Use of the Subcutaneous Triptorelin Stimulation Test for Diagnosis of Central Precocious Puberty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurements
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carel, J.C.; Léger, J. Clinical practice. Precocious puberty. N. Engl. J. Med. 2008, 358, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Bangalore Krishna, K.; Fuqua, J.S.; Rogol, A.D.; Klein, K.O.; Popovic, J.; Houk, C.P.; Charmandari, E.; Lee, P.A.; Freire, A.V.; Ropelato, M.G.; et al. Use of Gonadotropin-Releasing Hormone Analogs in Children: Update by an International Consortium. Horm. Res. Paediatr. 2019, 91, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Su, Z.; Pan, L.; Wang, L.; Xu, Z.; Peng, G.; Li, X. Factors affecting bone maturation in Chinese girls aged 4–8 years with isolated premature thelarche. BMC Pediatr. 2020, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, V.; Baronio, F.; Ortolano, R.; Maltoni, G.; Zucchini, S.; Di Natale, V.; Cassio, A. Transient central precocious puberty: A new entity among the spectrum of precocious puberty? Ital. J. Pediatr. 2021, 47, 210. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Busch, A.S.; Eckert-Lind, C.; Koch, T.; Hickey, M.; Juul, A. Trends in the Incidence of Central Precocious Puberty and Normal Variant Puberty Among Children in Denmark, 1998 to 2017. JAMA Netw. Open 2020, 3, e2015665. [Google Scholar] [CrossRef] [PubMed]
- Partsch, C.J.; Sippell, W.G. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum. Reprod. Update 2001, 7, 292–302. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, K.; Won, S.; Lee, K.W.; Park, M.J. A Significant Increase in the Incidence of Central Precocious Puberty among Korean Girls from 2004 to 2010. PLoS ONE 2015, 10, e0141844. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kwon, A.; Jung, M.K.; Kim, K.E.; Suh, J.; Chae, H.W.; Kim, D.H.; Ha, S.; Seo, G.H.; Kim, H.S. Incidence and Prevalence of Central Precocious Puberty in Korea: An Epidemiologic Study Based on a National Database. J. Pediatr. 2019, 208, 221–228. [Google Scholar] [CrossRef]
- Prosperi, S.; Chiarelli, F. Early and precocious puberty during the COVID-19 pandemic. Front. Endocrinol. 2022, 13, 1107911. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Sartori, C.; Catellani, C.; Righi, B. Precocious Puberty and Covid-19 Into Perspective: Potential Increased Frequency, Possible Causes, and a Potential Emergency to Be Addressed. Front. Pediatr. 2021, 9, 734899. [Google Scholar] [CrossRef]
- Poomthavorn, P.; Khlairit, P.; Mahachoklertwattana, P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm. Res. 2009, 72, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.V.; Escobar, M.E.; Gryngarten, M.G.; Arcari, A.J.; Ballerini, M.G.; Bergadá, I.; Ropelato, M.G. High diagnostic accuracy of subcutaneous Triptorelin test compared with GnRH test for diagnosing central precocious puberty in girls. Clin. Endocrinol. 2013, 78, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, R.; Milenkovic, T.; Soldatovic, I.; Pekic, S.; Mitrovic, K.; Todorovic, S. Triptorelin stimulated luteinizing hormone concentrations for diagnosing central precocious puberty: Study of diagnostic accuracy. Endocrine 2022, 75, 934–941. [Google Scholar] [CrossRef]
- Greulich, W.W.; Pyle, S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist, 2nd ed.; Stanford University Press: Stanford, CA, USA, 1959; pp. xvi, 256p. [Google Scholar]
- Kim, J.H.; Yun, S.; Hwang, S.S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean National Growth Charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatr. 2018, 61, 135–149. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Jansen, R.P.; Boylan, L.M.; Spaliviero, J.A.; Turtle, J.R. Pharmacokinetics of gonadotropin-releasing hormone: Comparison of subcutaneous and intravenous routes. J. Clin. Endocrinol. Metab. 1984, 59, 739–746. [Google Scholar] [CrossRef]
- Lahlou, N.; Carel, J.C.; Chaussain, J.L.; Roger, M. Pharmacokinetics and pharmacodynamics of GnRH agonists: Clinical implications in pediatrics. J. Pediatr. Endocrinol. Metab. 2000, 13 (Suppl. 1), 723–737. [Google Scholar] [CrossRef] [PubMed]
- Carel, J.C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R.; on behalf of the members of the ESPE-LWPES GnRH Analogs Consensus Conference Group. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef]
- Fuqua, J.S. Treatment and outcomes of precocious puberty: An update. J. Clin. Endocrinol. Metab. 2013, 98, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, G.; Antoniazzi, F.; Tatò, L. Use of the gonadotropin-releasing hormone agonist triptorelin in the diagnosis of delayed puberty in boys. J. Pediatr. 1995, 126 Pt 1, 756–758. [Google Scholar] [CrossRef]
- Mason-Garcia, M.; Vigh, S.; Comaru-Schally, A.M.; Redding, T.W.; Somogyvari-Vigh, A.; Horvath, J.; Schally, A.V. Radioimmunoassay for 6-D-tryptophan analog of luteinizing hormone-releasing hormone: Measurement of serum levels after administration of long-acting microcapsule formulations. Proc. Natl. Acad. Sci. USA 1985, 82, 1547–1551. [Google Scholar] [CrossRef]
- Stanhope, R.; Brook, C.C. Thelarche variant: A new syndrome of precocious sexual maturation? Acta Endocrinol. 1990, 123, 481–486. [Google Scholar] [CrossRef] [PubMed]
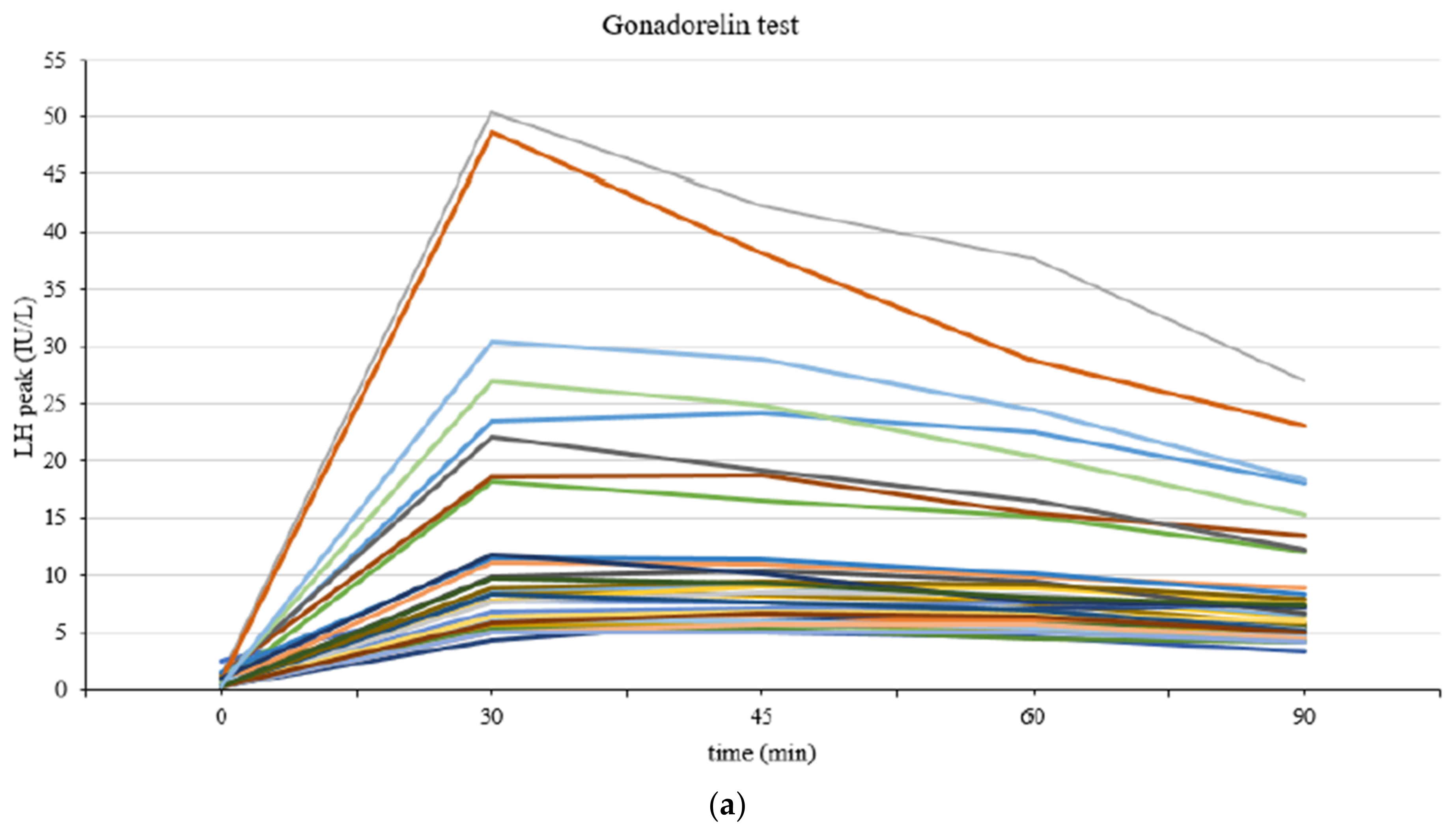
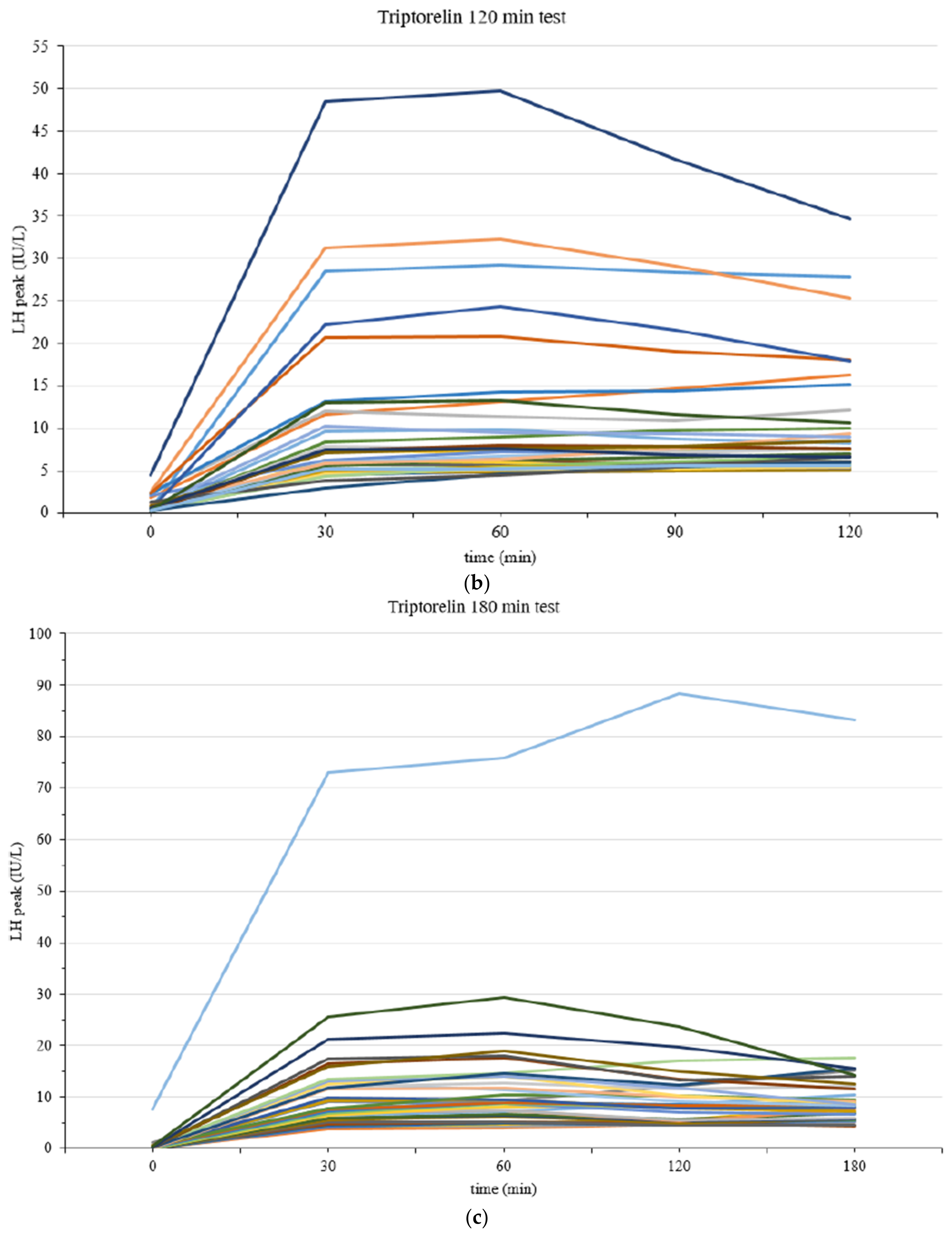
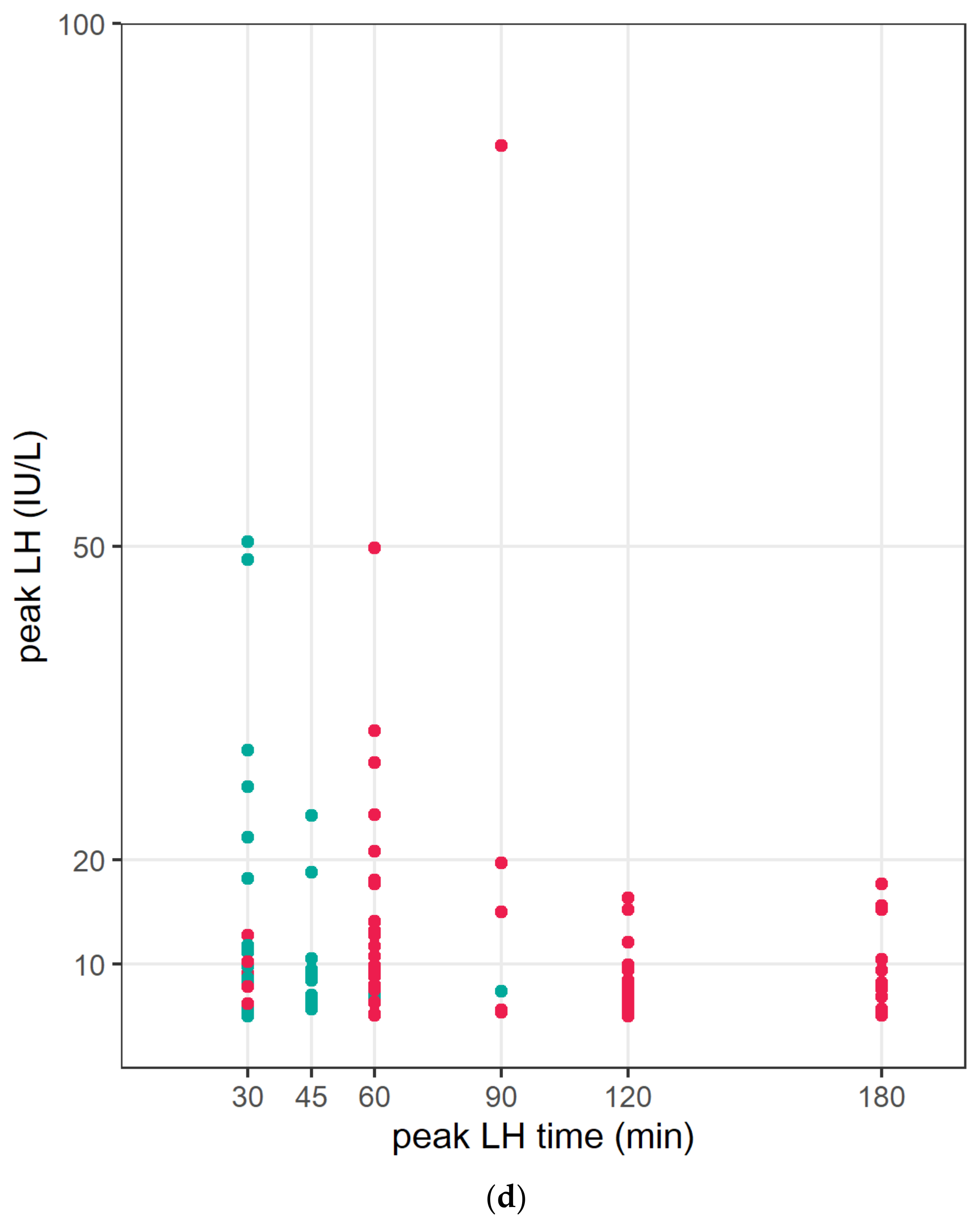

| CPP (n = 111) | IPT (n = 109) | p-Value | |
|---|---|---|---|
| Age (years) | 8.11 ± 0.73 | 8.06 ± 0.63 | 0.608 |
| Tanner stage | 2.28 ± 0.45 | 2.19 ± 0.39 | 0.098 |
| II | 79 | 88 | - |
| III | 32 | 21 | |
| BA–CA (years) | 2.16 ± 0.74 | 1.96 ± 0.67 | 0.039 * |
| Height SDS | 0.58 ± 0.92 | 0.75 ± 0.93 | 0.189 |
| BMI SDS | 0.51 ± 1.43 | 0.97 ± 1.30 | 0.014 * |
| Basal LH (IU/L) | 0.63 ± 0.93 | 0.25 ± 0.13 | <0.001 * |
| Basal FSH (IU/L) | 2.93 ± 1.43 | 1.69 ± 0.72 | <0.001 * |
| Peak LH (IU/L) | 12.13 ± 11.44 | 3.12 ± 1.05 | <0.001 * |
| Peak FSH (IU/L) | 16.94 ± 5.76 | 13.84 ± 4.52 | <0.001 * |
| Basal LH/FSH (IU/L) | 0.22 ± 0.28 | 0.19 ± 0.18 | 0.4121 |
| Peak LH/FSH (IU/L) | 0.76 ± 0.63 | 0.23 ± 0.09 | <0.001 * |
| Triptorelin Test | Gonadorelin Test | |||||
|---|---|---|---|---|---|---|
| CPP (n = 74) | IPT (n = 72) | p-Value | CPP (n = 37) | IPT (n = 37) | p-Value | |
| Age (years) | 8.11 ± 0.78 | 8.00 ± 0.56 | 0.477 | 8.11 ± 0.61 | 8.08 ± 0.73 | 0.880 |
| Tanner stage | 2.29 ± 0.46 | 2.00 ± 0.00 | <0.001 * | 2.27 ± 0.45 | 2.18 ± 0.39 | 0.414 |
| II | 52 | 58 | - | 27 | 31 | - |
| III | 22 | 14 | 10 | 6 | ||
| BA—CA (years) | 2.09 ± 0.77 | 1.78 ± 0.56 | 0.033 * | 2.30 ± 0.66 | 2.15 ± 0.72 | 0.352 |
| Height SDS | 0.74 ± 0.90 | 0.79 ± 0.97 | 0.721 | 0.27 ± 0.90 | 0.66 ± 0.87 | 0.064 |
| BMI SDS | 0.39 ± 1.59 | 0.85 ± 1.37 | 0.068 | 0.76 ± 1.01 | 1.22 ± 1.11 | 0.070 |
| Basal LH (IU/L) | 0.63 ± 1.09 | 0.20 ± 0.09 | <0.005 * | 0.62 ± 0.47 | 0.35 ± 0.16 | <0.005 * |
| Basal FSH (IU/L) | 2.93 ± 1.47 | 1.80 ± 0.73 | <0.001 * | 2.92 ± 1.36 | 1.46 ± 0.64 | <0.001 * |
| Peak LH (IU/L) | 11.94 ± 11.72 | 3.01 ± 1.02 | <0.001 * | 13.95 ± 13.44 | 3.33 ± 1.08 | <0.001 * |
| Peak FSH (IU/L) | 17.30 ± 5.82 | 14.06 ± 3.99 | <0.001 * | 16.22 ± 5.65 | 13.38 ± 5.53 | 0.042 * |
| Basal LH/FSH/ (IU/L) | 0.19 ± 0.24 | 0.13 ± 0.08 | 0.036 * | 0.27 ± 0.35 | 0.32 ± 0.25 | 0.521 |
| Peak LH/FSH (IU/L) | 0.73 ± 0.60 | 0.21 ± 0.08 | <0.001 * | 0.84 ± 0.69 | 0.25 ± 0.11 | <0.001 * |
| Blood Sampling Time | Gonadorelin | Triptorelin 120 min | Triptorelin 180 min |
|---|---|---|---|
| 0 min | 0 (0.0) | 0 (0.0) | |
| 30 min | 18 (48.7) | 3(8.1) | 3 (8.1) |
| 45 min | 17 (45.9) | - | |
| 60 min | 2 (5.4) | 10 (27.0) | 18 (48.7) |
| 90 min | 0 (0.0) | 2 (5.4) | - |
| 120 min | - | 22 (59.5) | 3 (8.1) |
| 180 min | - | 13 (35.1) |
| <120 Min (n = 38) | ≥120 Min (n = 36) | p-Value | |
|---|---|---|---|
| Age (years) | 8.22 ± 0.60 | 7.98 ± 0.93 | 0.188 |
| Tanner stage | 2.35 ± 0.48 | 2.22 ± 0.42 | 0.223 |
| II | 25 | 27 | |
| III | 14 | 8 | |
| BA–CA (years) | 1.97 ± 0.78 | 2.22 ± 0.75 | 0.174 |
| Height SDS | 0.64 ± 0.90 | 0.85 ± 0.89 | 0.307 |
| BMI SDS | 0.63 ± 0.91 | 0.16 ± 1.06 | 0.227 |
| Basal LH (IU/L) | 0.51 ± 0.82 | 0.75 ± 1.32 | 0.342 |
| Basal FSH (IU/L) | 2.74 ± 1.21 | 3.12 ± 1.69 | 0.267 |
| Peak LH (IU/L) | 13.34 ± 9.29 | 10.47 ± 13.81 | 0.295 |
| Peak FSH (IU/L) | 18.15 ± 5.88 | 16.40 ± 5.70 | 0.196 |
| Basal LH/FSH (IU/L) | 0.17 ± 0.15 | 0.21 ± 0.30 | 0.436 |
| Peak LH/FSH (IU/L) | 0.76 ± 0.45 | 0.69 ± 0.73 | 0.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Lee, Y.; Gim, S.; Jeong, H. Use of the Subcutaneous Triptorelin Stimulation Test for Diagnosis of Central Precocious Puberty. Children 2023, 10, 1830. https://doi.org/10.3390/children10111830
Ahn J, Lee Y, Gim S, Jeong H. Use of the Subcutaneous Triptorelin Stimulation Test for Diagnosis of Central Precocious Puberty. Children. 2023; 10(11):1830. https://doi.org/10.3390/children10111830
Chicago/Turabian StyleAhn, Jungmin, Youngin Lee, Seongmin Gim, and Hwalrim Jeong. 2023. "Use of the Subcutaneous Triptorelin Stimulation Test for Diagnosis of Central Precocious Puberty" Children 10, no. 11: 1830. https://doi.org/10.3390/children10111830
APA StyleAhn, J., Lee, Y., Gim, S., & Jeong, H. (2023). Use of the Subcutaneous Triptorelin Stimulation Test for Diagnosis of Central Precocious Puberty. Children, 10(11), 1830. https://doi.org/10.3390/children10111830







