Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework
Abstract
1. Introduction
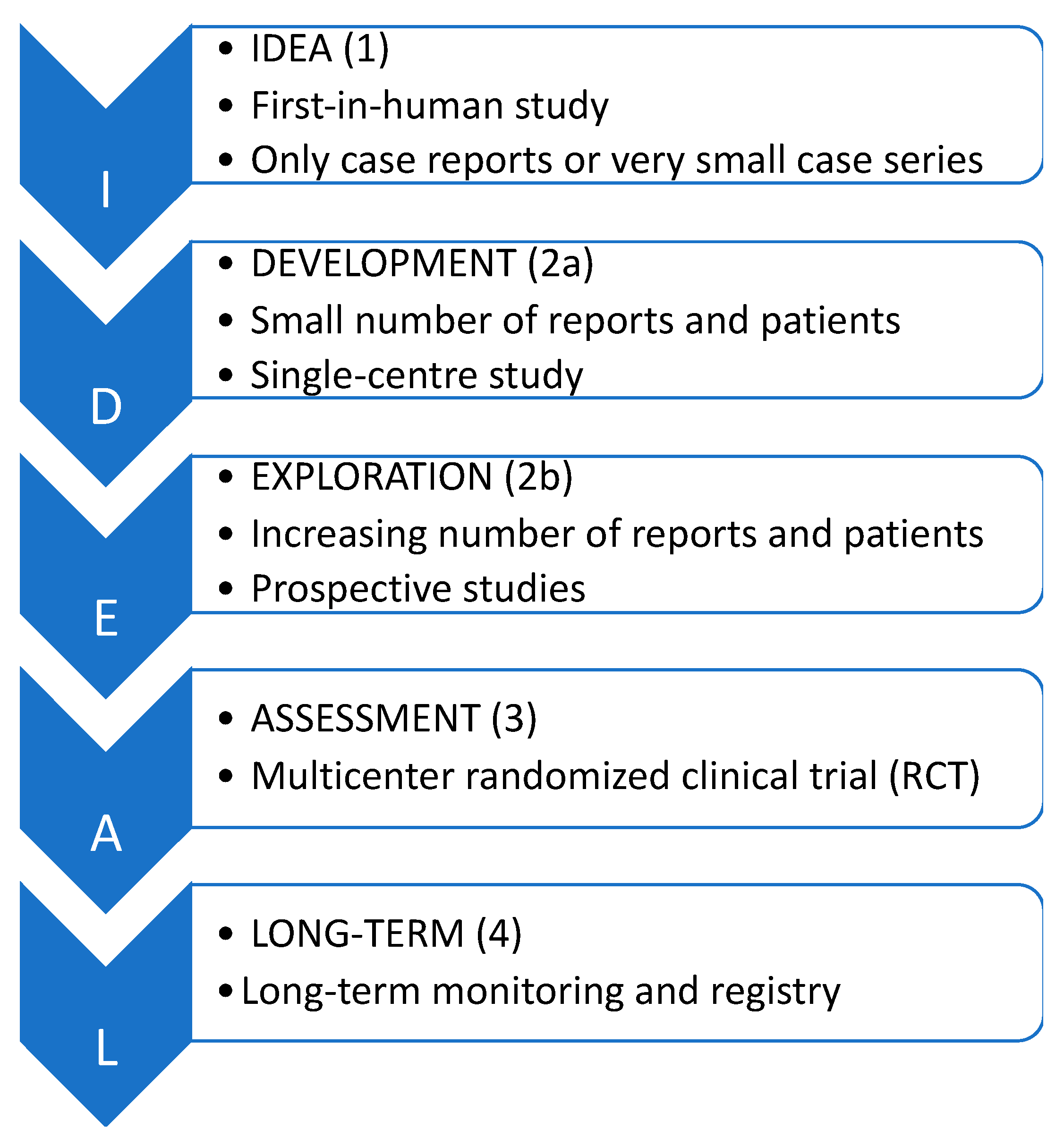
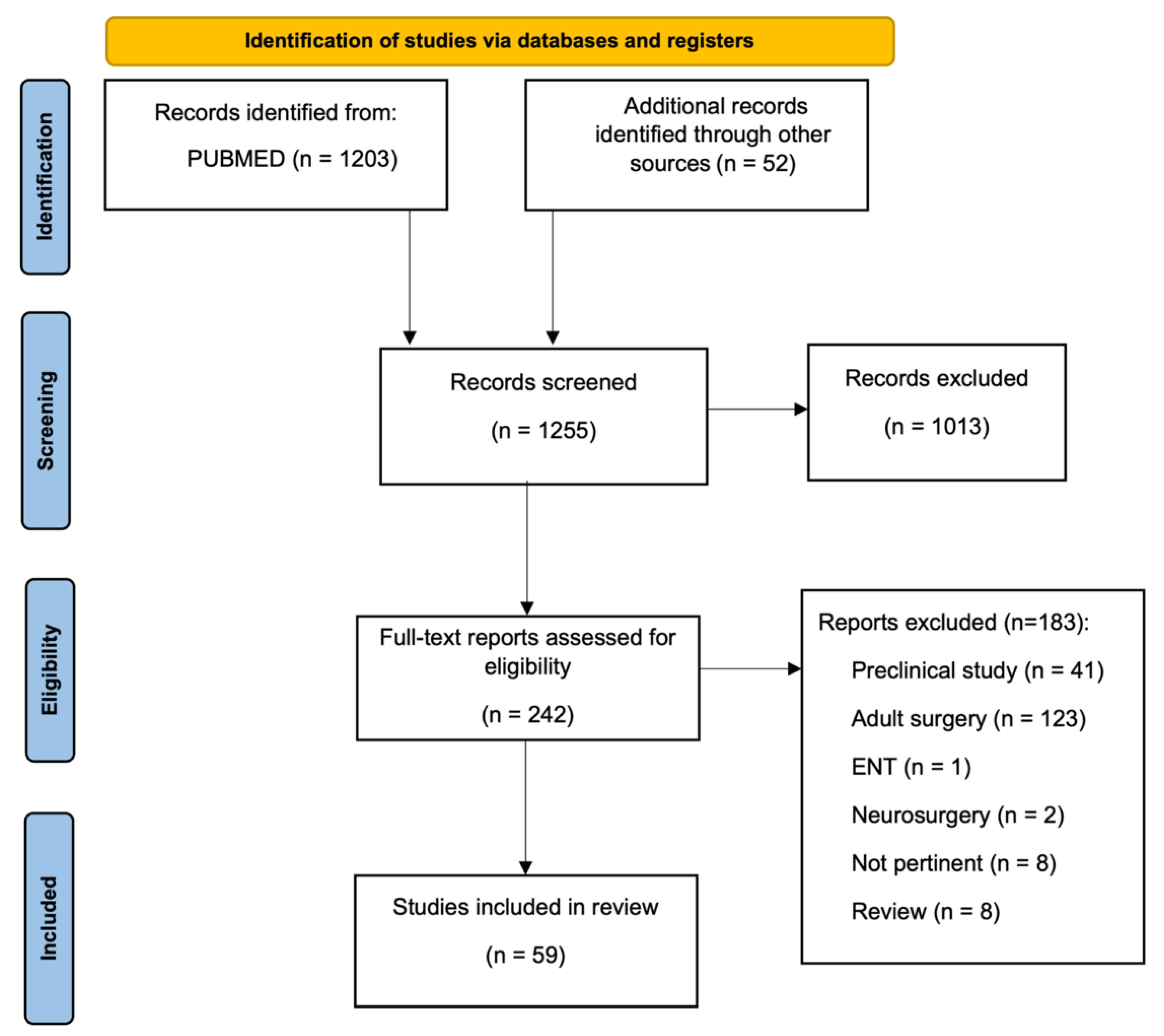
2. Material and Methods
3. Results
3.1. Current Applications and the IDEAL Stage for Each Category
3.1.1. Biliary Tree Imaging
| Authors | Year | No. of Procedures Performed | Mean pt Age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Esposito et al. [5] | 2022 | 21 | median 12.2 y | Cholelithiasis | Laparoscopic cholecystectomy | ICG 0.35 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Esposito et al. [10] | 2020 | 12 | 16.8 y | Cholelithiasis | Laparoscopic cholecystectomy | ICG 0.4 mg/kg iv | Karl Storz + da Vinci Firefly, Intuitive | Retrospective study | 2a |
| Esposito et al. [8] | 2019 | 15 | nd | Cholelithiasis | Laparoscopic cholecystectomy | ICG 0.4 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Esposito et al. [4] | 2019 | 5 | 15.8 y | Cholelithiasis | Laparoscopic cholecystectomy | ICG 0.4 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Fernandez Bautista et al. [9] | 2019 | 1 | 8.6 y | Cholelithiasis | Laparoscopic cholecystectomy | ICG 0.2 mg/kg iv | Stryker | Case series | 1 |
| Bryant et al. [6] | 2020 | 1 | 17 y | Gall bladder duplication | Laparoscopic cholecystectomy | nd | nd | Case report | 1 |
| Calabro et al. [7] | 2020 | 31 | 15 y | Symptomatic biliary diseases | Laparoscopic cholecystectomy | ICG 2.5 mg iv | Stryker | Prospective study | 2a |
| Yanagi et al. [11] | 2019 | 10 | 74.8 d | Biliary atresia | Kasai portoenterostomy (n = 9); hepaticojejunostomy (n = 1) | ICG 0.5 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Hirayama et al. [12] | 2015 | 5 | 52 d | Biliary atresia | Kasai portoenterostomy | ICG 0.1 mg/kg iv | Hamamatsu | Retrospective study | 2a |
| Hanaki et al. [13] | 2022 | 1 | nd | Biliary leakage after hepatectomy | Suture repair of biliary leak | ICG 2 mg iv | Stryker | Case report | 1 |
3.1.2. Vascular Perfusion for Gastrointestinal (GI) Procedures
| Authors | Year | No. of Procedures Performed | Mean pt Age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Paraboschi et al. [15] | 2022 | 1 | 6 m | ARM | PSARP | ICG 0.2 mg/kg iv | Medtronic | Case report | 1 |
| Yada et al. [18] | 2020 | 3 | 13.5 m | ARM | Colostomy closure | ICG 0.3 mg/kg iv | Stryker | Case series | 1 |
| Rentea et al. [16] | 2019 | 13 | 1.9 y | ARM, cloaca, Hirschsprung disease | PSARVUP (n = 9), primary and redo pull-through (n = 4) | ICG (range 0.1–0-3 mg/kg) iv | Stryker | Retrospective study | 2a |
| Paraboschi et al. [20] | 2022 | 1 | 17 y | GERD | Re-do Nissen | ICG 0.125 mg/kg iv | Medtronic | Case report | 1 |
| Onishi et al. [21] | 2022 | 1 | 16 d | Esophageal atresia | Thoracoscopic anastomosis | ICG 0.5 mg/kg iv | Stryker | Case report | 1 |
| Kisaoglu et al. [19] | 2019 | 1 | 4 y | Maple Syrup Urine Disease | Liver resection after transplantation | ICG 0.05 mg/kg iv | nd | Case report | 1 |
| Iinuma et al. [17] | 2013 | 1 | 15 y | Intestinal volvulus | Primary intestinal anastomosis | ICG 25 mg iv | Hamamatsu | Case report | 1 |
| Numanoglu et al. [14] | 2011 | 8 | 24.5 d | NEC | Laparoscopic bowel resection and stoma formation | Fluorescein 14 mg/kg iv | Karl Storz | Prospective study | 2a |
3.1.3. Lymphatic Flow Imaging
| Authors | Year | No. of Procedures Performed | Mean pt Age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Yokoyama et al. [27] | 2020 | 1 | 2.5 m | Chylous ascites | Repairing of leak fibrin sealant with polyglycolic acid felt | ICG 0.1 mL id | Hamamatsu Photonics | Case report | 1 |
| Otake et al. [26] | 2015 | 1 | 13 y | Chylous ascites | Laparoscopic ligation of the leakage site | nd | nd | Case report | 1 |
| Pham et al. [25] | 2020 | 1 | 6 m | Postoperative chylothorax | Transcatheter occlusion of the thoracic duct | ICG 0.05 mL of 0.25 mg/mL id | nd | Case report | 1 |
| Shirotsuki et al. [22] | 2018 | 11 | range 1–25 d | Esophageal atresia + postoperative chylothorax | First thoracoscopic TEF repair (n = 8) and thoracoscopic repair of chylous leakage points (n = 3) | ICG 0.025 mg inter-toe injection | Karl Storz | Retrospective study | 2a |
| Tan et al. [23] | 2014 | 1 | 1 m | Postoperative chylothorax | Bilateral pleurodesis | ICG (id, 1st inj. 25 mcg, 2nd inj. 12.5 mcg, 3rd inj. 12.5 mcg) | nd | Case report | 1 |
| Chang et al. [24] | 2014 | 1 | 3 m | Postoperative chylothorax | Open ligation of fistula | ICG 2 mL sc | nd | Case report | 1 |
| Cheng et al. [30] | 2020 | 8 | 9.2 y | Primary lymphedema | Lymphovenous anastomosis or vascularized lymph node transfer | ICG 0.5 mL sd | Sony Corporation | Retrospective study | 2a |
| Mihara et al. [29] | 2015 | 5 | 15.5 m | Generalized lymphatic dysplasia | Lymphovenous anastomosis | ICG 0.1 mL sc | Hamamatsu Photonics | Retrospective study | 2a |
| Ogata et al. [28] | 2007 | 1 | 12 y | Lymphedema of the lower extremities | Lymphaticovenular anastomosis | ICG 0.2 mL id | Hamamatsu Photonics | Case report | 1 |
| Drobot et al. [32] | 2020 | 1 | 14 y | Lymphatic malformation | Surgical excision | ICG 0.3 to 0.4 mL sc | Stryker | Case report | 1 |
| Kato et al. [33] | 2017 | 1 | 11 m | Lymphatic malformation | Lymphatic venous anastomosis | ICG 0.02 mL sc | Zeiss | Case report | 1 |
| Shirota et al. [31] | 2017 | 1 | 15 y | Lymphatic malformation | Surgical excision | ICG 0.125 mg sc and id | Hamamatsu Photonics | Case report | 1 |
3.1.4. Tumor Resection
| Authors | Year | No. of Procedures Performed | Mean pt age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Abdelhafeez et al. [34] | 2022 | 8 | median 2.5 y | Wilms tumor | Nephroureterectomy with lymph node sampling, MIS (n = 4), open (n = 4) | ICG 5 mg intraparenchymal (n = 4) or peri-hilar (n = 4) | Karl Storz (n = 4) + Visionsense Corp (n = 4) | Multicenter study | 2a |
| Abdelhafeez et al. [45] | 2022 | 12 | median 3 y | Bilateral Wilms tumor (n = 7) + epithelioid angiomyolipoma (n = 1) | Bilateral nephron-sparing surgery (n = 3) + radical nephrectomy (n = 1) + unilateral nephron-sparing surgery (n = 5) | ICG 1.5 mg/kg iv | Visionsense Corp | Retrospective study | 2a |
| Pachl et al. [46] | 2021 | 1 | 2 y | Wilms tumor | Laparoscopic nephroureterectomy + resection of nodes | 2 mL of 2.5mg/mL ICG | Karl Storz | Case report | 1 |
| Delgado-Miguel et al. [48] | 2022 | 1 | 3 m | Focal congenital hyperinsulinism | Laparoscopic resection of pancreatic nodule | ICG 2 mg/kg iv | Stryker | Case report | 1 |
| Chung et al. [24] | 2020 | 1 | 9 y | Hepatocellular carcinoma | Laparoscopic segment 5th resection | ICG 0.5 mg/kg iv | nd | Case report | 1 |
| Bada-Bosch et al. [49] | 2020 | 1 | 13 y | Splenic cyst | Laparoscopic partial splenectomy | ICG 0.2 mg/kg iv | Stryker | Case report | 1 |
| Mansfield et al. [50] | 2020 | 4 | 15.7 y | Paratesticular RMS | Retroperitoneal lymph node dissection | nd | nd | Retrospective study | 2a |
| Fung et al. [47] | 2020 | 1 | 4 y | Lung lesion | Thoracoscopic resection of the lesion | Methylene blue (0.5 mL) and ICG (0.5 mL) inj. around the lesion via the guiding needle | Karl Storz | Case report | 1 |
| Esposito et al. [10] | 2020 | 11 | 6.2 y | Abdominal lymphoma (n = 3) + ovarian tumors (n = 5) + CAM (n = 1) + pulmonary sequestration (n = 1) + lung hilar lymph node (n = 1) | Laparoscopic abdominal lymphoma excisions (n = 3) + robotic ovarian mass excision (n = 5) + thoracoscopic lobectomy (n = 2) + thoracoscopic biopsy (n = 1) | ICG 0.5 mg/mL/kg iv | Karl Storz + Da vinci Firefly, Intuitive | Retrospective study | 2a |
| Esposito et al. [8] | 2019 | 6 | 3.8 y | Abdominal lymphoma (n = 3) + abdominal tumor (n = 3) | Laparoscopic excision | ICG 0.5 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Shen et al. [43] | 2022 | 16 | median 15 m | HB | Liver resection | ICG 0.5 mg/kg iv | nd | Retrospective study | 2a |
| Abdelhafeez et al. [42] | 2021 | 65 | median 10 y | Thoracic lesions (n = 37) + abdomen masses (n = 19) + lesions trunk and extremities (n = 9) | Excision of HB (n = 9) + HCC (n = 2) + OS (n = 9) + NB (n = 6) + NRSTS (n = 6) + RMS (n = 5) + ES (n = 3) + GCT (n = 2) + CB (n = 1) + SPNP (n = 1) + Lymphoma (n = 1) + myoepithelial carcinoma of the chest wall (n = 1) + ACT (n = 2) + metastasectomy (n = 4) + non tumor resection (n = 13) | ICG 1.5 mg/kg iv | Visionsense Corp | Retrospective study | 2a |
| Cho et al. [41] | 2021 | 22 | 3 y | HB | Liver resections (n = 17) + liver transplants (n = 2) + lung metastasectomy (n = 2) + lymph-node metastasis sampling (n = 1) | ICG 0.3 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Souzaki et al. [35] | 2019 | 10 | 2.5 y | Primary liver tumors (n = 4), lung metastases (n = 6) | Extended right hepatectomy (n = 3) + liver transplantation (n = 1) + lung partial resection (n = 5) + lobectomy (n = 1) | ICG 0.5 mg/kg iv | Karl Storz | Retrospective study | 2a |
| Takahashi et al. [36] | 2019 | 1 | 14 y | HB peritoneal dissemination | Surgical excision + Living Donor Liver ReTransplantation | ICG 0.5 mg/kg iv | Hamamatsu Photonics | Case report | 1 |
| Yamada et al. [37] | 2019 | 36 | 5 y | Primary HB (n = 12), HB lung metastases (n = 7), mediastinal metastasis (n = 1), peritoneal metastasis (n = 1), pancreatic metastasis (n = 1), bone metastasis (n = 1) | Liver resection (n = 13), lung metastasectomies (n = 15), other metastasectomies (n = 5) | ICG 0.5 mg/kg iv | Hamamatsu Photonics | Retrospective study | 2a |
| Chen-Yoshikawa et al. [38] | 2017 | 1 | 3 y | HB lung metastasis | Lung metastasectomy | ICG 0.5 mg/kg iv | Panasonic AVC Networks Company | Case report | 1 |
| Kitagawa et al. [39] | 2015 | 37 | 3.5 y | HB lung metastases (n = 10) | Lung metastasectomy (n = 37) | ICG 0.5 mg/kg iv | Hamamatsu Photonics | Retrospective study | 2a |
| Yamamichi et al. [40] | 2015 | 3 | 3 y | Primary HB (n = 1), recurrent HB (n = 1), HB lung metastasis (n = 1) | Right hepatectomy (n = 1), residual tumor and diaphragm resection (n = 1), lung metastasectomy (n = 1) | ICG 0.5 mg/kg iv | Mizuho Medical Co | Case series | 1 |
| Mitani et al. [44] | 2014 | 1 | 2.6 y | HB | Tumor resection | ICG 0.5 mg/kg iv | Hamamatsu Photonics | Case report | 1 |
3.1.5. Urogenital Surgery
| Authors | Year | No. of Procedures Performed | Mean pt Age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Esposito et al. [54] | 2021 | 12 | median 4.1 y | Duplex kidney | Laparoscopic partial nephrectomy | ICG 0.3 mg/kg iv or into the ureteral catheter | Karl Storz | Retrospective study | 2a |
| Herz et al. [53] | 2016 | 6 | 5.6 y | Duplex kidney | Robot-assisted laparoscopic heminephrectomy | ICG 1.25–2.5 mg iv | Da Vinci Firefly, Intuitive | Retrospective study | 2a |
| Carty et al. [56] | 2021 | 3 | 8 y | Congenital ureteral stricture, mid-ureteral polyp disease, distal-ureteral polyp disease | Ureteral reconstruction | ICG 0.039–0.086 mg/kg iv | Da Vinci Firefly, Intuitive | Case series | 1 |
| Esposito et al. [55] | 2020 | 3 | nd | Solitary renal cyst | Robotic deroofing of cyst | ICG 0.35mg/kg iv | nd | Retrospective study | 1 |
| Esposito et al. [52] | 2020 | 57 | median 15.7 y (varicocele) + 8.7 y (nephrectomy) + 4.3 y (partial nephrectomy) + 10.8 y (renal cyst) | Varicocele (n = 41) + non-functioning kidney (n = 3) + symptomatic non-functioning obstructive upper pole moiety or lower pole moiety (n = 9) + simple renal cyst (n = 4) | Laparoscopic left varicocelectomy (n = 38) + robot-assisted left varicocelectomy (n = 3) + nephrectomy (n = 3) + partial nephrectomy (n = 9) + robot-assisted deroofing of simple renal cyst (n = 4) | ICG 0.3 mg/mL/kg iv | Karl Storz + Da Vinci Firefly, Intuitive | Retrospective study | 2a |
| Esposito et al. [10] | 2020 | 53 | 10.9 y | Varicocele (n = 40) + non-functioning kidney (n = 3) + non-functioning symptomatic obstructive upper pole or lower pole moiety (n = 7) + + simple renal cyst (n = 3) | Left varicocelectomy (n = 40), partial nephrectomy (n = 7), nephrectomy (n = 3), renal cyst deroofing (n = 3) | ICG 6.25 mg intratesticular (varicocele repair) + 0.3 mg/mL/kg iv (nephrectomy and renal cyst deroofing) | Karl Storz + Da Vinci Firefly, Intuitive | Retrospective study | 2a |
| Esposito et al. [51] | 2019 | 25 | 13.7 y | Varicocele | Laparoscopic Palomo left varicocelectomy | ICG 0.1 mg intratesticular | nd | Retrospective study | 2a |
| Esposito et al. [50] | 2019 | 35 | 8.4 y | Varicocele (n = 30) + non-functioning kidney (n = 3) + non-functioning upper pole in duplex kidney (n = 2) | Laparoscopic Palomo left varicocelectomy (n = 30) + nephrectomy (n = 3) + partial nephrectomy (n = 2) | ICG 2 mL intratesticular + 0.5 mg/kg iv (renal surgery) | Karl Storz | Retrospective study | 2a |
| Fernandez Bautista et al. [9] | 2019 | 3 | 8.6 y | Varicocele (n = 1) + renal failure (n = 2) | Laparoscopic Palomo varicocelectomy (n = 1) + laparoscopic nephrectomy (n = 2) | ICG 0.2 mg/kg iv | Stryker | Case series | 1 |
3.1.6. Plastic Surgery
| Authors | Year | No. of Procedures Performed | Mean pt Age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Fried et al. [59] | 2019 | 1 | 6 m | Teratoma | Free latissimum dorsi myocutaneous flap | nd | nd | Case report | 1 |
| Martins et al. [57] | 2016 | 11 | 8.8 y | nd | First-stage autologous rib cartilage ear reconstructions | ICG 5 mg iv | Stryker | Retrospective study | 2a |
| Hinchcliff et al. [60] | 2013 | 1 | 1 y | Anterior plagiocephaly | Reconstruction for left unilateral coronal synostosis | ICG 2.5 mg iv | Stryker | Case report | 1 |
| Ishikawa et al. [58] | 2013 | 13 | 10.5 y | Venous malformations | Sclerotherapy | 0.04 mL of ICG (0.01 mg/mL) injected inside the malformation | Hamamatsu Photonics | Retrospective study | 2a |
3.1.7. Miscellaneous
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishizawa, T.; McCulloch, P.; Muehrcke, D.; Carus, T.; Wiesel, O.; Dapri, G.; Schneider-Koriath, S.; Wexner, S.D.; Abu-Gazala, M.; Boni, L.; et al. Assessing the development status of intraoperative fluorescence imaging for perfusion assessments, using the IDEAL framework. BMJ Surg. Interv. Health Technol. 2021, 3, e000088. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, D.R.; Glasziou, P.; Marshall, J.C.; Nicholl, J. No surgical innovation without evaluation: The IDEAL recommendations. Lancet 2009, 374, 1105–1112. [Google Scholar] [CrossRef]
- Pennell, C.P.; Hirst, A.D.; Campbell, W.B.; Sood, A.; Agha, R.A.; Barkun, J.S.T.; McCulloch, P. Practical guide to the Idea, Development and Exploration stages of the IDEAL Framework and Recommendations. Br. J. Surg. 2016, 103, 607–615. [Google Scholar] [CrossRef]
- Esposito, C.; Corcione, F.; Settimi, A.; Farina, A.; Centonze, A.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Twenty-Five Year Experience with Laparoscopic Cholecystectomy in the Pediatric Population—From 10 mm Clips to Indocyanine Green Fluorescence Technology: Long-Term Results and Technical Considerations. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Settimi, A.; Cerulo, M.; Escolino, M. Efficacy of indocyanine green (ICG) fluorescent cholangiography to improve intra-operative visualization during laparoscopic cholecystectomy in pediatric patients: A comparative study between ICG-guided fluorescence and standard technique. Surg. Endosc. 2022, 36, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.K.; Marulanda, K.; Phillips, M.R. Laparoscopic Double Cholecystectomy in a Pediatric Patient for Gallbladder Duplication: An Unusual Case of Biliary Anatomy. Am. Surg. 2020, 86, 1531–1534. [Google Scholar] [CrossRef]
- Calabro, K.A.; Harmon, C.M.; Vali, K. Fluorescent Cholangiography in Laparoscopic Cholecystectomy and the Use in Pediatric Patients. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 586–589. [Google Scholar] [CrossRef]
- Esposito, C.; Del Conte, F.; Cerulo, M.; Gargiulo, F.; Izzo, S.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Clinical application and technical standardization of indocyanine green (ICG) fluorescence imaging in pediatric minimally invasive surgery. Pediatr. Surg. Int. 2019, 35, 1043–1050. [Google Scholar] [CrossRef]
- Fernández-Bautista, B.; Mata, D.P.; Parente, A.; Pérez-Caballero, R.; De Agustín, J.C. First Experience with Fluorescence in Pediatric Laparoscopy. Eur. J. Pediatr. Surg. Rep. 2019, 7, e43–e46. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Del Conte, F.; Cerulo, M.; Coppola, V.; Farina, A.; Crocetto, F.; Ricciardi, E.; Esposito, G.; Escolino, M. Image-Guided Pediatric Surgery Using Indocyanine Green (ICG) Fluorescence in Laparoscopic and Robotic Surgery. Front. Pediatr. 2020, 8, 314. [Google Scholar] [CrossRef]
- Yanagi, Y.; Yoshimaru, K.; Matsuura, T.; Shibui, Y.; Kohashi, K.; Takahashi, Y.; Obata, S.; Sozaki, R.; Izaki, T.; Taguchi, T. The outcome of real-time evaluation of biliary flow using near-infrared fluorescence cholangiography with Indocyanine green in biliary atresia surgery. J. Pediatr. Surg. 2019, 54, 2574–2578. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Iinuma, Y.; Yokoyama, N.; Otani, T.; Masui, D.; Komatsuzaki, N.; Higashidate, N.; Tsuruhisa, S.; Iida, H.; Nakaya, K.; et al. Near-infrared fluorescence cholangiography with indocyanine green for biliary atresia. Real-time imaging during the Kasai procedure: A pilot study. Pediatr. Surg. Int. 2015, 31, 1177–1182. [Google Scholar] [CrossRef]
- Hanaki, T.; Tokuyasu, N.; Sakamoto, T.; Fujiwara, Y. Hepatectomy guided by indocyanine green fluorescent imaging for visualizing bile leakage (with video). Clin. Case Rep. 2022, 10, e05942. [Google Scholar] [CrossRef]
- Numanoglu, A.; Millar, A.J.W. Necrotizing enterocolitis: Early conventional and fluorescein laparoscopic assessment. J. Pediatr. Surg. 2011, 46, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; Privitera, L.; Loukogeorgakis, S.; Giuliani, S. Indocyanine Green-Based Fluorescence-Guided Surgery in a Male Infant with Anorectal Malformation. Eur. J Pediatr. Surg. Rep. 2022, 10, e122–e125. [Google Scholar] [CrossRef] [PubMed]
- Rentea, R.M.; Halleran, D.R.; Ahmad, H.; Sanchez, A.V.; Gasior, A.C.; McCracken, K.; Hewitt, G.D.; Alexander, V.; Smith, C.; Weaver, L.; et al. Preliminary Use of Indocyanine Green Fluorescence Angiography and Value in Predicting the Vascular Supply of Tissues Needed to Perform Cloacal, Anorectal Malformation, and Hirschsprung Reconstructions. Eur. J. Pediatr. Surg. 2020, 30, 505–511. [Google Scholar] [CrossRef]
- Iinuma, Y.; Hirayama, Y.; Yokoyama, N.; Otani, T.; Nitta, K.; Hashidate, H.; Yoshida, M.; Iida, H.; Masui, D.; Manabe, S. Intraoperative near-infrared indocyanine green fluorescence angiography (NIR-ICG AG) can predict delayed small bowel stricture after ischemic intestinal injury: Report of a case. J. Pediatr. Surg. 2013, 48, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Migita, M.; Nakamura, R.; Abe, S.; Matsufuji, H. Indocyanine green fluorescence during pediatric stoma closure. J. Pediatr. Surg. Case Rep. 2020, 61, 101595. [Google Scholar] [CrossRef]
- Kisaoglu, A.; Demiryilmaz, I.; Dandin, O.; Ozkan, O.; Aydinli, B. Management of reperfusion deficiency with indocyanine green fluorescence imaging during deceased donor liver transplantation in a pediatric recipient. HPB 2020, 22, 633. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; Privitera, L.; Loukogeorgakis, S.; Giuliani, S. Fluorescence-Guided Surgery (FGS) during a Laparoscopic Redo Nissen Fundoplication: The First Case in Children. Children 2022, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Muto, M.; Yamada, K.; Murakami, M.; Kedoin, C.; Nagano, A.; Matsui, M.; Sugita, K.; Yano, K.; Harumatsu, T.; et al. Feasibility of delayed anastomosis for long gap esophageal atresia in the neonatal period using internal traction and indocyanine green-guided near-infrared fluorescence. Asian J. Endosc. Surg. 2022, 15, 877–881. [Google Scholar] [CrossRef]
- Shirotsuki, R.; Uchida, H.; Tanaka, Y.; Shirota, C.; Yokota, K.; Murase, N.; Hinoki, A.; Oshima, K.; Chiba, K.; Sumida, W.; et al. Novel thoracoscopic navigation surgery for neonatal chylothorax using indocyanine-green fluorescent lymphography. J. Pediatr. Surg. 2018, 53, 1246–1249. [Google Scholar] [CrossRef]
- Tan, I.-C.; Balaguru, D.; Rasmussen, J.C.; Guilliod, R.; Bricker, J.T.; Douglas, W.I.; Sevick-Muraca, E.M. Investigational Lymphatic Imaging at the Bedside in a Pediatric Postoperative Chylothorax Patient. Pediatr. Cardiol. 2014, 35, 1295–1300. [Google Scholar] [CrossRef]
- Chang, T.-I.; Chen, Y.-S.; Huang, S.-C. Intraoperative indocyanine green fluorescence lymphography to detect chylous leakage sites after congenital heart surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 739–740. [Google Scholar] [CrossRef]
- Pham, K.T.; Balaguru, D.; Tammisetti, V.S.; Guevara, C.J.; Rasmussen, J.C.; Zvavanjanja, R.C.; Hanfland, R.; Sevick-Muraca, E.M.; Aldrich, M.B. Multimodality lymphatic imaging of postoperative chylothorax in an infant with Noonan syndrome: A case report. Eur. J. Med. Res. 2020, 25, 55. [Google Scholar] [CrossRef] [PubMed]
- Otake, K.; Uchida, K.; Inoue, M.; Koike, Y.; Narushima, M.; Kusunoki, M. Use of computed tomography-lymphangiography with direct injection of water-soluble contrast medium to identify the origin of chylous ascites. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Nakaoka, T. Successful use of intraoperative ICG fluorescence lymphography and fibrin sealant with PGA felt for refractory chylous ascites in an infant: A novel procedure. Pediatr. Int. 2020, 62, 862–863. [Google Scholar] [CrossRef]
- Ogata, F.; Narushima, M.; Mihara, M.; Azuma, R.; Morimoto, Y.; Koshima, I. Intraoperative Lymphography Using Indocyanine Green Dye for Near-Infrared Fluorescence Labeling in Lymphedema. Ann. Plast. Surg. 2007, 59, 180–184. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Shibasaki, J.; Seki, Y.; Hayashi, A.; Iida, T.; Adachi, S.; Uchida, Y.; Kaneko, H.; Haragi, M.; et al. Indocyanine Green Lymphography and Lymphaticovenous Anastomosis for Generalized Lymphatic Dysplasia with Pleural Effusion and Ascites in Neonates. Ann. Vasc. Surg. 2015, 29, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liu, T.T. Lymphedema microsurgery improved outcomes of pediatric primary extremity lymphedema. Microsurgery 2020, 40, 766–775. [Google Scholar] [CrossRef]
- Shirota, C.; Hinoki, A.; Takahashi, M.; Tanaka, Y.; Tainaka, T.; Sumida, W.; Murase, N.; Oshima, K.; Shirotsuki, R.; Chiba, K.; et al. New Navigation Surgery for Resection of Lymphatic Malformations Using Indocyanine Green Fluorescence Imaging. Am. J. Case Rep. 2017, 18, 529–531. [Google Scholar] [CrossRef]
- Drobot, A.; Ganam, S.; Karra, N.; Bickel, A.; Abu Shakra, I.; Kakiashvili, E. Resection of an axillary macrocystic lymphatic malformation in a 14-year-old girl using intraoperative indocyanine green lymphography. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 504–507. [Google Scholar] [CrossRef]
- Kato, M.; Watanabe, S.; Iida, T.; Watanabe, A.; Megumi, F. Peri-orbital lymphangioma treated by lymphatic-venous anastomosis with indocyanine green lymphography analysis. J. Pediatr. Surg. Case Rep. 2017, 23, 9–14. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Davidoff, A.M.; Murphy, A.J.; Arul, G.S.; Pachl, M.J. Fluorescence-guided lymph node sampling is feasible during up-front or delayed nephrectomy for Wilms tumor. J. Pediatr. Surg. 2022, 57, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yamada, Y.; Hoshino, K.; Kawaida, M.; Mori, T.; Abe, K.; Fujimura, T.; Matsubara, K.; Hibi, T.; Shinoda, M.; et al. Living Donor Liver Re-Transplantation for Recurrent Hepatoblastoma in the Liver Graft following Complete Eradication of Peritoneal Metastases under Indocyanine Green Fluorescence Imaging. Cancers 2019, 11, 730. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Chen-Yoshikawa, T.F.; Hatano, E.; Yoshizawa, A.; Date, H. Clinical application of projection mapping technology for surgical resection of lung metastasis. Interact. CardioVascular Thorac. Surg. 2017, 25, 1010–1011. [Google Scholar] [CrossRef]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, T.; Oue, T.; Yonekura, T.; Owari, M.; Nakahata, K.; Umeda, S.; Nara, K.; Ueno, T.; Uehara, S.; Usui, N. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J. Pediatr. Surg. 2015, 50, 833–836. [Google Scholar] [CrossRef]
- Cho, Y.J.; Namgoong, J.-M.; Kwon, H.H.; Kwon, Y.J.; Kim, D.Y.; Kim, S.C. The Advantages of Indocyanine Green Fluorescence Imaging in Detecting and Treating Pediatric Hepatoblastoma: A Preliminary Experience. Front. Pediatr. 2021, 9, 635394. [Google Scholar] [CrossRef]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine Green–Guided Pediatric Tumor Resection: Approach, Utility, and Challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zheng, M.; Li, J.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Zou, Y.; Yang, T. Clinical Application of Indocyanine Green Fluorescence Imaging in the Resection of Hepatoblastoma: A Single Institution’s Experiences. Front. Surg. 2022, 9, 932721. [Google Scholar] [CrossRef]
- Mitani, Y.; Kubota, A.; Ueno, M.; Takifuji, K.; Watanabe, T.; Hayami, S.; Kounami, S.; Tsujimoto, H.; Yamaue, H. Real-time identification of hepatoblastoma using a near infrared imaging with indocyanine green. J. Pediatr. Surg. Case Rep. 2014, 2, 180–183. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Murphy, A.J.; Brennan, R.; Santiago, T.C.; Lu, Z.; Krasin, M.J.; Bissler, J.J.; Gleason, J.M.; Davidoff, A.M. Indocyanine green–guided nephron-sparing surgery for pediatric renal tumors. J. Pediatr. Surg. 2022, 57, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Pachl, M.J. Fluorescent Guided Lymph Node Harvest in Laparoscopic Wilms Nephroureterectomy. Urology 2021, 158, 189–192. [Google Scholar] [CrossRef]
- Fung, C.; Lau, C.; Wong, K.K. Indocyanine green fluorescence-guided pulmonary wedge resection in a child: A case report. Hong Kong Med. J. 2020, 26, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Miguel, C.; Muñoz-Serrano, A.; Moratilla, L.; del Carmen Sarmiento, M.; Miguel-Ferrero, M.; Leal, N.; Barrena, S.; Martínez, L. Indocyanine Green (ICG)-Guided Identification of Hypermetabolic Pancreatic Nodules in Focal Congenital Hyperinsulinism: A Case Report in a 3-Month-Old Infant. Eur. J. Pediatr. Surg. Rep. 2022, 10, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Bada-Bosch, I.; Mata, D.P.; de la Torre, M.; Ordóñez, J.; Blanco, M.D.; de Agustin, J. Laparoscopic Partial Splenectomy Assisted by Fluorescence in a 13-Year-Old Girl. Eur. J. Pediatr. Surg. Rep. 2020, 8, e81–e85. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Murphy, A.J.; Talbot, L.; Prajapati, H.; Maller, V.; Pappo, A.; Singhal, S.; Krasin, M.J.; Davidoff, A.M.; Abdelhafeez, A. Alternative approaches to retroperitoneal lymph node dissection for paratesticular rhabdomyosarcoma. J. Pediatr. Surg. 2020, 55, 2677–2681. [Google Scholar] [CrossRef]
- Esposito, C.; Turrà, F.; Del Conte, F.; Izzo, S.; Gargiulo, F.; Farina, A.; Severino, G.; Cerulo, M.; Escolino, M. Indocyanine Green Fluorescence Lymphography: A New Technique to Perform Lymphatic Sparing Laparoscopic Palomo Varicocelectomy in Children. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 564–567. [Google Scholar] [CrossRef]
- Esposito, C.; Coppola, V.; Del Conte, F.; Cerulo, M.; Esposito, G.; Farina, A.; Crocetto, F.; Castagnetti, M.; Settimi, A.; Escolino, M. Near-Infrared fluorescence imaging using indocyanine green (ICG): Emerging applications in pediatric urology. J. Pediatr. Urol. 2020, 16, 700–707. [Google Scholar] [CrossRef]
- Herz, D.; DaJusta, D.; Ching, C.; McLeod, D. Segmental arterial mapping during pediatric robot-assisted laparoscopic heminephrectomy: A descriptive series. J. Pediatr. Urol. 2016, 12, e1–e266. [Google Scholar] [CrossRef]
- Esposito, C.; Autorino, G.; Coppola, V.; Esposito, G.; Paternoster, M.; Castagnetti, M.; Cardone, R.; Cerulo, M.; Borgogni, R.; Cortese, G.; et al. Technical standardization of ICG near-infrared fluorescence (NIRF) laparoscopic partial nephrectomy for duplex kidney in pediatric patients. World J. Urol. 2021, 39, 4167–4173. [Google Scholar] [CrossRef]
- Esposito, C.; Soria-Gondek, A.; Castagnetti, M.; Cerulo, M.; Del Conte, F.; Esposito, G.; Pecoraro, C.; Cicala, D.; Farina, A.; Escolino, M. Laparoscopic or Robotic Deroofing Guided by Indocyanine Green Fluorescence and Perirenal Fat Tissue Wadding Technique of Pediatric Simple Renal Cysts. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Carty, K.N.; Hwang, A.; Gordon, A.; Locke, R.; DeMarco, R.T.; Bayne, C.E. Indocyanine green (ICG) assessment of ureteral perfusion during pediatric robotic surgery. J. Pediatr. Surg. Case Rep. 2021, 74, 102058. [Google Scholar] [CrossRef]
- Martins, D.B.; Farias-Eisner, G.; Mandelbaum, R.S.; Hoang, H.; Bradley, J.P.; Lee, J.C. Intraoperative Indocyanine Green Laser Angiography in Pediatric Autologous Ear Reconstruction. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e709. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Sasaki, S.; Furukawa, H.; Nagao, M.; Iwasaki, D.; Saito, N.; Yamamoto, Y. Preliminary Experience With Intraoperative Near-infrared Fluorescence Imaging in Percutaneous Sclerotherapy of Soft-Tissue Venous Malformations. Dermatol. Surg. 2013, 39, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Fried, F.W.; Beier, J.P.; Bohr, C.; Iro, H.; Horch, R.E.; Arkudas, A. Free Latissimus Dorsi Myocutaneous Flap in a 6-Month-Old Child for Reconstruction of a Temporal Fossa Defect After Teratoma Resection. Ann. Plast. Surg. 2019, 82, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, K.M.; Yao, A.; Taub, P.J. Laser-Assisted Indocyanine Green Imaging to Assess Perfusion of Scalp Closure in an Infant. J. Craniofacial Surg. 2013, 24, 2004–2006. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, Y.; Liao, J.; Hua, K.; Gu, Y.; Wang, D.; Tian, J.; Huang, J. Indocyanine green localization for laparoscopic duodenal web excision. Photodiagnosis Photodyn. Ther. 2022, 38, 102842. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; De Coppi, P.; Stoyanov, D.; Anderson, J.; Giuliani, S. Fluorescence imaging in pediatric surgery: State-of-the-art and future perspectives. J. Pediatr. Surg. 2021, 56, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Privitera, L.; Waterhouse, D.J.; Preziosi, A.; Paraboschi, I.; Ogunlade, O.; Da Pieve, C.; Barisa, M.; Ogunbiyi, O.; Weitsman, G.; Hutchinson, J.C.; et al. Short-wave infrared imaging enables high-contrast fluorescence-guided surgery in neuroblastoma. Cancer Res. 2023, CAN-22-2918. [Google Scholar] [CrossRef]
- Wellens, L.M.; Deken, M.M.; Sier, C.F.M.; Johnson, H.R.; de la Jara Ortiz, F.; Bhairosingh, S.S.; Houvast, R.D.; Kholosy, W.M.; Baart, V.M.; Pieters, A.M.M.J.; et al. Anti-GD2-IRDye800CW as a targeted probe for fluorescence-guided surgery in neuroblastoma. Sci. Rep. 2020, 10, 17667. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Verras, G.-I.; Anagnostopoulos, C.-N.; Kotis, K. A Smarter Health through the Internet of Surgical Things. Sensors 2022, 22, 4577. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | No. of Procedures Performed | Mean pt Age | Indication | Surgical Procedure | Dose of Dye | Imaging System | Study Design | IDEAL Framework Stage |
|---|---|---|---|---|---|---|---|---|---|
| Li S et al. [61] | 2022 | 1 | 13 d | Duodenal web | Laparoscopic resection of duodenal web | ICG 5 mL of a 0.125 mg/mL diluted solution through a nasogastric tube | Zhuhai Dipu Medical Technology Co | Zhuhai Dipu Medical Technology Co | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preziosi, A.; Paraboschi, I.; Giuliani, S. Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework. Children 2023, 10, 689. https://doi.org/10.3390/children10040689
Preziosi A, Paraboschi I, Giuliani S. Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework. Children. 2023; 10(4):689. https://doi.org/10.3390/children10040689
Chicago/Turabian StylePreziosi, Alessandra, Irene Paraboschi, and Stefano Giuliani. 2023. "Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework" Children 10, no. 4: 689. https://doi.org/10.3390/children10040689
APA StylePreziosi, A., Paraboschi, I., & Giuliani, S. (2023). Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework. Children, 10(4), 689. https://doi.org/10.3390/children10040689






