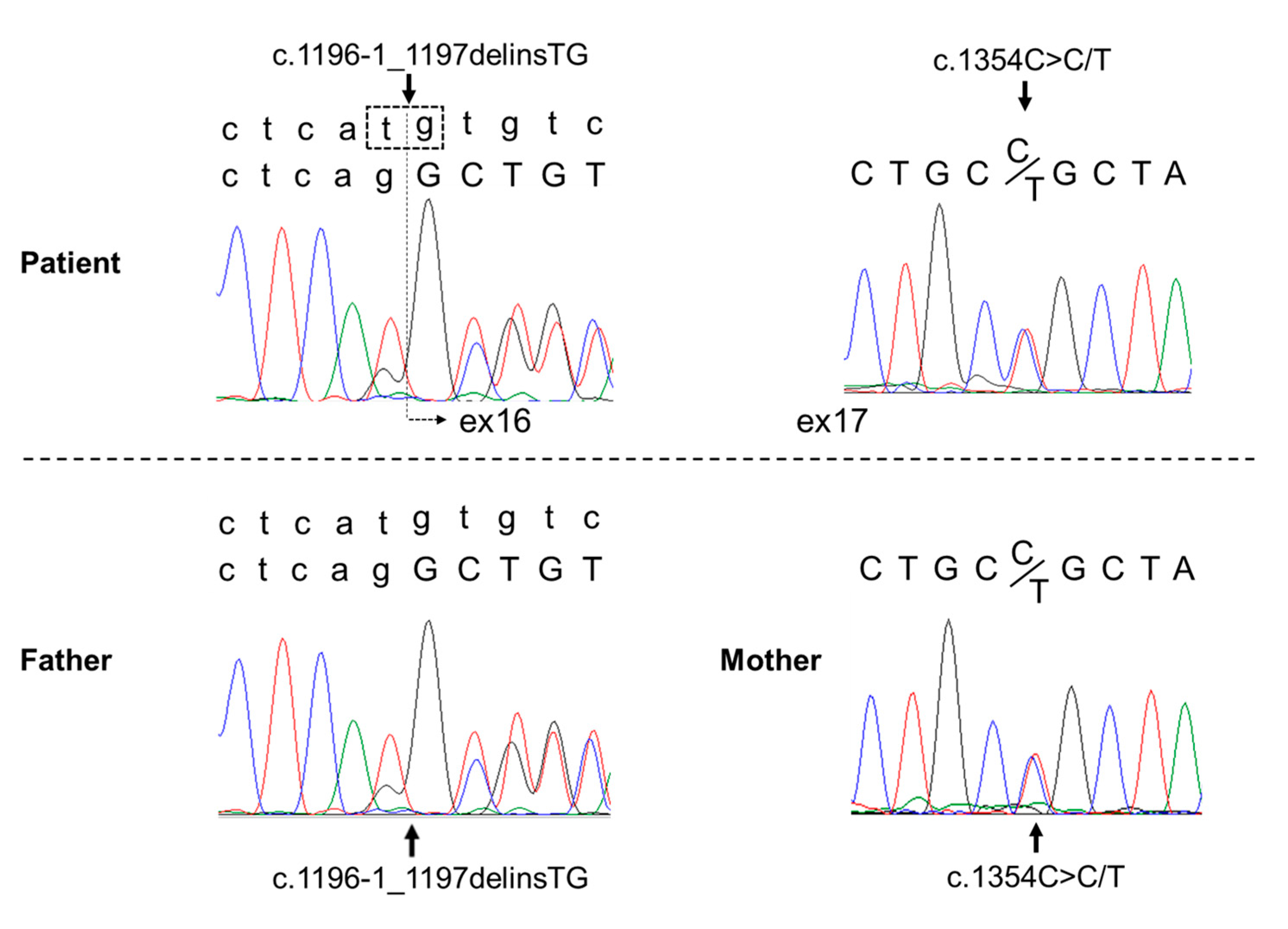
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farmakidis, C.; Pasnoor, M.; Barohn, R.J.; Dimachkie, M.M. Congenital myasthenic syndromes: A clinical and treatment approach. Curr. Treat Options Neurol. 2018, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Cruz, P.M.; Palace, J.; Beeson, D. The neuromuscular junction and wide heterogeneity of congenital myasthenic syndromes. Int. J. Mol. Sci. 2018, 19, 1677. [Google Scholar] [CrossRef] [PubMed]
- Della Marina, A.; Wibbeler, E.; Abicht, A.; Kölbel, H.; Lochmüller, H.; Roos, A.; Schara, U. Long term follow-up on pediatric cases with congenital myasthenic syndromes—A retrospective single centre cohort study. Front. Hum. Neurosci. 2020, 14, 560860. [Google Scholar] [CrossRef] [PubMed]
- Kinali, M.; Beeson, D.; Pitt, M.C.; Jungbluth, H.; Simonds, A.K.; Aloysius, A.; Cockerill, H.; Davis, T.; Palace, J.; Manzur, A.Y.; et al. Congenital myasthenic syndromes in childhood: Diagnostic and management challenges. J. Neuroimmunol. 2008, 201–202, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.C.; Milone, M.; Selcen, D.; Shen, X.M.; Engel, A.G.; Liewluck, T. Congenital myasthenic syndromes in adult neurology clinic: A long road to diagnosis and therapy. Neurology 2018, 91, e1770–e1777. [Google Scholar] [CrossRef]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef]
- Mihaylova, V.; Müller, J.S.; Vilchez, J.J.; Salih, M.A.; Kabiraj, M.M.; D’Amico, A.; Bertini, E.; Wölfle, J.; Schreiner, F.; Kurlemann, G.; et al. Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain 2008, 131, 747–759. [Google Scholar] [CrossRef]
- Irahara, K.; Komaki, H.; Honda, R.; Okumura, A.; Shiraishi, K.; Kobayashi, Y.; Azuma, Y.; Nakata, T.; Ohya, Y.; Sasaki, M. Clinical features of congenital myasthenic syndrome in Japan. No Hattatsu = Brain Dev. 2012, 44, 450–454. [Google Scholar]
- Thompson, R.; Bonne, G.; Missier, P.; Lochmüller, H. Targeted therapies for congenital myasthenic syndromes: Systematic review and steps towards a treatabolome. Emerg. Top Life Sci. 2019, 3, 19–37. [Google Scholar] [CrossRef]
- El Kadiri, Y.; Ratbi, I.; Sefiani, A.; Lyahyai, J. Novel copy number variation of COLQ gene in a Moroccan patient with congenital myasthenic syndrome: A case report and review of the literature. BMC Neurol. 2022, 22, 292. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kuruvilla, A. Repetitive compound muscle action potentials in electrophysiological diagnosis of congenital myasthenic syndromes: A case report and review of literature. Ann. Indian Acad. Neurol. 2010, 13, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebrouck, A.E.; Beeson, D. The congenital myasthenic syndromes: Expanding genetic and phenotypic spectrums and refining treatment strategies. Curr. Opin. Neurol. 2019, 32, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Wargon, I.; Richard, P.; Kuntzer, T.; Sternberg, D.; Nafissi, S.; Gaudon, K.; Lebail, A.; Bauche, S.; Hantaï, D.; Fournier, E.; et al. Long-term follow-up of patients with congenital myasthenic syndrome caused by COLQ mutations. Neuromuscul. Disord. 2012, 22, 318–324. [Google Scholar] [CrossRef]
- Lilleker, J.B.; Keh, Y.S.; Roncaroli, F.; Sharma, R.; Roberts, M. Metabolic myopathies: A practical approach. Pract. Neurol. 2018, 18, 14–26. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.G.; Lammers, G.J.; Wintzen, A.R.; Molenaar, P.C. Repetitive CMAPs: Mechanisms of neural and synaptic genesis. Muscle Nerve 1996, 19, 1127–1133. [Google Scholar] [CrossRef]
- Ding, Q.; Shen, D.; Dai, Y.; Hu, Y.; Guan, Y.; Liu, M.; Cui, L. Mechanism hypotheses for the electrophysiological manifestations of two cases of endplate acetylcholinesterase deficiency related congenital myasthenic syndrome. J. Clin. Neurosci. 2018, 48, 229–232. [Google Scholar] [CrossRef]
- Janas, J.S.; Barohn, R.J. A clinical approach to the congenital myasthenic syndromes. J. Child Neurol. 1995, 10, 168–169. [Google Scholar] [CrossRef]
- Harper, C.M.; Engel, A.G. Quinidine sulfate therapy for the slow-channel congenital myasthenic syndrome. Ann. Neurol. 1998, 43, 480–484. [Google Scholar] [CrossRef]
- Finsterer, J. Congenital myasthenic syndromes. Orphanet. J. Rare Dis. 2019, 14, 57. [Google Scholar] [CrossRef]
- Ohno, K.; Brengman, J.; Tsujino, A.; Engel, A.G. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc. Natl. Acad. Sci. USA 1998, 95, 9654–9659. [Google Scholar] [CrossRef]
- McMacken, G.M.; Spendiff, S.; Whittaker, R.G.; O’Connor, E.; Howarth, R.M.; Boczonadi, V.; Horvath, R.; Slater, C.R.; Lochmüller, H. Salbutamol modifies the neuromuscular junction in a mouse model of ColQ myasthenic syndrome. Hum. Mol. Genet. 2019, 28, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Navegantes, L.C.; Resano, N.M.; Migliorini, R.H.; Kettelhut, I.C. Role of adrenoceptors and cAMP on the catecholamine-induced inhibition of proteolysis in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E663–E668. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horibe, T.; Shimomura, H.; Tokunaga, S.; Taniguchi, N.; Lee, T.; Kimura, S.; Takeshima, Y. A Pediatric Case of COLQ-Related Congenital Myasthenic Syndrome with Marked Fatigue. Children 2023, 10, 769. https://doi.org/10.3390/children10050769
Horibe T, Shimomura H, Tokunaga S, Taniguchi N, Lee T, Kimura S, Takeshima Y. A Pediatric Case of COLQ-Related Congenital Myasthenic Syndrome with Marked Fatigue. Children. 2023; 10(5):769. https://doi.org/10.3390/children10050769
Chicago/Turabian StyleHoribe, Takuya, Hideki Shimomura, Sachi Tokunaga, Naoko Taniguchi, Tomoko Lee, Shigemi Kimura, and Yasuhiro Takeshima. 2023. "A Pediatric Case of COLQ-Related Congenital Myasthenic Syndrome with Marked Fatigue" Children 10, no. 5: 769. https://doi.org/10.3390/children10050769
APA StyleHoribe, T., Shimomura, H., Tokunaga, S., Taniguchi, N., Lee, T., Kimura, S., & Takeshima, Y. (2023). A Pediatric Case of COLQ-Related Congenital Myasthenic Syndrome with Marked Fatigue. Children, 10(5), 769. https://doi.org/10.3390/children10050769




