Trend of Bronchial Hyperresponsiveness to Methacholine as a Cost Predictor of Mild-to-Moderate Asthma: A Twelve-Month Survey in Teenagers
Abstract
1. Introduction
2. Materials and Methods
2.1. Economic Evaluation
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Heart Lung and Blood Institute—National Institute of Health. International Consensus Report on the Diagnosis and Management of Asthma; NIH Publication Number 92-3091.1992; National Institute of Health: Bethesda, MD, USA, 1992.
- Bousquet, J.; Jeffery, P.K.; Busse, W.W.; Johnson, M.; Vignola, A.M. Asthma: From bronchoconstriction to airways inflammation and remodelling. Am. J. Respir. Crit. Care Med. 2000, 161, 1720–1745. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Boulet, L.P. Structural changes in airway diseases. Chest 2006, 129, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Al-Ramli, W.; Hamid, A. Remodelling in asthma. Proc. Am. Thorac. Soc. 2009, 6, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Janson, C. The importance of airway remodelling in the natural course of asthma. Clin. Respir. J. 2010, 4 (Suppl. S1), 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the Asthma Control Test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Suh, D.I.; Song, D.J.; Baek, H.-S.; Shin, M.; Yoo, Y.; Kwon, J.-W.; Jang, G.C.; Yang, H.-J.; Lee, E.; et al. Asthma control test reflects not only lung function but also airway inflammation in children with stable asthma. J. Asthma 2020, 57, 648–653. [Google Scholar] [CrossRef]
- Dinakar, C. Monitoring asthma control in children. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 113–118. [Google Scholar] [CrossRef]
- Kalm-Stephens, P.; Malinovsci, A.; Janson, C.; Venge, P.; Nordvall, L.; Alving, K. Concurrence of elevated FeNO and hyperresponsiveness in non-asthmatic adolescents. Pediatr. Pulmonol. 2020, 55, 571–579. [Google Scholar] [CrossRef]
- Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis management of asthma—Summary report 2007. J. Allergy Clin. Immunol. 2007, 120, S94–S138. [CrossRef]
- Deschildre, A.; Pin, I.; El Abd, K.; Belmin-Larrar, S.; El Mourad, S.; Thumerelle, C.; Le Roux, P.; Langlois, C.; de Blic, J. Asthma control assessment in a pediatric population: Comparison between GINA/NAEPP guidelines, Childhood Asthma Control Test (C-ACT), and physician’s rating. J. Allergy 2014, 69, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Tzu-Ning Wen, T.N.; Lin, H.C.; Yeh, K.; Huang, J.-L.; Chiang, L.-C. Effectiveness of Asthma Care on Symptoms, Childhood Asthma Control Test, and Lung Function among Asthmatic Children. J. Med. Syst. 2022, 46, 71. [Google Scholar] [CrossRef]
- Grzelewska-Rzymowska, I.F.; Mikolajczyk, J.; Kroczynska-Bednarek, J.; Gorski, P. Association between asthma control test, pulmonary function tests and non-specific hyperresponsiveness in assessing the level of asthma control. Pneumonol. Alergol. 2015, 83, 266–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palacio da Silva, C.; Aretakis Cordeiro, J.S.; Amorim de Britto, M.C.; Bezerra, P.G.d.M.; de Andrade, L.B. Peack inspiratory flow in children adolescents with asthma using dry powdwer inhalers: A cross-sectional study. J. Bras. Pneumol. 2021, 47, e20200473. [Google Scholar] [CrossRef]
- Goeman, D.P.; Hogan, C.D.; Aroni, R.A.; Abramson, M.J.; Sawyer, S.M.; Stewart, K.; Sanci, L.A. Barriers to delivering asthma care: A qualitative study of general practitioners. Med. J. Aust. 2005, 183, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Chipps, B.E.; Spahn, J.D. What are the determinants of asthma control? J. Asthma 2006, 43, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Naimi, D.R.; Freedman, T.G.; Ginsburg, K.R.; Bogen, D.; Rand, C.S.; Apter, A.J. Adolescents asthma: Why bother with our meds? J. Allergy Clin. Immunol. 2009, 123, 1335–1341. [Google Scholar] [CrossRef]
- Fidler, A.; Sweenie, R.; Ortega, A.; Cushing, C.C.; Ramsey, R.; Fedele, D. Meta-analysis of adherence promotion interventions in pediatric asthma. J. Pediatr. Psychol. 2021, 46, 1195–1212. [Google Scholar] [CrossRef]
- Jebrak, G.; Houdouin, V.; Terrioux, P.; Lambert, N.; Maitre, B.; Ruppert, A.-M. Therapeutic adherence among asthma patients: Variations according to age groups. How can it be improved? The potential contributions of new technologies. Rev. Mal. Respir. 2022, 39, 442–454. [Google Scholar] [PubMed]
- Avital, A.; Springer, C.; Bar-Yishay, E.; Godfrey, S. Adenosine, methacholine, and exercise challenges in children with asthma or paediatric obstructive pulmonary disease. Thorax 1995, 5, 511–516. [Google Scholar] [CrossRef]
- Sweenie, R.; Cushing, C.C.; Fleming, K.K. Daily adherence variability psychosocial differences in adolescents with asthma: A pilot study Daily adherence variability psychosocial differences in adolescents with asthma: A pilot study. J. Behav. Med. 2022, 45, 148–158. [Google Scholar] [CrossRef]
- Munoz, X.; Sanchez-Vidaurre, S.; Roca, O.; Torres, F.; Morell, F.; Cruz, M.J. Bronchial inflammation and hyperresponsiveness in well controlled asthma. Clin. Exp. Allergy 2012, 42, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, M.; Hermansen, M.N.; Hansen, K.; Chawes, B.L. Assessment of adherence to asthma controllers in children and adolescents. Pediatr. Allergy Immunol. 2020, 31, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Dal Negro, R.W.; Turco, P. Trend of lung function, bronchial hyperreactivity, and health outcomes in adolescents with mild-to-moderate asthma by their adherence to once-daily treatment: A twelve-month survey. Children 2022, 9, 1854. [Google Scholar] [CrossRef]
- Boole, G. Mathematical Analysis of Logic, Being an Essay towards a Calculus of Deductive Reasoning; MacMillan: Cambridge, UK, 1847. [Google Scholar]
- Crompton, G.K. Problems patients have using pressurized aerosol inhalers. Eur. J. Respir. Dis. 1982, 63 (Suppl. S119), 101–104. [Google Scholar]
- Italian Medicines Agency. Lists of Class A and Class H Medicinal products. Available online: https://www.aifa.gov.it/en/liste-farmaci-a-h (accessed on 1 September 2023).
- GU Serie Generale n.23 del 28 January 2013—Suppl. Ordinario n. 8. Decreto 18 Ottobre 2012. Remunerazione Prestazioni di Assistenza Ospedaliera per Acuti, Assistenza Ospedaliera di Riabilitazione e di Lungodegenza Post Acuzie e di Assistenza Specialistica Ambulatoriale. (13A00528). Available online: https://www.gazzettaufficiale.it/eli/id/2013/01/28/13A00528/sg (accessed on 1 September 2023).
- Italian Ministry of Health. Schede di Dimissione Ospedaliera (SDO). 2020. Available online: https://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1237&area=ricoveriOspedalieri&menu=vuotolink (accessed on 1 September 2023).
- Pradelli, L.; Ghetti, G. A general model for the estimation of societal costs of lost production and informal care in Italy. Farmecon. Health Econ. Ther. Pathw. 2017, 18, 5–14. [Google Scholar] [CrossRef][Green Version]
- Harmonized Indices of Consumer Prices (HICP), European Commission EuroStat 2022. Available online: https://ec.europa.eu/eurostat/web/hicp/data/main-tables (accessed on 1 September 2022).
- Keene, O.N.; Davis, R.L.; Koch, G.G. Use of generalized estimating equations in a trial in influenza to explore treatment effects over time. Pharm. Stat. 2004, 3, 281–287. [Google Scholar] [CrossRef]
- Couriel, J. Asthma in adolescents. Paediatr. Respir. Rev. 2003, 4, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.M.; Stoloff, S. Is mild asthma really “mild”? Int. J. Clin. Pract. 2005, 59, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.S.; Irving, L.B. Evidence-based pharmacologic treatment of mild asthma. Int. J. Clin. Pract. 2007, 61, 1375–1379. [Google Scholar] [CrossRef]
- Sheikh, S.; Ryan-Wenger, N.A.; Pitts, J.; Nemastil, C.J.; Palacios, S. Impact of asthma severity on long-term asthma control. J. Asthma 2021, 58, 725–734. [Google Scholar] [CrossRef]
- Sumino, K.; Sugar, E.A.; Irvin, C.G.; Kaminsky, D.A.; Shade, D.; Wei, C.Y.; Holbrook, J.T.; Wise, R.A.; Castro, M. Variability of methacholine bronchoprovocation and the effect of inhaled corticosteroids in mild asthma. Ann. Allergy Asthma Immunol. 2014, 112, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Y.; Kang, H.; Yoo, Y.; Yu, J.; Nah, K.M.; Kim, C.K. Peak expiratory flow variability exercise responsiveness in methacholine-hyperresponsive adolescents with asthma remission. J. Asthma 2005, 42, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.J. Interpretation of the positive methacholine challenge. Am. J. Ind. Med. 2008, 51, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, H.; Bender, B.; Ackerson, L.; Bowrya, P.; Smith, B.; Rand, C. Noncompliance treatment failure in children with asthma. J. Allergy Clin. Immunol. 1996, 98, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, C.; Brimer, A.G.; Adams, C.D.; Malhi, K. Social perceptions and adherence of youth in asthma. Mo. Med. 2006, 103, 553–556. [Google Scholar]
- Bender, B.; Zhang, L. Negative affect, medication adherence, and asthma control in children. J. Allergy Clin. Immunol. 2008, 122, 490–495. [Google Scholar] [CrossRef]
- Makela, M.J.; Becker, V.; Hedegaard, M.; Larsson, K. Adherence to inhaled therapies health outcomes costs in patients with asthma and COPD. Respir. Med. 2013, 107, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Chongmelaxme, B.; Chayakunapruk, N.; Dilokthornsakul, P. Incorporating adherence in cost-effectiveness analysis of asthma: A systemic review. J. Med. Econ. 2019, 22, 554–566. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, C.E.; Sossa-Briceno, M.P.; Sinha, I.P. When adherence and inhalation technique matter: Difficult-to-control pediatric asthma in low-to-midlle income countries. Pediatr. Pulmonol. 2021, 56, 1366–1373. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Ghushchyan, V.; Navaratnam, P.; Friedman, H.S.; Kavati, A.; Ortiz, B.; Lanier, B. The national cost of asthma among school-aged children in the United States. Ann. Allergy Asthma Immunol. 2017, 119, 246–252. [Google Scholar] [CrossRef]
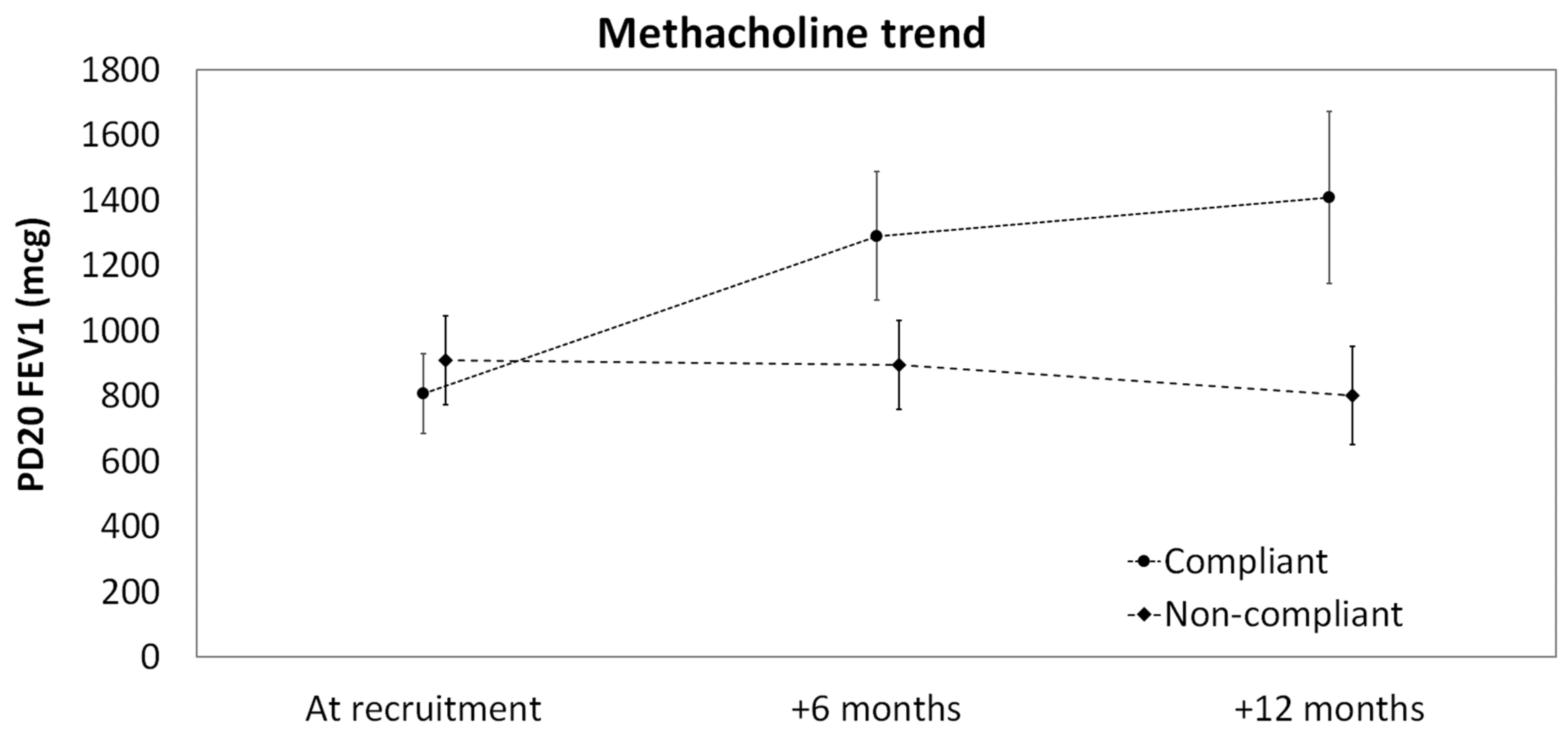
| Variables | Total (n = 106) | Compliant (n = 53) | Non-Compliant (n = 53) | p-Value |
|---|---|---|---|---|
| % male | 60.4% | 54.7% | 66.0% | 0.160 |
| Mean age (SD) | 15.9 (1.6) | 15.5 (1.7) | 16.2 (1.5) | 0.050 |
| Mean FEV1 (SD) | 85.8 (14.7) | 85.2 (15.5) | 87.4 (14.1) | 0.532 |
| Mean MMEF (SD) | 52.8 (18.7) | 51.4 (17.3) | 52.9 (19.4) | 0.745 |
| Mean MEF25 (SD) | 45.2 (19.1) | 44.8 (18.1) | 45.1 (19.5) | 0.953 |
| Mean PD20 FEV1 (SD) | 857.6 (482.9) | 807.1 (411.0) | 908.1 (544.7) | 0.358 |
| Predictors | Estimate (95% CI) |
|---|---|
| Compliant vs. non-compliant at baseline | −107.9 (−308.3 to 92.5) |
| Time effect (vs. at recruitment) in non-compliant patients | |
| +6 months post-treatment | −7.31 (−168.8 to 154.2) |
| +12 months post-treatment | −97.6 (−286.2 to 91.1) |
| Interaction: time effect (vs. at recruitment) in compliant patients | |
| +6 months post-treatment | 493.8 (260.8 to 726.9) |
| +12 months post-treatment | 710.2 (453.8 to 966.7) |
| Clinical Outcomes | At Recruitment | Δ 6 Months vs. at Recruitment | Δ 12 Months vs. at Recruitment | ||
|---|---|---|---|---|---|
| Compliant | Non-Compliant | Compliant | Non-Compliant | ||
| Exacerbation rate | 0.87 (0.85) | −0.47 (−0.76 to −0.18) | 0 (−0.29 to 0.29) | −0.66 (−0.92 to −0.40) | 0.13 (−0.19 to 0.46) |
| Antibiotics courses | 0.89 (0.87) | −0.53 (−0.78 to −0.27) | 0.04 (−0.24 to 0.31) | −0.75 (−0.99 to −0.52) | −0.11 (−0.42 to 0.19) |
| Systemic steroids courses | 0.84 (0.76) | −0.66 (−0.88 to −0.44) | −0.09 (−0.35 to 0.16) | −0.79 (−1.01 to −0.57) | −0.08 (−0.41 to 0.26) |
| GP visits | 1.44 (1.06) | −0.91 (−1.19 to −0.62) | 0.23 (−0.10 to 0.55) | −1.25 (−1.51 to −0.98) | 0.11 (−0.22 to 0.45) |
| Specialist visits | 1.58 (1.18) | −0.72 (−1.06 to −0.38) | 0.11 (−0.19 to 0.42) | −0.81 (−1.15 to −0.48) | 0.09 (−0.32 to 0.50) |
| Hospitalizations | 0.05 (0.21) | −0.04 (−0.14 to 0.07) | 0.08 (0.004 to 0.15) | - | - |
| Number of days off | 2.60 (2.18) | −2.15 (−2.69 to −1.61) | 0.23 (−0.27 to 0.72) | −2.34 (−2.94 to −1.74) | 0.02 (−0.67 to 0.71) |
| Cost Item (EUR) | At Recruitment | Δ 6 Months vs. at Recruitment | Δ 12 Months vs. at Recruitment | ||
|---|---|---|---|---|---|
| Compliant | Non-Compliant | Compliant | Non-Compliant | ||
| Drugs | 21.32 (17.75) | −13.64 (−18.9 to −8.4) | 0.14 (−5.1 to 5.3) | −18.60 (−23.4 to −13.8) | −2.54 (−8.5 to 3.4) |
| Visits and hospitalization | 128.94 (298.47) | −86.62 (−236 to 62.3) | 113.21 (11.9 to 215) | −175.2 (−287 to −63.9) | 4.29 (−6.4 to 15.0) |
| Indirect | 213.9 (178.79) | −176.70 (−221 to −132) | 18.6 (−22.0 to 59.2) | −192.20 (−241 to −143) | 1.55 (−55.1 to 58.2) |
| Total | 364.15 (385.81) | −276.96 (−443 to −111) | 131.95 (18.0 to 246) | −386.03 (−520 to −252) | 3.30 (−55.7 to 62.3) |
| Clinical Outcome | Estimate (95% CI) |
|---|---|
| Exacerbation rate | −0.36 (−0.66 to −0.07) |
| Antibiotics courses | −0.31 (−0.49 to −0.12) |
| Systemic steroids courses | −0.24 (−0.46 to −0.01) |
| GP visits | −0.73 (−1.23 to −0.23) |
| Specialist visits | −0.15 (−0.39 to 0.10) |
| Hospitalizations | −0.05 (−0.08 to −0.02) |
| Number of days off | −1.02 (−1.91 to −0.12) |
| Clinical Outcome | PD20 FEV1 Strata | Estimate (95% CI) |
|---|---|---|
| Exacerbation rate | 400–800 vs. <400 mcg | −0.43 (−0.78 to −0.09) |
| >800 vs. <400 mcg | −0.53 (−0.81 to −0.24) | |
| Antibiotics courses | 400–800 vs. <400 mcg | −0.19 (−0.51 to 0.13) |
| >800 vs. <400 mcg | −0.34 (−0.58 to −0.10) | |
| Systemic steroids courses | 400–800 vs. <400 mcg | −0.29 (−0.66 to 0.08) |
| >800 vs. <400 mcg | −0.24 (−0.53 to 0.06) | |
| GP visits | 400–800 vs. <400 mcg | −0.50 (−0.97 to −0.02) |
| >800 vs. <400 mcg | −0.68 (−1.09 to −0.26) | |
| Specialist visits | 400–800 vs. <400 mcg | 0.00 (−0.47 to 0.47) |
| >800 vs. <400 mcg | −0.20 (−0.53 to 0.13) | |
| Hospitalizations | 400–800 vs. <400 mcg | −0.07 (−0.16 to 0.02) |
| >800 vs. <400 mcg | −0.06 (−0.14 to 0.02) | |
| Number of days off | 400–800 vs. <400 mcg | −0.25 (−1.14 to 0.64) |
| >800 vs. <400 mcg | −1.02 (−1.72 to −0.32)) |
| Model 1 | Estimate (95% CI) |
| Log10 PD20 FEV1 | −184.90 (−305.89 to −63.90) |
| Model 2 | Estimate (95% CI) |
| PD20 FEV1 400–800 vs. <400 mcg | −141.67 (−305.74 to 22.40) |
| PD20 FEV1 > 800 vs. <400 mcg | −208.55 (−658.48 to −58.61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Negro, R.W.; Turco, P.; Povero, M. Trend of Bronchial Hyperresponsiveness to Methacholine as a Cost Predictor of Mild-to-Moderate Asthma: A Twelve-Month Survey in Teenagers. Children 2023, 10, 1876. https://doi.org/10.3390/children10121876
Dal Negro RW, Turco P, Povero M. Trend of Bronchial Hyperresponsiveness to Methacholine as a Cost Predictor of Mild-to-Moderate Asthma: A Twelve-Month Survey in Teenagers. Children. 2023; 10(12):1876. https://doi.org/10.3390/children10121876
Chicago/Turabian StyleDal Negro, Roberto W., Paola Turco, and Massimiliano Povero. 2023. "Trend of Bronchial Hyperresponsiveness to Methacholine as a Cost Predictor of Mild-to-Moderate Asthma: A Twelve-Month Survey in Teenagers" Children 10, no. 12: 1876. https://doi.org/10.3390/children10121876
APA StyleDal Negro, R. W., Turco, P., & Povero, M. (2023). Trend of Bronchial Hyperresponsiveness to Methacholine as a Cost Predictor of Mild-to-Moderate Asthma: A Twelve-Month Survey in Teenagers. Children, 10(12), 1876. https://doi.org/10.3390/children10121876






