Platelet Counts and Risk of Severe Retinopathy of Prematurity: A Bayesian Model-Averaged Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Search Strategy
2.2. Study Selection and Definitions
2.3. Extraction od Data and Study Quality Assessment
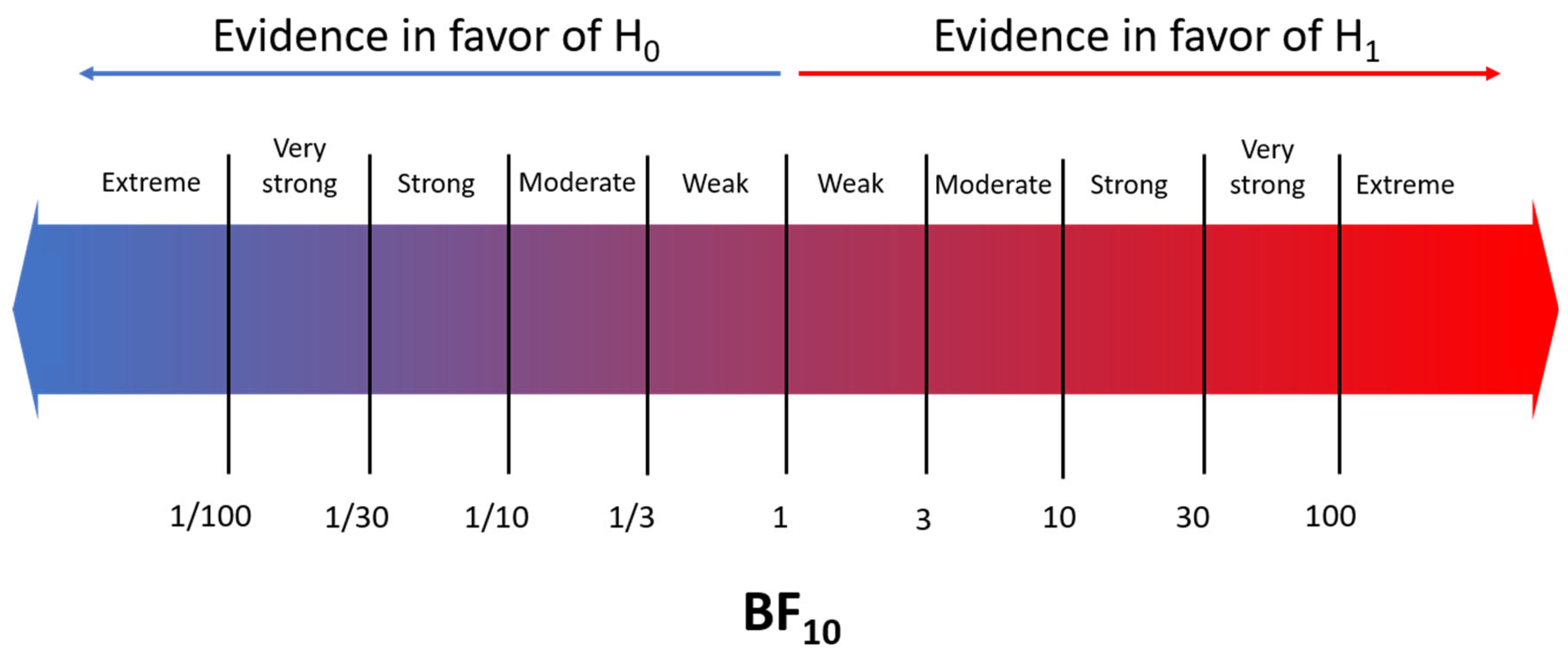
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Studies and Risk of Bias Assessment
3.2. Bayesian Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavallaro, G.; Filippi, L.; Bagnoli, P.; La Marca, G.; Cristofori, G.; Raffaeli, G.; Padrini, L.; Araimo, G.; Fumagalli, M.; Groppo, M. The pathophysiology of retinopathy of prematurity: An update of previous and recent knowledge. Acta Ophthalmol. 2014, 92, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Cavallaro, G.; Raffaeli, G.; Mohammed Rahim, O.M.; Gulden, S.; Ghazi, A.M.; Mosca, F.; Degraeuwe, P.; Villamor, E. Chorioamnionitis as a risk factor for retinopathy of prematurity: An updated systematic review and meta-analysis. PLoS ONE 2018, 13, e0205838. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.M.; Huizing, M.J.; Villamor-Martinez, E.; Bartoš, F.; Villamor, E. Association of funisitis with short-term outcomes of prematurity: A frequentist and bayesian meta-analysis. Antioxidants 2023, 12, 534. [Google Scholar] [CrossRef]
- Hundscheid, T.M.; Gulden, S.; Almutairi, M.F.; Bartoš, F.; Cavallaro, G.; Villamor, E. Sex differences in the risk of retinopathy of prematurity: A systematic review, frequentist and Bayesian meta-analysis, and meta-regression. World J. Pediatr. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, G.; Lundgren, P.; Pivodic, A.; Löfqvist, C.; Nilsson, A.K.; Ley, D.; Sävman, K.; Smith, L.E.; Hellström, A. Decreased platelet counts and serum levels of VEGF-A, PDGF-BB, and BDNF in extremely preterm infants developing severe ROP. Neonatology 2021, 118, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Parrozzani, R.; Nacci, E.B.; Bini, S.; Marchione, G.; Salvadori, S.; Nardo, D.; Midena, E. Severe retinopathy of prematurity is associated with early post-natal low platelet count. Sci. Rep. 2021, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Seliniotaki, A.K.; Haidich, A.B.; Moutzouri, S.; Lithoxopoulou, M.; Ziakas, N.; Lundgren, P.; Hellström, A.; Mataftsi, A. Association of platelet deficiency with severe retinopathy of prematurity: A review. Acta Paediatr. 2022, 111, 2056–2070. [Google Scholar] [CrossRef]
- Vinekar, A.; Hegde, K.; Gilbert, C.; Braganza, S.; Pradeep, M.; Shetty, R.; Shetty, K.B. Do platelets have a role in the pathogenesis of aggressive posterior retinopathy of prematurity? Retina 2010, 30, S20–S23. [Google Scholar] [CrossRef]
- Cakir, B.; Liegl, R.; Hellgren, G.; Lundgren, P.; Sun, Y.; Klevebro, S.; Löfqvist, C.; Mannheimer, C.; Cho, S.; Poblete, A. Thrombocytopenia is associated with severe retinopathy of prematurity. JCI Insight 2018, 3, e99448. [Google Scholar] [CrossRef]
- Grant, R.L. The uptake of Bayesian methods in biomedical meta-analyses: A scoping review (2005–2016). J. Evid. Based Med. 2019, 12, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, F.; Otte, W.M.; Gronau, Q.F.; Timmers, B.; Ly, A.; Wagenmakers, E.-J. Empirical prior distributions for Bayesian meta-analyses of binary and time to event outcomes. arXiv 2023, arXiv:2306.11468. [Google Scholar]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.-J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 2020, 23, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics notes: Absence of evidence is not evidence of absence. BMJ 1995, 311, 485. [Google Scholar] [CrossRef] [PubMed]
- Gronau, Q.F.; Heck, D.W.; Berkhout, S.W.; Haaf, J.M.; Wagenmakers, E.-J. A primer on Bayesian model-averaged meta-analysis. Adv. Methods Pract. Psychol. Sci. 2021, 4, 25152459211031256. [Google Scholar] [CrossRef]
- Bartoš, F.; Gronau, Q.F.; Timmers, B.; Otte, W.M.; Ly, A.; Wagenmakers, E.J. Bayesian model-averaged meta-analysis in medicine. Stat. Med. 2021, 40, 6743–6761. [Google Scholar] [CrossRef] [PubMed]
- González-Luis, G.; Ghirardello, S.; Bas-Suárez, P.; Cavallaro, G.; Mosca, F.; Clyman, R.I.; Villamor, E. Platelet counts and patent ductus arteriosus in preterm infants: An updated systematic review and meta-analysis. Front. Pediatr. 2021, 8, 613766. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Husain, S. (Eds.) Primary Prevention of ROP and the Oxygen Saturation Targeting Trials. Seminars in Perinatology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kumawat, D.; Sachan, A.; Shah, P.; Chawla, R.; Chandra, P. Aggressive posterior retinopathy of prematurity: A review on current understanding. Eye 2021, 35, 1140–1158. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Heck, D.; Gronau, F.; Wagenmakers, E. metaBMA: Bayesian Model Averaging for Random and Fixed Effects Meta-Analysis, version 0.6. 7; [Computer software]; JASP Team: Amsterdam, The Netherlands, 2019. [Google Scholar]
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon. Bull. Rev. 2021, 28, 813–826. [Google Scholar] [CrossRef]
- Lee, M.; Wagenmakers, E.-J. Bayesian Data Analysis for Cognitive Science: A Practical Course; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Bartoš, F.; Maier, M.; Wagenmakers, E.J.; Doucouliagos, H.; Stanley, T. Robust Bayesian meta-analysis: Model-averaging across complementary publication bias adjustment methods. Res. Synth. Methods 2023, 14, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Vevea, J.L.; Hedges, L.V. A general linear model for estimating effect size in the presence of publication bias. Psychometrika 1995, 60, 419–435. [Google Scholar] [CrossRef]
- Stanley, T.D.; Doucouliagos, H. Meta-regression approximations to reduce publication selection bias. Res. Synth. Methods 2014, 5, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Choręziak, A.; Szpecht, D.; Chmielarz-Czarnocińska, A.; Pawłowska, I.; Gotz-Więckowska, A. The association of platelet counts with development and treatment for retinopathy of prematurity–is thrombocytopenia a risk factor? Arch. Med. Sci. AMS 2022, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.K.; Ying, G.-s.; Huang, J.; Karp, K.; Quinn, G.E.; Binenbaum, G. Thrombocytopenia and retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 447–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okur, N.; Buyuktiryaki, M.; Uras, N.; Oncel, M.Y.; Ertekin, O.; Canpolat, F.E.; Oguz, S.S. Platelet mass index in very preterm infants: Can it be used as a parameter for neonatal morbidities? J. Matern. Fetal Neonatal Med. 2016, 29, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Dong, Y.; Lu, C.-w.; Yang, W.; Li, Q. Relationship between mean platelet volume and retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1791–1794. [Google Scholar] [CrossRef]
- Cekmez, F.; Tanju, I.; Canpolat, F.; Aydinoz, S.; Aydemir, G.; Karademir, F.; Sarici, S. Mean platelet volume in very preterm infants: A predictor of morbidities. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 134–137. [Google Scholar] [CrossRef]
- Hengartner, T.; Adams, M.; Pfister, R.E.; Snyers, D.; McDougall, J.; Waldvogel, S.; Held-Egli, K.; Spring, L.; Rogdo, B.; Riedel, T. Associations between red blood cell and platelet transfusions and retinopathy of prematurity. Neonatology 2021, 117, 562–568. [Google Scholar] [CrossRef]
- Fevereiro-Martins, M.; Santos, A.C.; Marques-Neves, C.; Guimarães, H.; Bicho, M. Complete blood count parameters as biomarkers of retinopathy of prematurity: A Portuguese multicenter study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 2997–3006. [Google Scholar] [CrossRef]
- Abdel Salam Gomaa, N.; Helmy, Y.A.; Maher, S.; Hassanein, D.; Shuaib, A.; Hegazy, A.I.; Ali, A.A. Clinical characteristics of preterm neonates with aggressive posterior retinopathy of prematurity. Clin. Ophthalmol. 2021, 15, 2263–2277. [Google Scholar] [CrossRef]
- Sahinoglu-Keskek, N.; Akkoyun, I.; Çetinkaya, B. Platelet distribution width is a predictive marker for development of severe retinopathy of prematurity. Eye 2021, 35, 3435–3436. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.D.; Pheng, E.; Min, E.T.L.; Van Rostenberghe, H.; Shatriah, I. Comparison of Mean Platelet Counts in Preterm Infants with and without Retinopathy of Prematurity. Int. J. Environ. Res. Public Health 2021, 18, 3783. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, P.; Lundberg, L.; Hellgren, G.; Holmström, G.; Hård, A.-L.; Smith, L.E.; Wallin, A.; Hallberg, B.; Hellström, A. Aggressive posterior retinopathy of prematurity is associated with multiple infectious episodes and thrombocytopenia. Neonatology 2016, 111, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sancak, S.; Toptan, H.H.; Yildirim, T.G.; Karatekin, G.; Ovali, F. Thrombocytopenia as a risk factor for retinopathy of prematurity. Retina 2019, 39, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Panchal H, S.A. Thrombocytopenia and severe retinopathy of prematurity. J. Paediatr. Child Health 2020, 56, 59. [Google Scholar] [CrossRef]
- Ünsal, A.İ.A.; Key, Ö.; Güler, D.; Omurlu, İ.K.; Anık, A.; Demirci, B.; Dündar, S. Can complete blood count parameters predict retinopathy of prematurity? Turk. J. Ophthalmol. 2020, 50, 87. [Google Scholar] [CrossRef]
- Korkmaz, L.; Baştuğ, O.; Özdemir, A.; Korkut, S.; Karaca, Ç.; Akın, M.A.; Öztürk, M.A. Platelet mass index can be a reliable marker in predicting the prognosis of retinopathy of prematurity in very preterm infants. Pediatr. Neonatol. 2018, 59, 455–463. [Google Scholar] [CrossRef]
- Celik, K.; Ekinci, D.; Asena, M.; Matur, N.O. Can Hematological Parameters be a Indicator Risk Factor in the Development of Retinopathy of Prematurity? Klin. Pädiatrie 2021, 233, 216–220. [Google Scholar] [CrossRef]
- Yüksel, H.; Şahin, A.; Şahin, M.; Türkcü, F.M.; ÇINAR, Y.; Gürsel-Özkurt, Z.; Karaalp, Ü.; Uluca, Ü.; İhsan, Ç. Mean trombosit volume in patients with retinopathy of prematurity. J. Clin. Exp. Investig. 2014, 5, 276–279. [Google Scholar]
- Kicinski, M.; Springate, D.A.; Kontopantelis, E. Publication bias in meta-analyses from the Cochrane Database of Systematic Reviews. Stat. Med. 2015, 34, 2781–2793. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Carpenter, J.R.; Schwarzer, G. Detecting and adjusting for small-study effects in meta-analysis. Biom. J. 2011, 53, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Assessing risk of bias due to missing results in a synthesis. Cochrane Handb. Syst. Rev. Interv. 2019, 349–374. [Google Scholar]
- Maier, M.; Bartoš, F.; Wagenmakers, E.-J. Robust Bayesian meta-analysis: Addressing publication bias with model-averaging. Psychol. Methods 2023, 28, 107. [Google Scholar] [CrossRef] [PubMed]
- Gunnink, S.F.; Vlug, R.; Fijnvandraat, K.; Van Der Bom, J.G.; Stanworth, S.J.; Lopriore, E. Neonatal thrombocytopenia: Etiology, management and outcome. Expert Rev. Hematol. 2014, 7, 387–395. [Google Scholar] [CrossRef]
- Sola, M.C.; Del Vecchio, A.; Rimsza, L.M. Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit. Clin. Perinatol. 2000, 27, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Dhindsa, Y.; Sim, M.S.; Altendahl, M.; Tsui, I. Prenatal intrauterine growth restriction and risk of retinopathy of prematurity. Sci. Rep. 2020, 10, 17591. [Google Scholar] [CrossRef]
- Lee, J.W.; VanderVeen, D.; Allred, E.N.; Leviton, A.; Dammann, O. Prethreshold retinopathy in premature infants with intrauterine growth restriction. Acta Paediatr. 2015, 104, 27–31. [Google Scholar] [CrossRef]
- Spekman, J.A.; El Emrani, S.; Schalij-Delfos, N.E.; Slaghekke, F.; van Klink, J.M.; Lopriore, E.; Groene, S.G. Association between fetal growth-restriction and retinopathy of prematurity using a unique identical twin model. Pediatr. Res. 2023, 94, 1738–1743. [Google Scholar] [CrossRef]
- Chiang, M.F.; Arons, R.R.; Flynn, J.T.; Starren, J.B. Incidence of retinopathy of prematurity from 1996 to 2000: Analysis of a comprehensive New York state patient database. Ophthalmology 2004, 111, 1317–1325. [Google Scholar] [CrossRef]
- Tolsma, K.W.; Allred, E.N.; Chen, M.L.; Duker, J.; Leviton, A.; Dammann, O. Neonatal bacteremia and retinopathy of prematurity: The ELGAN study. Arch. Ophthalmol. 2011, 129, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Isaza, G.; Arora, S.; Bal, M.; Chaudhary, V. Incidence of retinopathy of prematurity and risk factors among premature infants at a neonatal intensive care unit in Canada. J. Pediatr. Ophthalmol. Strabismus 2013, 50, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E. Updates in red blood cell and platelet transfusions in preterm neonates. Am. J. Perinatol. 2019, 36, S37–S40. [Google Scholar] [CrossRef]
- Moore, C.M.; D’Amore, A.; Fustolo-Gunnink, S.; Hudson, C.; Newton, A.; Santamaria, B.L.; Deary, A.; Hodge, R.; Hopkins, V.; Mora, A. Two-year outcomes following a randomised platelet transfusion trial in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Curley, A.; Stanworth, S.J.; Willoughby, K.; Fustolo-Gunnink, S.F.; Venkatesh, V.; Hudson, C.; Deary, A.; Hodge, R.; Hopkins, V.; Lopez Santamaria, B. Randomized trial of platelet-transfusion thresholds in neonates. N. Engl. J. Med. 2019, 380, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Ohto, H.; Nollet, K.E.; Takano, S.; Kashiwabara, N.; Chishiki, M.; Maeda, H.; Imamura, T.; Kawasaki, Y.; Momoi, N. Using platelet parameters to anticipate morbidity and mortality among preterm neonates: A retrospective study. Front. Pediatr. 2020, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Poggi, C.; Barp, J.; Berti, E.; Fontanelli, G. Mean platelet volume and risk of bronchopulmonary dysplasia and intraventricular hemorrhage in extremely preterm infants. Am. J. Perinatol. 2011, 28, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Grevsen, A.K.; Hviid, C.V.; Hansen, A.K.; Hvas, A.-M. (Eds.) The Role of Platelets in Premature Neonates with Intraventricular Hemorrhage: A Systematic Review and Meta-Analysis. Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2020. [Google Scholar]
- Wang, J.; Wang, Z.; Zhang, M.; Lou, Z.; Deng, J.; Li, Q. Diagnostic value of mean platelet volume for neonatal sepsis: A systematic review and meta-analysis. Medicine 2020, 99, e21649. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yoon, J.; Lim, C.S.; Choi, B.M.; Yoon, S.-Y. Clinical significance of platelet-associated hematological parameters as an early supplementary diagnostic tool for sepsis in thrombocytopenic very-low-birth-weight infants. Platelets 2015, 26, 620–626. [Google Scholar] [CrossRef]
- Kannar, V.; Deepthi, A.; Kumar, M.L.H.; Junjegowda, K.; Mariyappa, N. Effect of gestational age, prematurity and birth asphyxia on platelet indices in neonates. J. Clin. Neonatol. 2014, 3, 144–147. [Google Scholar] [CrossRef]


| Condition | Variable | Phase | k | Effect Size | SD | 95% Credible Interval | BF10 | Evidence for | p-Value Frequentist nalysis a | BFrf | Evidence for | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | H1 | H0 | Random Effects | Fixed Effects | ||||||||||
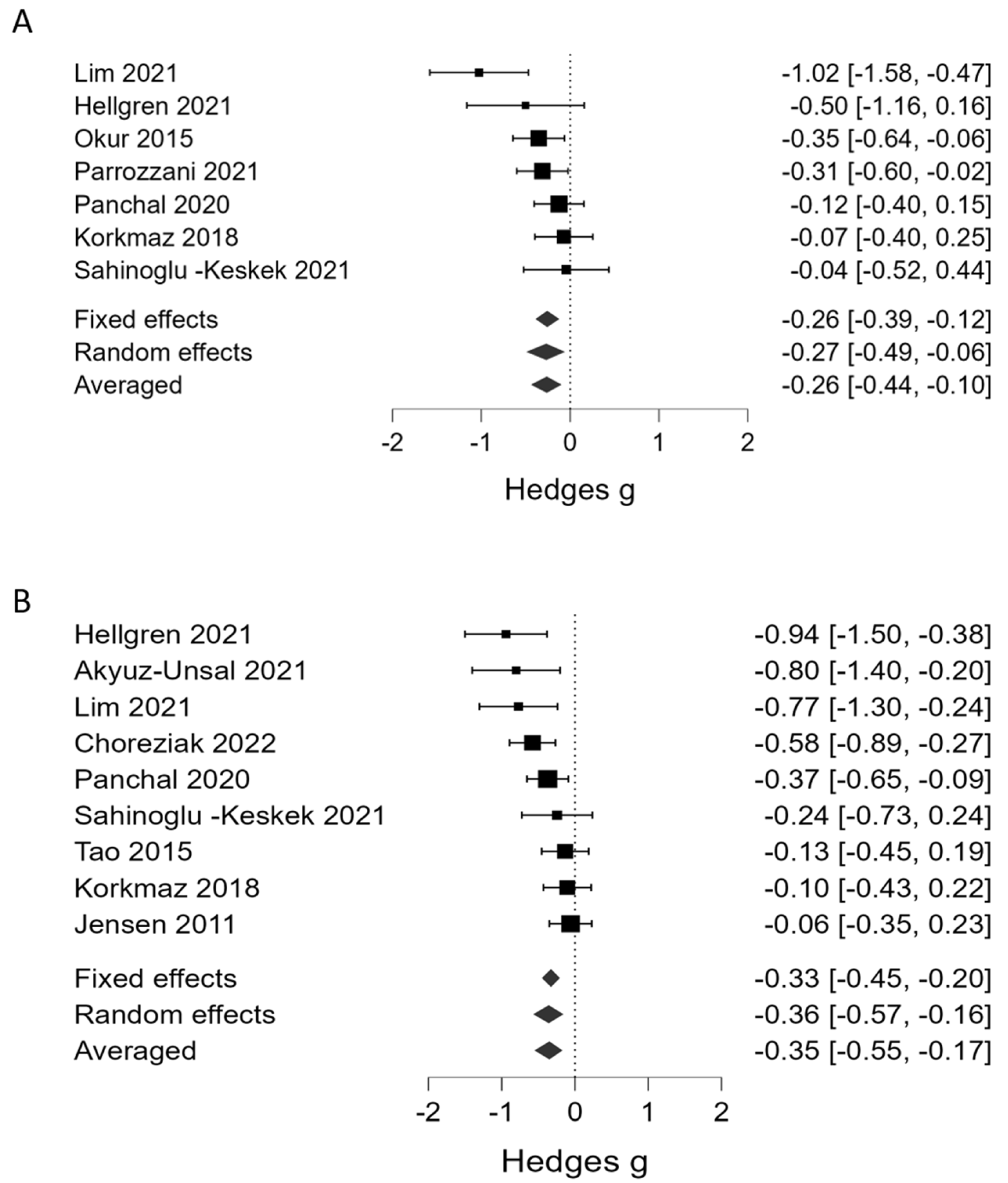
| Severe ROP | Platelet counts | 1 | 7 | Hedges’ g | −0.26 | 0.09 | −0.42 | −0.10 | 13.5 | strong | <0.001 | 0.79 | weak | ||
| 2 | 9 | Hedges’ g | −0.34 | 0.10 | −0.55 | −0.15 | 51.0 | very strong | <0.001 | 2.75 | weak | ||||
| Thrombocytopenia (<100 × 109/L) | 1 | 3 | Log OR | 0.59 | 0.31 | −0.03 | 1.19 | 6.01 | mod. | <0.001 | 1.33 | weak | |||
| 2 | 4 | Log OR | 1.17 | 0.36 | 0.29 | 1.79 | 28.2 | strong | <0.001 | 1.00 | weak | ||||
| PMI | 1 | 4 | Hedges’ g | −0.12 | 0.12 | −0.35 | 0.15 | 0.49 | weak | 0.14 | 1.10 | weak | |||
| 2 | 2 | Hedges’ g | 0.01 | 0.24 | −0.54 | 0.45 | 0.48 | weak | 0.50 | 3.47 | mod. | ||||
| MPV | 1 | 4 | Hedges’ g | −0.05 | 0.16 | −0.36 | 0.29 | 0.36 | weak | 0.32 | 4.95 | mod. | |||
| 2 | 4 | Hedges’ g | −0.04 | 0.21 | −0.48 | 0.37 | 0.42 | weak | 0.88 | 257.0 | extr. | ||||
| Platelet transfusion | both | 5 | Log OR | 0.78 | 0.30 | 0.06 | 1.30 | 12.0 | strong | <0.001 | 2.72 | weak | |||
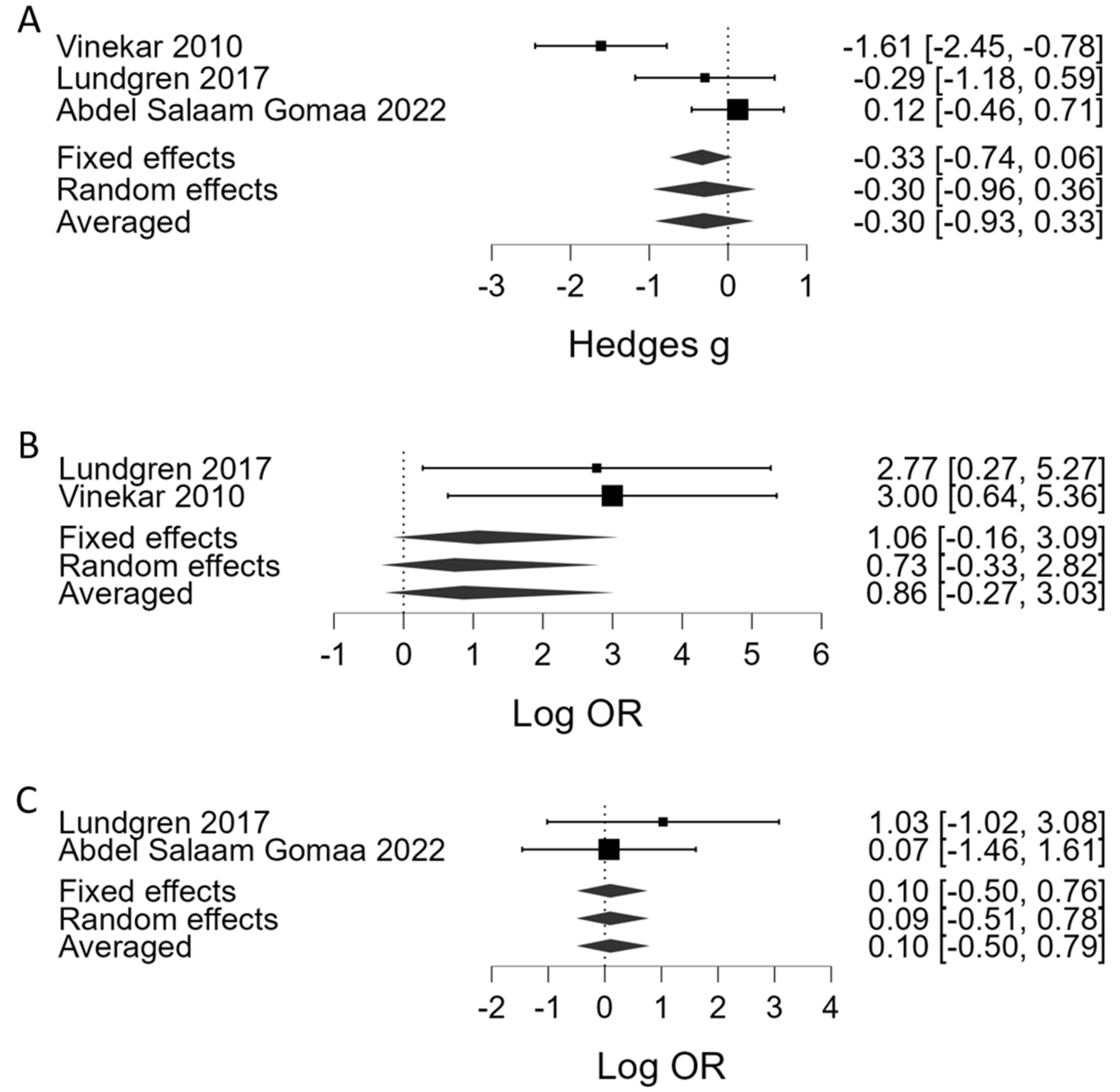
| APROP | Platelet counts | 2 | 3 | Hedges’ g | −0.30 | 0.31 | −0.93 | 0.33 | 1.20 | weak | 0.55 | 6.54 | mod. | ||
| Thrombocytopenia (<100 × 109/L) | 2 | 2 | Log OR | 0.86 | 0.88 | −0.27 | 3.03 | 2.34 | weak | <0.001 | 1.32 | weak | |||
| Platelet transfusion | both | 2 | Log OR | 0.10 | 0.32 | −0.50 | 079 | 0.90 | weak | 0.51 | 0.74 | weak | |||
| Condition | Variable | Phase | k | Effect Size | 95% Credible Interval | BF10 | BFrf | BFbias | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||||
| Severe ROP | Platelet counts | 1 | 7 | Hedges’ g | −0.18 | −0.41 | 0.18 | 1.17 | 0.74 | 3.27 |
| 2 | 9 | Hedges g | −0.14 | −0.46 | 0.32 | 0.64 | 0.95 | 12.4 | ||
| Thrombocytopenia (<100 × 109/L) | 1 | 3 | Log OR | 0.45 | −0.20 | 1.10 | 2.75 | 1.43 | 1.86 | |
| 2 | 4 | Log OR | 0.83 | −0.14 | 1.67 | 4.30 | 1.24 | 2.00 | ||
| PMI | 1 | 4 | Hedges’ g | −0.09 | −0.34 | 0.20 | 0.43 | 0.95 | 0.77 | |
| 2 | 2 | Hedges’ g | 0.10 | −0.42 | 0.71 | 0.52 | 2.23 | 1.34 | ||
| MPV | 1 | 4 | Hedges’ g | −0.02 | −0.33 | 0.35 | 0.34 | 3.71 | 0.70 | |
| 2 | 4 | Hedges’ g | 0.07 | −0.44 | 0.63 | 0.55 | 118.1 | 1.22 | ||
| Platelet transfusion | both | 5 | Log OR | 0.28 | −0.38 | 1.07 | 1.21 | 1.99 | 11.65 | |
| APROP | Platelet counts | 2 | 3 | Hedges’ g | −0.21 | −0.81 | 0.49 | 1.01 | 4.91 | 1.18 |
| Thrombocytopenia (<100 × 109/L) | 3 | 2 | Log OR | 0.30 | −0.54 | 1.86 | 1.20 | 1.10 | 8.98 | |
| Platelet transfusion | both | 2 | Log OR | 0.04 | −0.63 | 0.76 | 0.90 | 0.85 | 0.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, M.F.; Gulden, S.; Hundscheid, T.M.; Bartoš, F.; Cavallaro, G.; Villamor, E. Platelet Counts and Risk of Severe Retinopathy of Prematurity: A Bayesian Model-Averaged Meta-Analysis. Children 2023, 10, 1903. https://doi.org/10.3390/children10121903
Almutairi MF, Gulden S, Hundscheid TM, Bartoš F, Cavallaro G, Villamor E. Platelet Counts and Risk of Severe Retinopathy of Prematurity: A Bayesian Model-Averaged Meta-Analysis. Children. 2023; 10(12):1903. https://doi.org/10.3390/children10121903
Chicago/Turabian StyleAlmutairi, Mohamad F., Silvia Gulden, Tamara M. Hundscheid, František Bartoš, Giacomo Cavallaro, and Eduardo Villamor. 2023. "Platelet Counts and Risk of Severe Retinopathy of Prematurity: A Bayesian Model-Averaged Meta-Analysis" Children 10, no. 12: 1903. https://doi.org/10.3390/children10121903
APA StyleAlmutairi, M. F., Gulden, S., Hundscheid, T. M., Bartoš, F., Cavallaro, G., & Villamor, E. (2023). Platelet Counts and Risk of Severe Retinopathy of Prematurity: A Bayesian Model-Averaged Meta-Analysis. Children, 10(12), 1903. https://doi.org/10.3390/children10121903








