Targeted Diode Laser Therapy for Oral and Perioral Capillary-Venous Malformation in Pediatric Patients: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
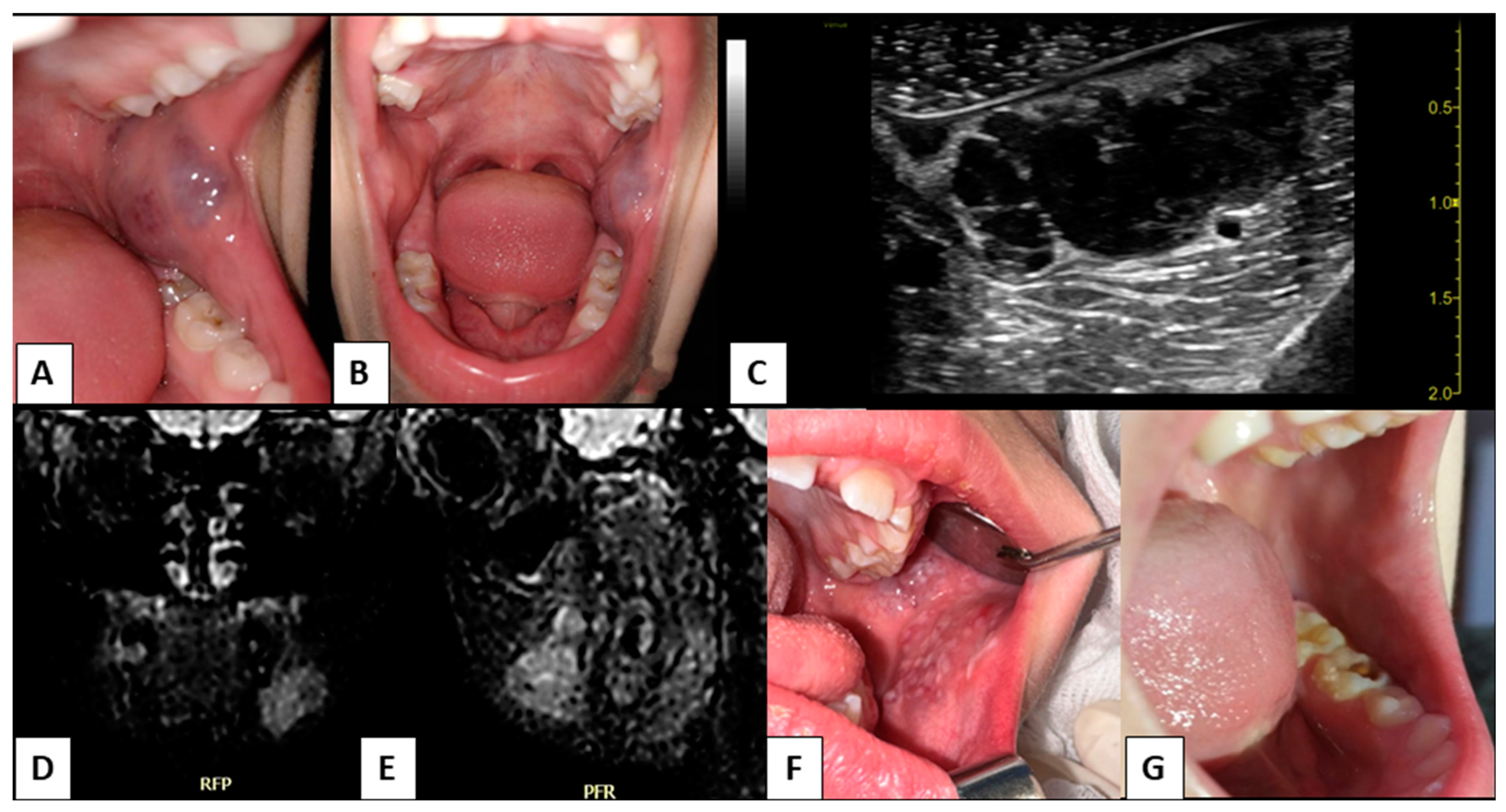
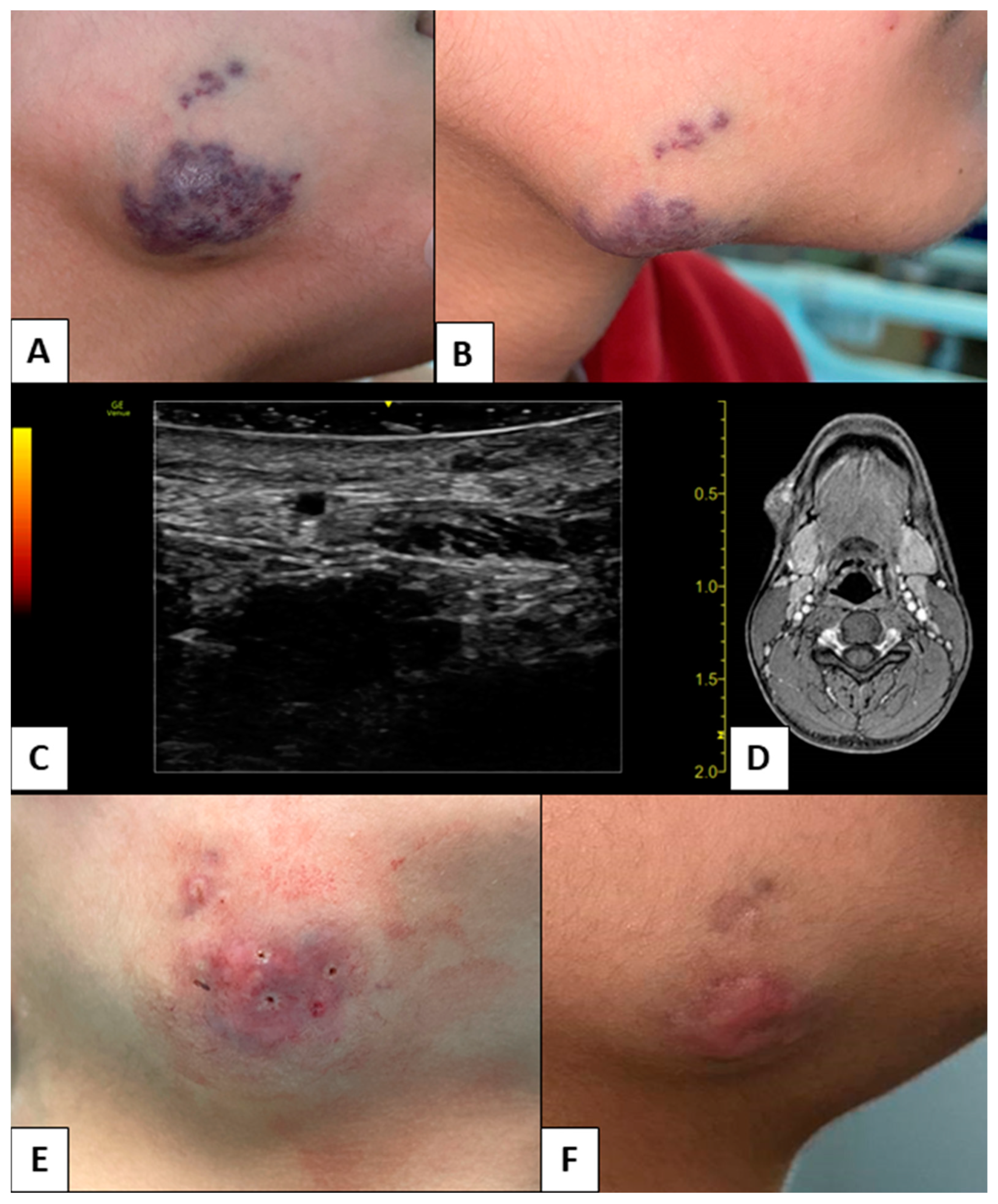
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putra, J.; Al-Ibraheemi, A. Vascular Anomalies of the Head and Neck: A Pediatric Overview. Head Neck Pathol. 2021, 15, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Mahady, K.; Thust, S.; Berkeley, R.; Stuart, S.; Barnacle, A.; Robertson, F.; Mankad, K. Vascular Anomalies of the Head and Neck in Children. Quant. Imaging Med. Surg. 2015, 5, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Sadick, M.; Müller-Wille, R.; Wildgruber, M.; Wohlgemuth, W.A. Vascular Anomalies (Part I): Classification and Diagnostics of Vascular Anomalies. RöFo—Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2018, 190, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Müller-Wille, R.; Wildgruber, M.; Sadick, M.; Wohlgemuth, W.A. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular Malformations. RöFo—Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2018, 190, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Garzon, M.C.; Huang, J.T.; Enjolras, O.; Frieden, I.J. Vascular Malformations: Part II: Associated Syndromes. J. Am. Acad. Dermatol. 2007, 56, 541–564. [Google Scholar] [CrossRef]
- Limongelli, L.; Tempesta, A.; De Caro, A.; Maiorano, E.; Angelelli, G.; Capodiferro, S.; Favia, G. Diode Laser Photocoagulation of Intraoral and Perioral Venous Malformations after Tridimensional Staging by High Definition Ultrasonography. Photobiomodul. Photomed. Laser Surg. 2019, 37, 722–728. [Google Scholar] [CrossRef]
- Angelelli, G.; Moschetta, M.; Limongelli, L.; Albergo, A.; Lacalendola, E.; Brindicci, F.; Favia, G.; Maiorano, E. Endocavitary Sonography of Early Oral Cavity Malignant Tumors. Head Neck 2017, 39, 1349–1356. [Google Scholar] [CrossRef]
- Lee, J.W.; Chung, H.Y. Vascular Anomalies of the Head and Neck: Current Overview. Arch. Craniofac. Surg. 2018, 19, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Ow, A.; Yang, W.; Hu, Y.; Wang, L.; Zhang, C. Surgical Management of Solitary Venous Malformation in the Midcheek Region. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 160–166. [Google Scholar] [CrossRef]
- Greene, A.K. Current Concepts of Vascular Anomalies. J. Craniofac. Surg. 2012, 23, 220. [Google Scholar] [CrossRef]
- Reddy, G.S.P.; Reddy, G.V.; Reddy, K.S.K.; Priyadarshini, B.S.; Sree, P.K. Intralesional Sclerotherapy—A Novel Approach for The Treatment of Intraoral Haemangiomas. J. Clin. Diagn. Res. JCDR 2016, 10, ZD13–ZD14. [Google Scholar] [CrossRef]
- Sham, M.E.; Sultana, N. Vascular Anomalies in Maxillofacial Region—Review. J. Oral Maxillofac. Surg. Med. Pathol. 2012, 24, 137–146. [Google Scholar] [CrossRef]
- Maruani, A.; Tavernier, E.; Boccara, O.; Mazereeuw-Hautier, J.; Leducq, S.; Bessis, D.; Guibaud, L.; Vabres, P.; Carmignac, V.; Mallet, S.; et al. Sirolimus (Rapamycin) for Slow-Flow Malformations in Children. JAMA Dermatol. 2021, 157, 1289–1298. [Google Scholar] [CrossRef]
- Zheng, J.W.; Mai, H.M.; Zhang, L.; Wang, Y.A.; Fan, X.D.; Su, L.X.; Qin, Z.P.; Yang, Y.W.; Jiang, Y.H.; Zhao, Y.F.; et al. Guidelines for the Treatment of Head and Neck Venous Malformations. Int. J. Clin. Exp. Med. 2013, 6, 377–389. [Google Scholar]
- Mitra, S.; Shen, D.; Afshar, S. Assessing the Dental and Oral Health Needs at Vascular Anomalies Centers across the United States. J. Vasc. Anom. 2022, 3, e031. [Google Scholar] [CrossRef]
- Miyazaki, H.; Romeo, U.; Ohshiro, T.; Kudo, T.; Makiguchi, T.; Kawachi, N.; Ogawa, M.; Inoue, Y.; Yokoo, S. Treatment Strategies for Large Oral Venous Malformations Using Intralesional Laser Photocoagulation. Lasers Med. Sci. 2014, 29, 1987–1990. [Google Scholar] [CrossRef]
- IARC Publications Website—Pathology and Genetics of Tumours of Soft Tissue and Bone. Available online: https://publications.iarc.fr/book-and-report-series/who-classification-of-tumours/pathology-and-genetics-of-tumours-of-soft-tissue-and-bone-2002 (accessed on 22 February 2023).
- Zhou, Q.; Zheng, J.W.; Mai, H.M.; Luo, Q.F.; Fan, X.D.; Su, L.X.; Wang, Y.A.; Qin, Z.P. Treatment Guidelines of Lymphatic Malformations of the Head and Neck. Oral Oncol. 2011, 47, 1105–1109. [Google Scholar] [CrossRef]
- Piram, M.; Lorette, G.; Sirinelli, D.; Herbreteau, D.; Giraudeau, B.; Maruani, A. Sturge-Weber Syndrome in Patients with Facial Port-Wine Stain. Pediatr. Dermatol. 2012, 29, 32–37. [Google Scholar] [CrossRef]
- John Libbey Eurotext—European Journal of Dermatology—Port-Wine Stains Are More than Skin-Deep! Expanding the Spectrum of Extracutaneous Manifestations of Nevi Flammei of the Head and Neck. Available online: https://www.jle.com/fr/revues/ejd/e-docs/port_wine_stains_are_more_than_skin_deep_expanding_the_spectrum_of_extracutaneous_manifestations_of_nevi_flammei_of_the_head_and_neck_292369/article.phtml (accessed on 22 February 2023).
- Fiorella, M.L.; Lillo, L.; Fiorella, R. Diode Laser in the Treatment of Epistaxis in Patients with Hereditary Haemorrhagic Telangiectasia. Acta Otorhinolaryngol. Ital. 2012, 32, 164. [Google Scholar]
- Hopp, R.N.; de Siqueira, D.C.; Sena-Filho, M.; Jorge, J. Oral Vascular Malformation in a Patient with Hereditary Hemorrhagic Telangiectasia: A Case Report. Spec. Care Dentist. 2013, 33, 150–153. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Suppressa, P.; Sabbà, C.; Maiorano, E. Diode Laser Treatment and Clinical Management of Multiple Oral Lesions in Patients with Hereditary Haemorrhagic Telangiectasia. Br. J. Oral Maxillofac. Surg. 2016, 54, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Buckmiller, L.; Richter, G.; Suen, J. Diagnosis and Management of Hemangiomas and Vascular Malformations of the Head and Neck. Oral Dis. 2010, 16, 405–418. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Settings |
|---|---|
| Type of Laser | GaA1As-A2G Diode |
| Mean Wavelength (nm) | 800 ± 10 nm |
| Type and Gauge (µm) of the Fiber | Quartz; 320 µm |
| Power Output for TMT (W/cm2) | 8–12 W/cm2 |
| Power Output for ILP (W/cm2) | 13 W/cm2 |
| Modality | Pulsed Mode |
| t-on (ms) | 190 ms |
| t-off (ms) | 250 ms |
| Characteristic | Data |
|---|---|
| n | 36 |
| Male/female | 14/22 |
| Mean age and age range | 14 years; range 4–18 years |
| Syndromes (n) | SWS, 5 patients; HHT, 7 patients |
| Angiomatoses (n) | 5 patients |
| Patients without comorbidities (n) | 24 patients |
| Number of CVM | 63 |
| Group A CVM (n; %) | 23 (36.51%) |
| Group B1 CVM (n; %) | 8 (12.70%) |
| Group B2 CVM (n; %) | 10 (15.87%) |
| Group C1 CVM (n; %) | 9 (14.29%) |
| Group C2 CVM (n; %) | 13 (20.63%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tempesta, A.; Dell’Olio, F.; Siciliani, R.A.; Favia, G.; Capodiferro, S.; Limongelli, L. Targeted Diode Laser Therapy for Oral and Perioral Capillary-Venous Malformation in Pediatric Patients: A Prospective Study. Children 2023, 10, 611. https://doi.org/10.3390/children10040611
Tempesta A, Dell’Olio F, Siciliani RA, Favia G, Capodiferro S, Limongelli L. Targeted Diode Laser Therapy for Oral and Perioral Capillary-Venous Malformation in Pediatric Patients: A Prospective Study. Children. 2023; 10(4):611. https://doi.org/10.3390/children10040611
Chicago/Turabian StyleTempesta, Angela, Fabio Dell’Olio, Rosaria Arianna Siciliani, Gianfranco Favia, Saverio Capodiferro, and Luisa Limongelli. 2023. "Targeted Diode Laser Therapy for Oral and Perioral Capillary-Venous Malformation in Pediatric Patients: A Prospective Study" Children 10, no. 4: 611. https://doi.org/10.3390/children10040611
APA StyleTempesta, A., Dell’Olio, F., Siciliani, R. A., Favia, G., Capodiferro, S., & Limongelli, L. (2023). Targeted Diode Laser Therapy for Oral and Perioral Capillary-Venous Malformation in Pediatric Patients: A Prospective Study. Children, 10(4), 611. https://doi.org/10.3390/children10040611









