Use of COVID-19 Convalescent Plasma for Treatment of Symptomatic SARS-CoV-2 Infection at a Children’s Hospital: A Contribution to a Still Inadequate Body of Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serum/Plasma Samples
CCP Donors
2.3. Study Population
2.4. SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT)
2.5. Outcomes Measurement
2.6. Statistical Analysis
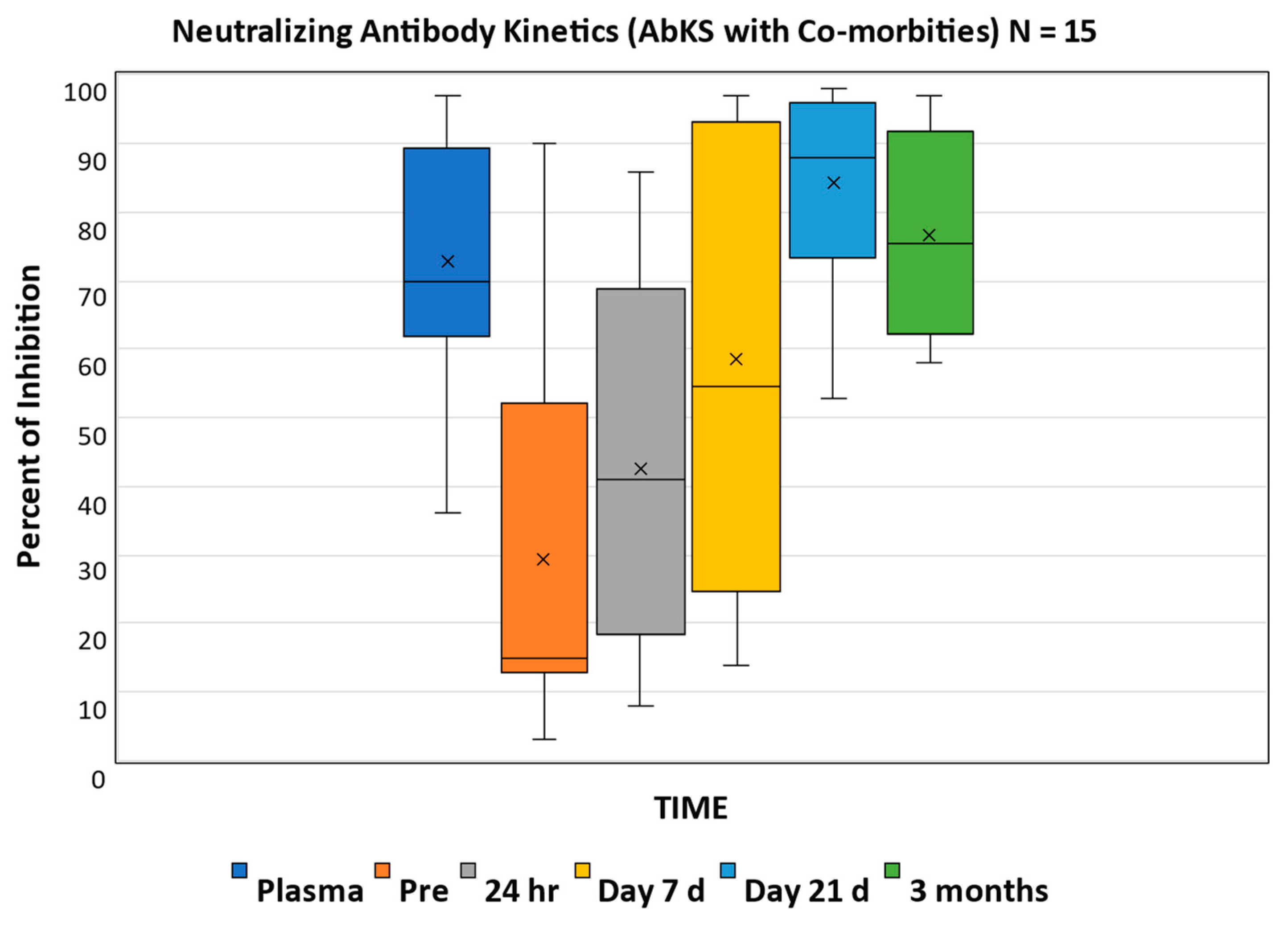
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO World Health Organization COVID-19 Detailed Surveillance Data Dashboard. Available online: https://covid19.who.int/ (accessed on 17 August 2022).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with COVID-19—Preliminary report. N. Engl. J. Med. 2020, 384, 693–704. [Google Scholar]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar]
- Joyner, M.J.; Bruno, K.A.; Klassen, S.A.; Kunze, K.L.; Johnson, P.W.; Lesser, E.R.; Wiggins, C.C.; Senefeld, J.W.; Klompas, A.M.; Hodge, D.O.; et al. Safety update. Mayo Clin. Proc. 2020, 95, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Abolghasemi, H.; Eshghi, P.; Cheraghali, A.M.; Imani Fooladi, A.A.; Bolouki Moghaddam, F.; Imanizadeh, S.; Moeini Maleki, M.; Ranjkesh, M.; Rezapour, M.; Bahramifar, A.; et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus. Apher. Sci. 2020, 59, 102875. [Google Scholar] [CrossRef]
- Arrieta, A.; Galvis, A.E.; Morphew, T.; Ehwerhemuepha, L.; Osborne, S.; Enriquez, C.; Imfeld, K.; Hoang, J.; Nieves, D.; Ashouri, N.; et al. Safety and antibody kinetics of COVID-19 convalescent plasma for the treatment of moderate to severe cases of SARS-CoV-2 infection in pediatric patients. Pediatr. Infect. Dis. J. 2021, 40, 606–611. [Google Scholar] [CrossRef]
- Ogden, C.L.; Flegal, K.M. Changes in terminology for childhood overweight and obesity. Natl. Health Stat. Rep. 2010, 25, 1–5. [Google Scholar]
- Norman, H.; Nie, D.H.B.C.; Hadlai, H. IBM SPSS Statistics, Version 27. IBM Software Group’s Business Analytics Portfolio. IBM: Armonk, NY, USA, 2020.
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Song, X.; Delaney, M.; Bell, M.; Smith, K.; Pershad, J.; Ansusinha, E.; Hahn, A.; Hamdy, R.; Harik, N.; et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J. Pediatr. 2020, 223, 199–203.e1. [Google Scholar] [CrossRef]
- Liguoro, I.; Pilotto, C.; Bonanni, M.; Ferrari, M.E.; Pusiol, A.; Nocerino, A.; Vidal, E.; Cogo, P. SARS-CoV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020, 179, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef]
- Kim, L.; Whitaker, M.; O’Halloran, A.; Kambhampati, A.; Chai, S.J.; Reingold, A.; Armistead, I.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 States, 1 March–25 July 2020. Morb. Mortal Wkly. Rep. 2020, 2020, 1081–1088. [Google Scholar]
- Jean, S.S.; Lee, P.I.; Hsueh, P.R. Treatment options for COVID-19: The reality and challenges. J. Microbiol. Immunol. Infect. 2020, 53, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Javorac, D.; Grahovac, L.; Manic, L.; Stojilkovic, N.; Andelkovic, M.; Bulat, Z.; Dukic-Cosic, D.; Curcic, M.; Djordjevic, A.B. An overview of the safety assessment of medicines currently used in the COVID-19 disease treatment. Food Chem. Toxicol. 2020, 144, 111639. [Google Scholar] [CrossRef]
- Lopez-Medina, E.; Lopez, P.; Hurtado, I.C.; Davalos, D.M.; Ramirez, O.; Martinez, E.; Diazgranados, J.A.; Onate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial. JAMA 2021, 325, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Dequin, P.F.; Heming, N.; Meziani, F.; Plantefeve, G.; Voiriot, G.; Badie, J.; Francois, B.; Aubron, C.; Ricard, J.D.; Ehrmann, S.; et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA 2020, 324, 1298–1306. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.; et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020, 324, 1317–1329. [Google Scholar]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.P.T.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis. JAMA 2021, 326, 499–518. [Google Scholar]
- Guimaraes, P.O.; Quirk, D.; Furtado, R.H.; Maia, L.N.; Saraiva, J.F.; Antunes, M.O.; Kalil Filho, R.; Junior, V.M.; Soeiro, A.M.; Tognon, A.P.; et al. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N. Engl. J. Med. 2021, 385, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Mariette, X.; Tharaux, P.L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Group, C.-C. Effect of tocilizumab vs. usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Intern. Med. 2021, 181, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.C.; Sarkar, N.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; Cohen, M.S.; et al. COVID-19 phase 3 prevention trial, T. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N. Engl. J. Med. 2021, 385, 1184–1195. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Pannaraj, P.S.; Boom, J.A.; Sahni, L.C.; Chiotos, K.; Cameron, M.A.; et al. Overcoming Covid, I. Maternal vaccination and risk of hospitalization for COVID-19 among infants. N. Engl. J. Med. 2022, 387, 109–119. [Google Scholar] [CrossRef]
- Casadevall, A.; Joyner, M.J.; Pirofski, L.A. A randomized trial of convalescent plasma for COVID-19-potentially hopeful signals. JAMA 2020, 324, 455–457. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Imai, M.; Maeda, K.; Nakajima, N.; Higashi-Kuwata, N.; Iwatsuki-Horimoto, K.; Ito, M.; Kiso, M.; Maemura, T.; Takeda, Y.; et al. Highly neutralizing COVID-19 convalescent plasmas potently block SARS-CoV-2 replication and pneumonia in Syrian hamsters. J. Virol. 2022, 96, e0155121. [Google Scholar] [CrossRef]
- Diorio, C.; Anderson, E.M.; Mcnerney, K.O.; Goodwin, E.C.; Chase, J.C.; Bolton, M.J.; Arevalo, C.P.; Weirick, M.E.; Gouma, S.; Vella, L.A.; et al. Convalescent plasma for pediatric patients with SARS-CoV-2-associated acute respiratory distress syndrome. Pediat. Blood Cancer 2020, 67, e28693. [Google Scholar] [CrossRef]
- Malecki, P.; Faltin, K.; Mania, A.; Mazur-Melewska, K.; Cwalinska, A.; Zawadzka, A.; Bukowska, A.; Lisowska, K.; Graniczna, K.; Figlerowicz, M. Effects and safety of convalescent plasma administration in a group of Polish pediatric patients with COVID-19: A case series. Life 2021, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Piacentini, G.; Nosetti, L.; Franchini, M. The use of convalescent plasma for pediatric patients with SARS-CoV-2: A systematic literature review. Transfus. Apher. Sci. 2021, 60, 103043. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Brosnan, M.K.; Yoon, S.; Jung, D.; Littlefield, K.; Ganesan, A.; Caputo, C.A.; Li, M.; Morgenlander, W.R.; Henson, S.N.; et al. Pharmacokinetics of high-titer anti-SARS-CoV-2 human convalescent plasma in high-risk children. JCI Insight 2022, 7, e151518. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; Lesser, E.R.; et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medRxiv 2020. [Google Scholar] [CrossRef]
- Menichetti, F.; Popoli, P.; Puopolo, M.; Spila Alegiani, S.; Tiseo, G.; Bartoloni, A.; De Socio, G.V.; Luchi, S.; Blanc, P.; Puoti, M.; et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: A randomized clinical trial. JAMA Netw. Open 2021, 4, e2136246. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vazquez, C.; Savoy, N.; Giunta, D.H.; Perez, L.G.; Sanchez, M.D.L.; et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. In National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 13 January 2023).
- Semple, J.W.R.J.; Kapur, R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 2019, 133, 1840–1853. [Google Scholar] [CrossRef]
- CDC. National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol. 2021. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwizjfibiYr9AhVTM94KHbXcAJoQFnoECDYQAQ&url=https%3A%2F%2Fwww.reginfo.gov%2Fpublic%2Fdo%2FDownloadDocument%3FobjectID%3D35876801&usg=AOvVaw1_MHgDxTeTX5MImfCmqtcM (accessed on 13 January 2023).
- Vlaar, A.P.; Toy, P.; Fung, M.; Looney, M.R.; Juffermans, N.P.; Bux, J.; Bolton-Maggs, P.; Peters, A.L.; Silliman, C.C.; Kor, D.J.; et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion 2019, 59, 2465–2476. [Google Scholar] [CrossRef]
- Wiersum-Osselton, J.; Whitaker, B.; Grey, S.; Land, K.; Perez, G.; Rajbhandary, S.; Andrzejewski, C., Jr.; Bolton-Maggs, P.; Lucero, H.; Renaudier, P.; et al. Revised international surveillance case definition of transfusion-associated circulatory overload: A classification agreement validation study. Lancet Haematol. 2019, 6, e350–e358. [Google Scholar] [CrossRef]
- Arvin, A.M.; Fink, K.; Schmid, M.A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–363. [Google Scholar] [CrossRef]


| Age (Years) | |
| median (IQR) | 14 (8.18) |
| Range: N (%) | |
| <2 | 3 (7.0%) |
| 2–6 | 1 (2.3%) |
| 6–<12 | 12 (27.9%) |
| 12–<18 | 17 (39.5%) |
| >18 | 10 (23%) |
| Male (N, %) | 24 (55.8%) |
| Hispanic | 34 (79%) |
| Weight (kg) | |
| Median (IQR) | 70.3 (8.18) |
| Range | 3.4–159 |
| BMI | |
| Median (IQR) | 27.35 (18.7, 38.1) |
| N (%) > 30 | 19 (45.2) |
| Co-morbidities * N (%) | 41 (95.4) |
| Obesity | 17 (39.6) |
| Oncology | 7 (16.3) |
| Immune deficiency | 5 (11.6) |
| Neuromuscular | 6 (13.9) |
| CLD | 5 (11.6) |
| CKD | 1 (2.3) |
| Prematurity | 2 (4.6) |
| Cardiac | 2 (4.6) |
| Baseline | 24 h | 7 Days | 21 Days | |
|---|---|---|---|---|
| WBC (K/UL) | 6.1 (4.0) | 5.8 (4.3) | 7.0 (4.2) | 6.6 (4.2) |
| Hgb (gm/dL) | 12.2 (2.3) | 12.5 (3.4) | 12.5 (3.4) | 122.5 (3.4) |
| Platelets (K/UL) | 173.2 (99.9) | 206.9 (125.8) | 294.3 (184.4) | 247.3 (196.9) |
| Creatine (mg/dL) | 0.8 (1.7) | 0.8 (1.7) | 0.5 (0.8) | 0.5 (0.7) |
| AST (units/L) | 63.7 (51.8) | 52 (35.4) | 54.9 (47.4) | 68.6 (116.7) |
| ALT (units/L) | 54.1 (69.4) | 43 (49.1) | 59.5 (65.5) | 90 (137.4) |
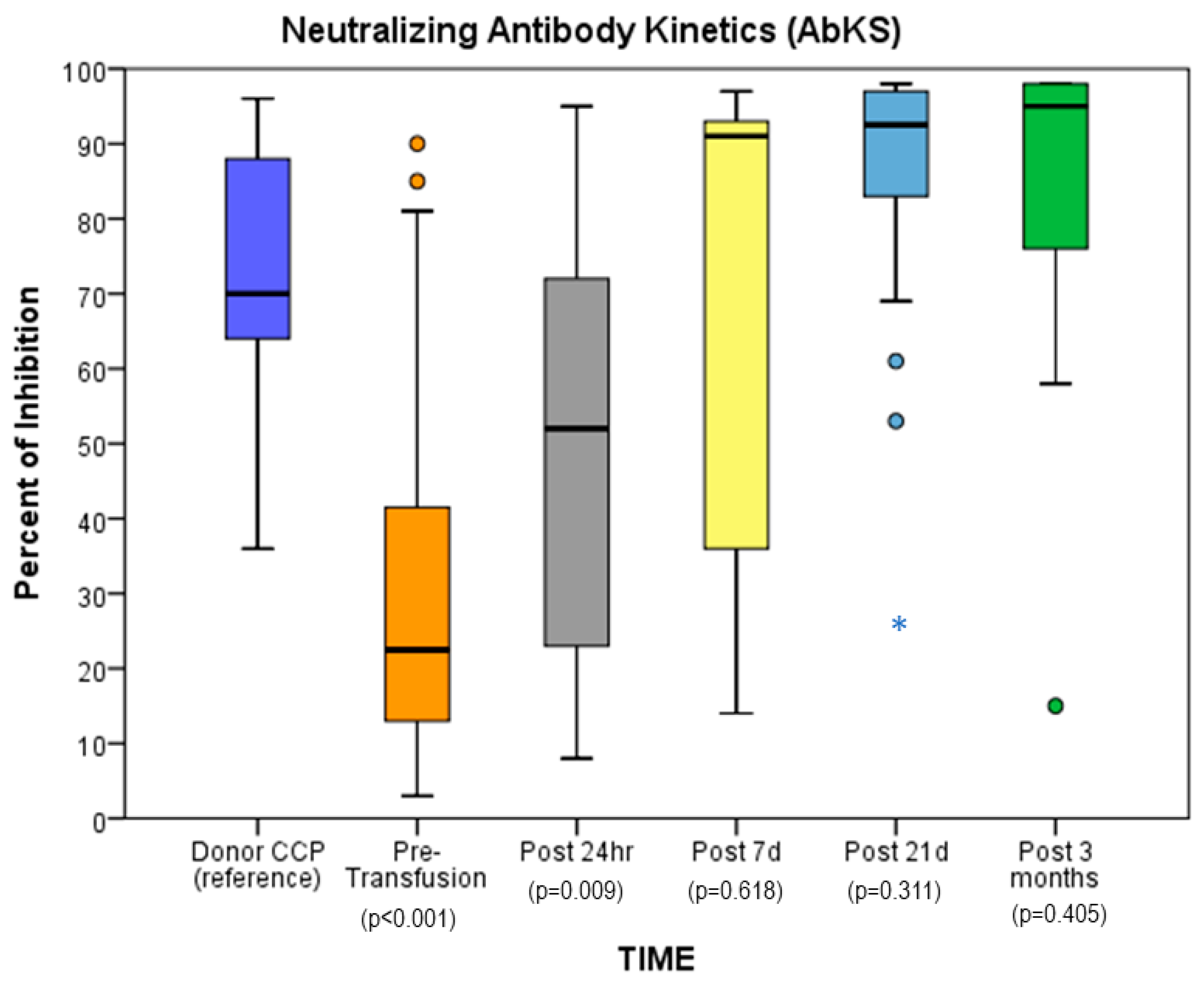
| CCP 1 | Pre | 24 h Post 1 | CCP 2 | 24 h Post 2 | Post 7 d 2 | Post d 21 | |
|---|---|---|---|---|---|---|---|
| Median | 79 | 18.5 | 57.1 | 74.5 | 73.5 | 94.5 | 95.5 |
| Q1 | 56.25 | 11 | 39.75 | 57.75 | 67.25 | 92.5 | 94.25 |
| Q3 | 90.25 | 32 | 77.75 | 66.75 | 81.75 | 95.25 | 96.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta, A.; Galvis, A.E.; Osborne, S.; Morphew, T.; Imfeld, K.; Enriquez, C.; Hoang, J.; Swearingen, M.; Nieves, D.J.; Ashouri, N.; et al. Use of COVID-19 Convalescent Plasma for Treatment of Symptomatic SARS-CoV-2 Infection at a Children’s Hospital: A Contribution to a Still Inadequate Body of Evidence. Children 2023, 10, 350. https://doi.org/10.3390/children10020350
Arrieta A, Galvis AE, Osborne S, Morphew T, Imfeld K, Enriquez C, Hoang J, Swearingen M, Nieves DJ, Ashouri N, et al. Use of COVID-19 Convalescent Plasma for Treatment of Symptomatic SARS-CoV-2 Infection at a Children’s Hospital: A Contribution to a Still Inadequate Body of Evidence. Children. 2023; 10(2):350. https://doi.org/10.3390/children10020350
Chicago/Turabian StyleArrieta, Antonio, Alvaro E. Galvis, Stephanie Osborne, Tricia Morphew, Karen Imfeld, Claudia Enriquez, Janet Hoang, Marcia Swearingen, Delma J. Nieves, Negar Ashouri, and et al. 2023. "Use of COVID-19 Convalescent Plasma for Treatment of Symptomatic SARS-CoV-2 Infection at a Children’s Hospital: A Contribution to a Still Inadequate Body of Evidence" Children 10, no. 2: 350. https://doi.org/10.3390/children10020350
APA StyleArrieta, A., Galvis, A. E., Osborne, S., Morphew, T., Imfeld, K., Enriquez, C., Hoang, J., Swearingen, M., Nieves, D. J., Ashouri, N., Singh, J., & Nugent, D. (2023). Use of COVID-19 Convalescent Plasma for Treatment of Symptomatic SARS-CoV-2 Infection at a Children’s Hospital: A Contribution to a Still Inadequate Body of Evidence. Children, 10(2), 350. https://doi.org/10.3390/children10020350







