Distal Tibial Hemimelia in Fetal Methotrexate Syndrome: A Case Study and Literature Review
Abstract
1. Introduction
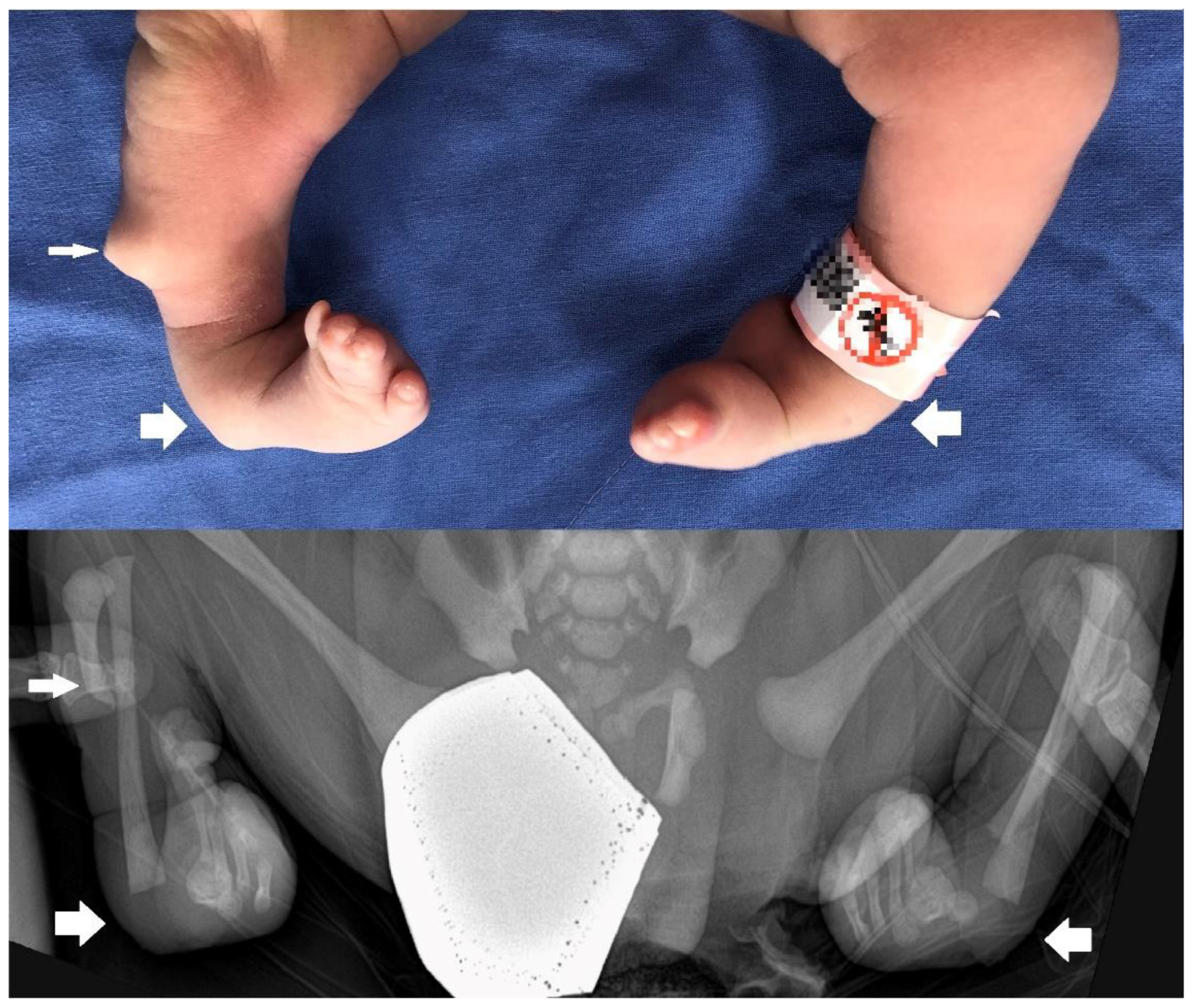
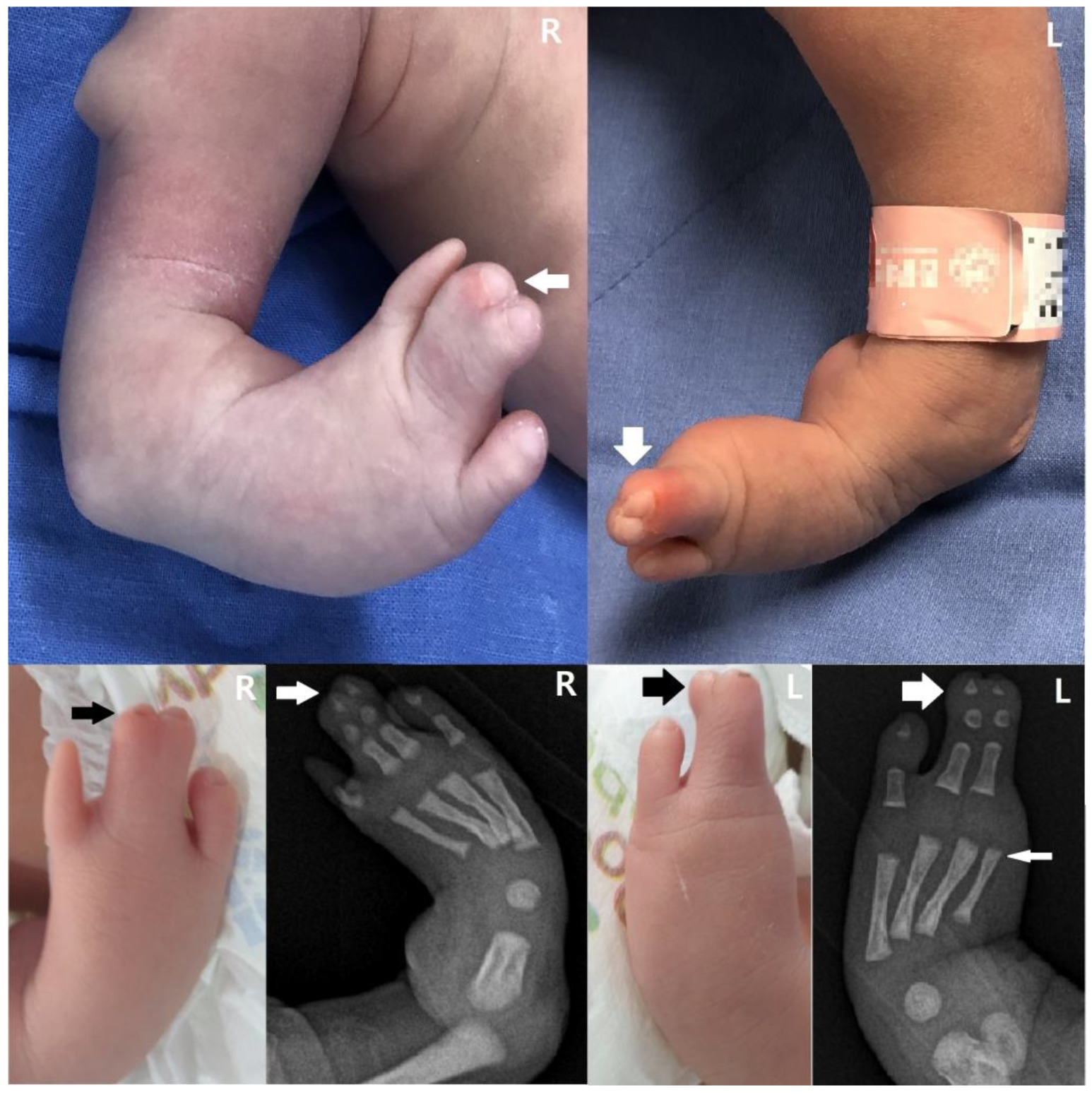
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hyoun, S.C.; Obican, S.G.; Scialli, A.R. Teratogen update: Methotrexate. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 187–207. [Google Scholar] [CrossRef]
- Moreno-Ruiz, N.L.; Borgatta, L.; Yanow, S.; Kapp, N.; Wiebe, E.R.; Winikoff, B. Alternatives to mifepristone for early medical abortion. Int. J. Gynaecol. Obstet. 2007, 96, 212–218. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet. Gynecol. 2008, 111, 1479–1485. [Google Scholar] [CrossRef]
- Barnhart, K.T. Clinical practice. Ectopic pregnancy. N. Engl. J. Med. 2009, 361, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Hoover, K.W.; Tao, G.; Kent, C.K. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet. Gynecol. 2010, 115, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Milunsky, A.; Graef, J.W.; Gaynor, M.F., Jr. Methotrexate-induced congenital malformations. J. Pediatr. 1968, 72, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Verberne, E.A.; de Haan, E.; van Tintelen, J.P.; Lindhout, D.; van Haelst, M.M. Fetal methotrexate syndrome: A systematic review of case reports. Reprod. Toxicol. 2019, 87, 125–139. [Google Scholar] [CrossRef]
- Weber-Schoendorfer, C.; Chambers, C.; Wacker, E.; Beghin, D.; Bernard, N.; Shechtman, S.; Johnson, D.; Cuppers-Maarschalkerweerd, B.; Pistelli, A.; Clementi, M.; et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: A prospective multicenter cohort study. Arthritis Rheumatol. 2014, 66, 1101–1110. [Google Scholar] [CrossRef]
- Nurmohamed, L.; Moretti, M.E.; Schechter, T.; Einarson, A.; Johnson, D.; Lavigne, S.V.; Erebara, A.; Koren, G.; Finkelstein, Y. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am. J. Obstet. Gynecol. 2011, 205, 533.e1–533.e3. [Google Scholar] [CrossRef]
- Lewden, B.; Vial, T.; Elefant, E.; Nelva, A.; Carlier, P.; Descotes, J.; French Network of Regional Pharmacovigilance Centers. Low dose methotrexate in the first trimester of pregnancy: Results of a French collaborative study. J. Rheumatol. 2004, 31, 2360–2365. [Google Scholar] [PubMed]
- Adam, M.P.; Manning, M.A.; Beck, A.E.; Kwan, A.; Enns, G.M.; Clericuzio, C.; Hoyme, E.H. Methotrexate/misoprostol embryopathy: Report of four cases resulting from failed medical abortion. Am. J. Med. Genet. A 2003, 123, 72–78. [Google Scholar] [CrossRef]
- Chapa, J.B.; Hibbard, J.U.; Weber, E.M.; Abramowicz, J.S.; Verp, M.S. Prenatal diagnosis of methotrexate embryopathy. Obstet. Gynecol. 2003, 101, 1104–1107. [Google Scholar] [CrossRef]
- Yedlinsky, N.T.; Morgan, F.C.; Whitecar, P.W. Anomalies associated with failed methotrexate and misoprostol termination. Obstet. Gynecol. 2005, 105, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Seidahmed, M.Z.; Shaheed, M.M.; Abdulbasit, O.B.; Al Dohami, H.; Babiker, M.; Abdullah, M.A.; Abomelha, A.A. A case of methotrexate embryopathy with holoprosencephaly, expanding the phenotype. Birth Defects Res. A Clin. Mol. Teratol. 2006, 76, 138–142. [Google Scholar] [CrossRef]
- Corona-Rivera, J.R.; Rea-Rosas, A.; Santana-Ramirez, A.; Acosta-Leon, J.; Hernandez-Rocha, J.; Miguel-Jimenez, K. Holoprosencephaly and genitourinary anomalies in fetal methotrexate syndrome. Am. J. Med. Genet. A 2010, 152, 1741–1746. [Google Scholar] [CrossRef]
- Piggott, K.D.; Sorbello, A.; Riddle, E.; DeCampli, W. Congenital cardiac defects: A possible association of aminopterin syndrome and in utero methotrexate exposure? Pediatr. Cardiol. 2011, 32, 518–520. [Google Scholar] [CrossRef]
- Poggi, S.H.; Ghidini, A. Importance of timing of gestational exposure to methotrexate for its teratogenic effects when used in setting of misdiagnosis of ectopic pregnancy. Fertil. Steril. 2011, 96, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Addar, M.H. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: Case report. J. Obstet. Gynaecol. Can. 2004, 26, 1001–1003. [Google Scholar] [CrossRef]
- Goffman, D.; Cole, D.S.; Bobby, P.; Garry, D.J. Failed methotrexate termination of pregnancy: A case report. J. Perinatol. 2006, 26, 645–647. [Google Scholar] [CrossRef]
- Donnenfeld, A.E.; Pastuszak, A.; Noah, J.S.; Schick, B.; Rose, N.C.; Koren, G. Methotrexate exposure prior to and during pregnancy. Teratology 1994, 49, 79–81. [Google Scholar] [CrossRef]
- Buckley, L.M.; Bullaboy, C.A.; Leichtman, L.; Marquez, M. Multiple congenital anomalies associated with weekly low-dose methotrexate treatment of the mother. Arthritis Rheum. 1997, 40, 971–973. [Google Scholar] [CrossRef]
- Nguyen, C.; Duhl, A.J.; Escallon, C.S.; Blakemore, K.J. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet. Gynecol. 2002, 99, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Usta, I.M.; Nassar, A.H.; Yunis, K.A.; Abu-Musa, A.A. Methotrexate embryopathy after therapy for misdiagnosed ectopic pregnancy. Int. J. Gynaecol. Obstet. 2007, 99, 253–255. [Google Scholar] [CrossRef]
- Feldkamp, M.; Carey, J.C. Clinical teratology counseling and consultation case report: Low dose methotrexate exposure in the early weeks of pregnancy. Teratology 1993, 47, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.C.; Barbero, P.; Groisman, B.; Aguirre, M.A.; Koren, G. Methotrexate embryopathy after exposure to low weekly doses in early pregnancy. Reprod. Toxicol. 2014, 43, 26–29. [Google Scholar] [CrossRef]
- Dalrymple, J.M.; Stamp, L.K.; O’Donnell, J.L.; Chapman, P.T.; Zhang, M.; Barclay, M.L. Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2008, 58, 3299–3308. [Google Scholar] [CrossRef]
- Lloyd, M.E.; Carr, M.; McElhatton, P.; Hall, G.M.; Hughes, R.A. The effects of methotrexate on pregnancy, fertility and lactation. QJM 1999, 92, 551–563. [Google Scholar] [CrossRef]
- Visser, K.; Katchamart, W.; Loza, E.; Martinez-Lopez, J.A.; Salliot, C.; Trudeau, J.; Bombardier, C.; Carmona, L.; van der Heijde, D.; Bijlsma, J.W.J.; et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: Integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann. Rheum. Dis. 2009, 68, 1086–1093. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, D.-S.; Lee, S.-H. Distal Tibial Hemimelia in Fetal Methotrexate Syndrome: A Case Study and Literature Review. Children 2023, 10, 228. https://doi.org/10.3390/children10020228
Jo D-S, Lee S-H. Distal Tibial Hemimelia in Fetal Methotrexate Syndrome: A Case Study and Literature Review. Children. 2023; 10(2):228. https://doi.org/10.3390/children10020228
Chicago/Turabian StyleJo, Dae-Sik, and Seung-Hyun Lee. 2023. "Distal Tibial Hemimelia in Fetal Methotrexate Syndrome: A Case Study and Literature Review" Children 10, no. 2: 228. https://doi.org/10.3390/children10020228
APA StyleJo, D.-S., & Lee, S.-H. (2023). Distal Tibial Hemimelia in Fetal Methotrexate Syndrome: A Case Study and Literature Review. Children, 10(2), 228. https://doi.org/10.3390/children10020228




