Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy
Abstract
1. Introduction
1.1. Precocious Puberty
- -
- central or true precocious puberty (CPP), if it is determined by early activation of the HPG axis with the production of gonadotropins;
- -
- peripheral or precocious pseudopuberty (PPP), unrelated to the production of gonadotropins.
1.2. Central Precocious Puberty
1.3. Peripherical Precocious Puberty
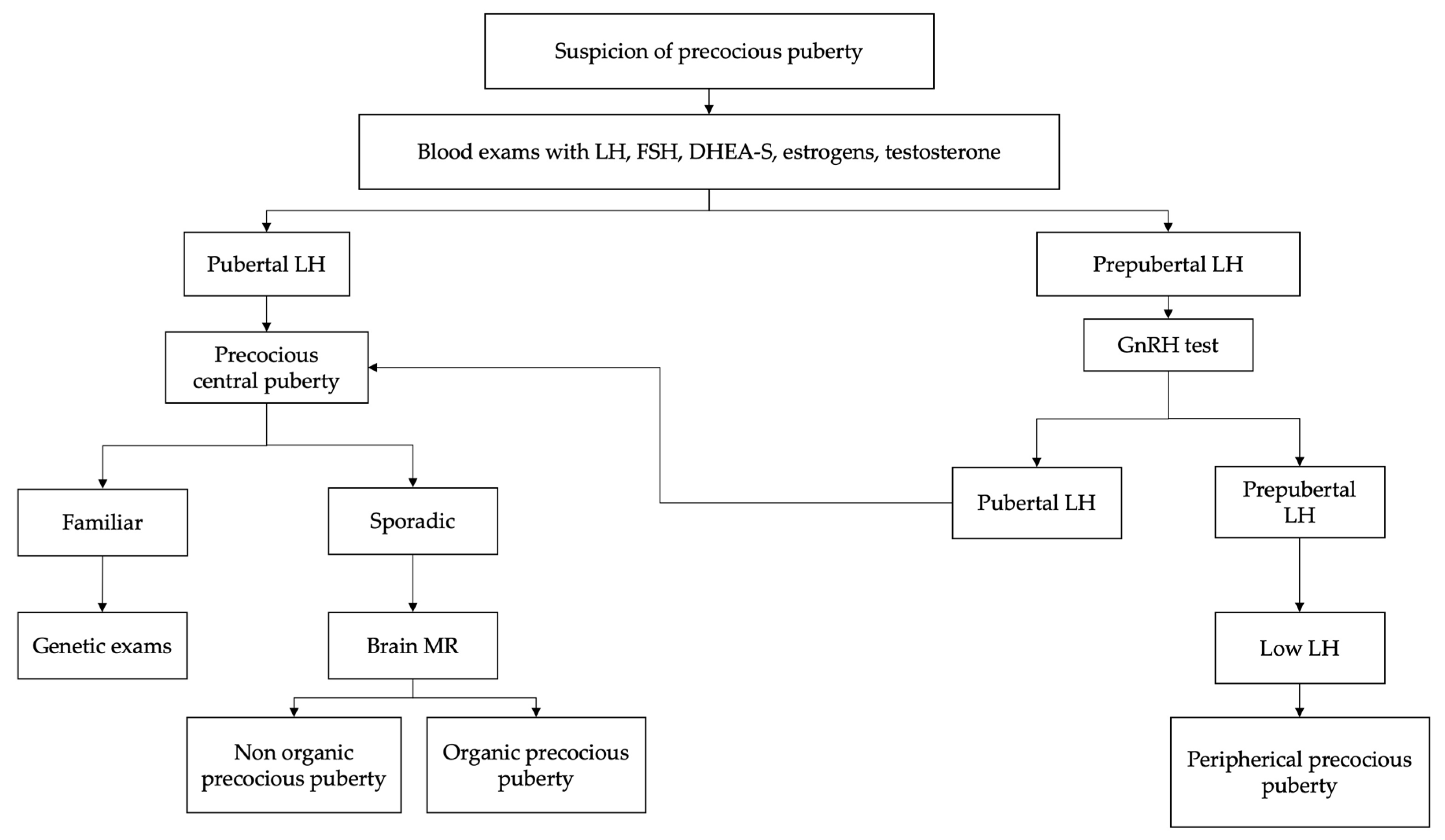
2. Diagnosis
3. Treatment
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Eugster, E.A. Treatment of Central Precocious Puberty. J. Endocr. Soc. 2019, 3, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Lora, A.L.; Torres-Tamayo, M.; Zurita-Cruz, J.N.; Aguilar-Herrera, B.E.; Calzada-León, R.; Rivera-Hernández, A.J.; Morales-Pérez, M.A.; Padrón-Martínez, M.M.; Ruiz-Reyes, M.L.; García-Morales, L.M.; et al. Diagnosis of precocious puberty: Clinical guideline for the diagnosis and treatment of precocious puberty. Bol. Med. Hosp. Infant. Mex. 2020, 77, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, R.S.; Eugster, E.A. Central precocious puberty: From genetics to treatment. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Loomba-Albrecht, L.A.; Styne, D.M. The Physiology of Puberty and its Disorders. Pediatr. Ann. 2012, 41, e1–e9. [Google Scholar] [CrossRef]
- Wood, C.L.; Lane, L.C.; Cheetham, T. Puberty: Normal physiology (brief overview). Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101265. [Google Scholar] [CrossRef]
- Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testicular growth and development in puberty. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 215–224. [Google Scholar] [CrossRef]
- Latronico, A.C.; Brito, V.N.; Carel, J.-C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Klein, D.A.; Emerick, J.E.; Sylvester, J.; Vogt, K.S. Disorders of Puberty: An Approach to Diagnosis and Management. Am. Fam. Physician 2017, 96, 590–599. [Google Scholar]
- Brito, V.N.; Canton, A.P.M.; Seraphim, C.E.; Abreu, A.P.; Macedo, D.B.; Mendonca, B.B.; Kaiser, U.B.; Argente, J.; Latronico, A.C. The Congenital and Acquired Mechanisms Implicated in the Etiology of Central Precocious Puberty. Endocr. Rev. 2023, 44, 193–221. [Google Scholar] [CrossRef]
- Brito, V.N.; Spinola-Castro, A.M.; Kochi, C.; Kopacek, C.; da Silva, P.C.A.; Guerra-Júnior, G. Central precocious puberty: Revisiting the diagnosis and therapeutic management. Arch. Endocrinol. Metab. 2016, 60, 163–172. [Google Scholar] [CrossRef]
- Sultan, C.; Gaspari, L.; Maimoun, L.; Kalfa, N.; Paris, F. Disorders of puberty. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 62–89. [Google Scholar] [CrossRef] [PubMed]
- Manotas, M.C.; González, D.M.; Céspedes, C.; Forero, C.; Moreno, A.P.R. Genetic and Epigenetic Control of Puberty. Sex. Dev. 2022, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Nenna, R.; Petrarca, L.; Tarani, F.; Paparella, R.; Mancino, E.; Di Mattia, G.; Conti, M.G.; Matera, L.; Bonci, E.; et al. Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study. Diagnostics 2022, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, S.; Chiarelli, F. Early and precocious puberty during the COVID-19 pandemic. Front. Endocrinol. 2023, 13, 1107911. [Google Scholar] [CrossRef] [PubMed]
- Chioma, L.; Bizzarri, C.; Verzani, M.; Fava, D.; Salerno, M.; Capalbo, D.; Guzzetti, C.; Penta, L.; Di Luigi, L.; di Iorgi, N.; et al. Sedentary lifestyle and precocious puberty in girls during the COVID-19 pandemic: An Italian experience. Endocr. Connect. 2022, 11, e210650. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, M.I. Psychosocial Aspects in Girls with Idiopathic Precocious Puberty. Psychiatry Investig. 2012, 9, 25–28. [Google Scholar] [CrossRef]
- Tremblay, L.; Frigon, J.-Y. Precocious Puberty in Adolescent Girls: A Biomarker of Later Psychosocial Adjustment Problems. Child Psychiatry Hum. Dev. 2005, 36, 73–94. [Google Scholar] [CrossRef]
- de Oliveira Neto, C.P.; de Sousa Azulay, R.S.; de Almeida, A.G.F.P.; Tavares, M.d.G.R.; Vaz, L.H.G.; Leal, I.R.L.; Gama, M.E.A.; Ribeiro, M.R.C.; Nascimento, G.C.; Magalhães, M.; et al. Differences in Puberty of Girls before and during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 4733. [Google Scholar] [CrossRef]
- Shim, Y.S.; Lee, H.S.; Hwang, J.S. Genetic factors in precocious puberty. Clin. Exp. Pediatr. 2022, 65, 172–181. [Google Scholar] [CrossRef]
- Tauber, M.; Hoybye, C. Endocrine disorders in Prader-Willi syndrome: A model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021, 9, 235–246. [Google Scholar] [CrossRef]
- Tinano, F.R.; Canton, A.P.M.; Montenegro, L.R.; de Castro Leal, A.; Faria, A.G.; Seraphim, C.E.; Brauner, R.; Jorge, A.A.; Mendonca, B.B.; Argente, J.; et al. Clinical and Genetic Characterization of Familial Central Precocious Puberty. J. Clin. Endocrinol. Metab. 2023, 108, 1758–1767. [Google Scholar] [CrossRef]
- Tajima, T. Genetic causes of central precocious puberty. Clin. Pediatr. Endocrinol. 2022, 31, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Carlomagno, F.; Cangiano, B.; Kanakis, G.; Pozza, C.; Sbardella, E.; Isidori, A.M.; Krausz, C.; Gianfrilli, D. Somatotropic-Testicular Axis: A crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology 2021, 9, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Q.; Li, G.-M.; Li, Y. Advanced bone age as an indicator facilitates the diagnosis of precocious puberty. J. Pediatr. 2018, 94, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; Mohn, A.; Chiarelli, F.; Giannini, C. Evaluation of Bone Age in Children: A Mini-Review. Front. Pediatr. 2021, 9, 580314. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Tadokoro-Cuccaro, R.; Hannema, S.E.; Acerini, C.L.; Hughes, I.A. Frequency of gonadal tumours in complete androgen insensitivity syndrome (CAIS): A retrospective case-series analysis. J. Pediatr. Urol. 2017, 13, 498.e1–498.e6. [Google Scholar] [CrossRef]
- Ahmed, M.L.; Ong, K.K.; Dunger, D.B. Childhood obesity and the timing of puberty. Trends Endocrinol. Metab. 2009, 20, 237–242. [Google Scholar] [CrossRef]
- Silventoinen, K.; Jelenkovic, A.; Palviainen, T.; Dunkel, L.; Kaprio, J. The Association Between Puberty Timing and Body Mass Index in a Longitudinal Setting: The Contribution of Genetic Factors. Behav. Genet. 2022, 52, 186–194. [Google Scholar] [CrossRef]
- Haddad, N.G.; Eugster, E.A. Peripheral precocious puberty including congenital adrenal hyperplasia: Causes, consequences, management and outcomes. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101273. [Google Scholar] [CrossRef]
- Tarani, L.; Rasio, D.; Tarani, F.; Parlapiano, G.; Valentini, D.; Dylag, K.A.; Spalice, A.; Paparella, R.; Fiore, M. Pediatrics for Disability: A Comprehensive Approach to Children with Syndromic Psychomotor Delay. Curr. Pediatr. Rev. 2021, 18, 110–120. [Google Scholar] [CrossRef]
- Jørgensen, A.; Rajpert-De Meyts, E. Regulation of meiotic entry and gonadal sex differentiation in the human: Normal and disrupted signaling. Biomol. Concepts 2014, 5, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Buttterfield, D.A. Unraveling the complexity of neurodegeneration in brains of subjects with Down syndrome: Insights from proteomics. Proteom. Clin. Appl. 2014, 8, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Profeta, G.; Micangeli, G.; Tarani, F.; Paparella, R.; Ferraguti, G.; Spaziani, M.; Isidori, A.M.; Menghi, M.; Ceccanti, M.; Fiore, M.; et al. Sexual Developmental Disorders in Pediatrics. Clin. Ter. 2022, 173, 475–488. [Google Scholar] [CrossRef]
- Yu, T.; Yu, Y.; Li, X.; Xue, P.; Yu, X.; Chen, Y.; Kong, H.; Lin, C.; Wang, X.; Mei, H.; et al. Effects of childhood obesity and related genetic factors on precocious puberty: Protocol for a multi-center prospective cohort study. BMC Pediatr. 2022, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Lee, P.A. Endocrinology of male puberty. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 5–9. [Google Scholar] [CrossRef]
- Korkmaz, O.; Sari, G.; Mecidov, I.; Ozen, S.; Goksen, D.; Darcan, S. The Gonadotropin-Releasing Hormone Analogue Therapy May Not Impact Final Height in Precocious Puberty of Girls With Onset of Puberty Aged 6–8 Years. J. Clin. Med. Res. 2019, 11, 133–136. [Google Scholar] [CrossRef]
- Macedo, D.B.; Cukier, P.; Mendonca, B.B.; Latronico, A.C.; Brito, V.N. Advances in the etiology, diagnosis and treatment of central precocious puberty. Arq. Bras. Endocrinol. Metabol. 2014, 58, 108–117. [Google Scholar] [CrossRef]
- Liimatta, J.; Utriainen, P.; Voutilainen, R.; Jääskeläinen, J. Girls with a History of Premature Adrenarche Have Advanced Growth and Pubertal Development at the Age of 12 Years. Front. Endocrinol. 2017, 8, 291. [Google Scholar] [CrossRef]
- Novello, L.; Speiser, P.W. Premature Adrenarche. Pediatr. Ann. 2018, 47, E7–E11. [Google Scholar] [CrossRef]
- Wirth, T. Fibröse Dysplasie. Orthopade 2020, 49, 929–940. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Soto, J.; Pereira, A.; Busch, A.S.; Almstrup, K.; Corvalan, C.; Iñiguez, G.; Juul, A.; Mericq, V. Reproductive hormones during pubertal transition in girls with transient Thelarche. Clin. Endocrinol. 2020, 93, 296–304. [Google Scholar] [CrossRef]
- Balzer, B.W.R.; Garden, F.L.; Amatoury, M.; Luscombe, G.M.; Paxton, K.; Hawke, C.I.; Handelsman, D.J.; Steinbeck, K.S. Self-rated Tanner stage and subjective measures of puberty are associated with longitudinal gonadal hormone changes. J. Pediatr. Endocrinol. Metab. 2019, 32, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, T.H.; Main, K.M.; Ljubicic, M.L.; Jensen, T.K.; Andersen, H.R.; Andersen, M.S.; Petersen, J.H.; Andersson, A.-M.; Juul, A. Sex Differences in Reproductive Hormones During Mini-Puberty in Infants With Normal and Disordered Sex Development. J. Clin. Endocrinol. Metab. 2018, 103, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Walia, R.; Bhansali, A.; Dayal, D.; Sachdeva, N.; Singh, T.; Bhadada, S.K. FSH-stimulated Inhibin B (FSH-iB): A Novel Marker for the Accurate Prediction of Pubertal Outcome in Delayed Puberty. J. Clin. Endocrinol. Metab. 2021, 106, e3495–e3505. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Argente, J. Central precocious puberty: Epidemiology, etiology, diagnosis and treatment. An. Pediatr. 2011, 74, 336.e1–336.e13. [Google Scholar] [CrossRef]
- Tomlinson, C.; Macintyre, H.; Dorrian, C.A.; Ahmed, S.F.; Wallace, A.M. Testosterone measurements in early infancy. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F558–F559. [Google Scholar] [CrossRef]
- Alotaibi, M.F. Physiology of puberty in boys and girls and pathological disorders affecting its onset. J. Adolesc. 2019, 71, 63–71. [Google Scholar] [CrossRef]
- Muerköster, A.-P.; Frederiksen, H.; Juul, A.; Andersson, A.-M.; Jensen, R.C.; Glintborg, D.; Kyhl, H.B.; Andersen, M.S.; Timmermann, C.A.G.; Jensen, T.K. Maternal phthalate exposure associated with decreased testosterone/LH ratio in male offspring during mini-puberty. Odense Child Cohort. Environ. Int. 2020, 144, 106025. [Google Scholar] [CrossRef]
- DiVall, S.A.; Radovick, S. Endocrinology of female puberty. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 1–4. [Google Scholar] [CrossRef]
- Willemsen, R.H.; Elleri, D.; Williams, R.M.; Ong, K.K.; Dunger, D.B. Pros and cons of GnRHa treatment for early puberty in girls. Nat. Rev. Endocrinol. 2014, 10, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Cheuiche, A.V.; da Silveira, L.G.; de Paula, L.C.P.; Lucena, I.R.S.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Razzaghy-Azar, M.; Ghasemi, F.; Hallaji, F.; Ghasemi, A.; Ghasemi, M. Sonographic measurement of uterus and ovaries in premenarcheal healthy girls between 6 and 13 years old: Correlation with age and pubertal status. J. Clin. Ultrasound 2011, 39, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Haber, H.P.; Wollmann, H.A.; Ranke, M.B. Pelvic ultrasonography: Early differentiation between isolated premature thelarche and central precocious puberty. Eur. J. Pediatr. 1995, 154, 182–186. [Google Scholar] [CrossRef]
- Badouraki, M.; Christoforidis, A.; Economou, I.; Dimitriadis, A.S.; Katzos, G. Evaluation of pelvic ultrasonography in the diagnosis and differentiation of various forms of sexual precocity in girls. Ultrasound Obstet. Gynecol. 2008, 32, 819–827. [Google Scholar] [CrossRef]
- Yu, J.; Shin, H.Y.; Lee, S.H.; Kim, Y.S.; Kim, J.H. Usefulness of pelvic ultrasonography for the diagnosis of central precocious puberty in girls. Korean J. Pediatr. 2015, 58, 294–300. [Google Scholar] [CrossRef][Green Version]
- Messina, M.P.; Piccioni, M.G.; Petrella, C.; Vitali, M.; Greco, A.; Ralli, M.; Ceccanti, M.; Ferraguti, G.; Neri, I.; Ricchi, A.; et al. Advanced midwifery practice: Intrapartum ultrasonography to assess fetal head station and comparison with vaginal digital examination. Minerva Obstet. Gynecol. 2021, 73, 253–260. [Google Scholar] [CrossRef]
- Spaziani, M.; Lecis, C.; Tarantino, C.; Sbardella, E.; Pozza, C.; Gianfrilli, D. The role of scrotal ultrasonography from infancy to puberty. Andrology 2021, 9, 1306–1321. [Google Scholar] [CrossRef]
- Oehme, N.H.B.; Roelants, M.; Bruserud, I.S.; Eide, G.E.; Bjerknes, R.; Rosendahl, K.; Júlíusson, P.B. Ultrasound-based measurements of testicular volume in 6- to 16-year-old boys—Intra- and interobserver agreement and comparison with Prader orchidometry. Pediatr. Radiol. 2018, 48, 1771–1778. [Google Scholar] [CrossRef]
- Pozza, C.; Kanakis, G.; Carlomagno, F.; Lemma, A.; Pofi, R.; Tenuta, M.; Minnetti, M.; Tarsitano, M.; Sesti, F.; Paoli, D.; et al. Testicular ultrasound score: A new proposal for a scoring system to predict testicular function. Andrology 2020, 8, 1051–1063. [Google Scholar] [CrossRef]
- Chadha, N.K.; Forte, V. Pediatric head and neck malignancies. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.; McHugh, K. The role of radiology in head and neck tumours in children. Cancer Imaging 2010, 10, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983. [Google Scholar] [CrossRef] [PubMed]
- Bulcao Macedo, D.; Nahime Brito, V.; Latronico, A.C. New Causes of Central Precocious Puberty: The Role of Genetic Factors. Neuroendocrinology 2014, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dorn, L.D. Psychological and Social Problems in Children with Premature Adrenarche and Precocious Puberty. Curr. Clin. Neurol. 2007, 309–327. [Google Scholar] [CrossRef]
- Klein, K.O.; Lee, P.A. Gonadotropin-Releasing Hormone (GnRHa) Therapy for Central Precocious Puberty (CPP): Review of Nuances in Assessment of Height, Hormonal Suppression, Psychosocial Issues, and Weight Gain, with Patient Examples. Pediatr. Endocrinol. Rev. 2018, 15, 298–312. [Google Scholar] [CrossRef]
- Censani, M.; Feuer, A.; Orton, S.; Askin, G.; Vogiatzi, M. Changes in body mass index in children on gonadotropin-releasing hormone agonist therapy with precocious puberty, early puberty or short stature. J. Pediatr. Endocrinol. Metab. 2019, 32, 1065–1070. [Google Scholar] [CrossRef]
- Song, W.; Zhao, F.; Liang, S.; Li, G.; Xue, J. Is a Combination of a GnRH Agonist and Recombinant Growth Hormone an Effective Treatment to Increase the Final Adult Height of Girls with Precocious or Early Puberty? Int. J. Endocrinol. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Cantas-Orsdemir, S.; Eugster, E.A. Update on central precocious puberty: From etiologies to outcomes. Expert Rev. Endocrinol. Metab. 2019, 14, 123–130. [Google Scholar] [CrossRef]
- Partsch, C.-J.; Sippell, W.G. Treatment of central precocious puberty. Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 165–189. [Google Scholar] [CrossRef]
- Głąb, E.; Wikiera, B.; Bieniasz, J.; Barg, E. The Influence of GnRH Analog Therapy on Growth in Central Precocious Puberty. Adv. Clin. Exp. Med. 2016, 25, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Głab, E.; Barg, E.; Wikiera, B.; Grabowski, M.; Noczyńska, A. Influence of GnRH analog therapy on body mass in central precocious puberty. Pediatr. Endocrinol. Diabetes Metab. 2009, 15, 7–11. [Google Scholar] [PubMed]
- Şahin, N.M.; Dikmen, A.U.; Çetinkaya, S.; Aycan, Z. Subnormal Growth Velocity and Related Factors During GnRH Analog Therapy for Idiopathic Central Precocious Puberty. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 239–246. [Google Scholar] [CrossRef]
- Delemarre-Van De Waal, H.A.; Cohen-Kettenis, P.T. Clinical management of gender identity disorder in adolescents: A protocol on psychological and paediatric endocrinology aspects. Eur. J. Endocrinol. 2006, 155, S131–S137. [Google Scholar] [CrossRef]
- Mouat, F.; Hofman, P.L.; Jefferies, C.; Gunn, A.J.; Cutfield, W.S. Initial growth deceleration during GnRH analogue therapy for precocious puberty. Clin. Endocrinol. 2009, 70, 751–756. [Google Scholar] [CrossRef]
- Pienkowski, C.; Tauber, M. Gonadotropin-Releasing Hormone Agonist Treatment in Sexual Precocity. Endocr. Dev. 2016, 29, 214–229. [Google Scholar] [CrossRef]
- Fiore, M.; Tarani, L.; Radicioni, A.; Spaziani, M.; Ferraguti, G.; Putotto, C.; Gabanella, F.; Maftei, D.; Lattanzi, R.; Minni, A.; et al. Serum prokineticin-2 in prepubertal and adult Klinefelter individuals. Can. J. Physiol. Pharmacol. 2021, 100, 151–157. [Google Scholar] [CrossRef]
- Ferraguti, G.; Fanfarillo, F.; Tarani, L.; Blaconà, G.; Tarani, F.; Barbato, C.; Minni, A.; Ralli, M.; Francati, S.; Greco, A.; et al. NGF and the Male Reproductive System: Potential Clinical Applications in Infertility. Int. J. Mol. Sci. 2022, 23, 13127. [Google Scholar] [CrossRef]
- Ferraguti, G.; Terracina, S.; Micangeli, G.; Lucarelli, M.; Tarani, L.; Ceccanti, M.; Spaziani, M.; D’orazi, V.; Petrella, C.; Fiore, M. NGF and BDNF in pediatrics syndromes. Neurosci. Biobehav. Rev. 2023, 145, 105015. [Google Scholar] [CrossRef]
- Roberts, S.A.; Kaiser, U.B. Genetics in endocrinology: Genetic etiologies of central precocious puberty and the role of imprinted genes. Eur. J. Endocrinol. 2020, 183, R107–R117. [Google Scholar] [CrossRef]
- Paparella, R.; Menghi, M.; Micangeli, G.; Leonardi, L.; Profeta, G.; Tarani, F.; Petrella, C.; Ferraguti, G.; Fiore, M.; Tarani, L. Autoimmune Polyendocrine Syndromes in the Pediatric Age. Children 2023, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Spaziani, M.; D’orazi, V.; Tarani, L.; Terracina, S.; Tarani, F.; Micangeli, G.; Barbato, C.; Minni, A.; Greco, A.; et al. Prokineticin 2/PROK2 and Male Infertility. Biomedicines 2022, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, A.; Michala, L. Autonomous Ovarian Cysts in Prepubertal Girls. How Aggressive Should We Be? A Review of the Literature. J. Pediatr. Adolesc. Gynecol. 2015, 28, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, L.S.; Shenker, A.; Gejman, P.V.; Merino, M.J.; Friedman, E.; Spiegel, A.M. Activating Mutations of the Stimulatory G Protein in the McCune–Albright Syndrome. N. Engl. J. Med. 1991, 325, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.T.; Singer, F.R.; Eugster, E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J. Rare Dis. 2012, 7, S4. [Google Scholar] [CrossRef]
- Estrada, A.; Boyce, A.M.; Brillante, B.A.; Guthrie, L.C.; Gafni, R.I.; Collins, M.T. Long-term outcomes of letrozole treatment for precocious puberty in girls with McCune–Albright syndrome. Eur. J. Endocrinol. 2016, 175, 477–483. [Google Scholar] [CrossRef]
- Haddad, N.; Eugster, E. An Update on the Treatment of Precocious Puberty in McCune-Albright Syndrome and Testotoxicosis. J. Pediatr. Endocrinol. Metab. 2007, 20, 653–661. [Google Scholar] [CrossRef]
| Tanner Stage | Breast (Female) | Pubic Hair (Female, Male) | Genitalia (Male) |
|---|---|---|---|
| I | Preadolescent | None | Preadolescent |
| II | Breast bud palpable under the areola | Sparse, long, straight | Enlargement of scrotum/testes |
| III | Breast tissue palpable outside areola; no areolar development | Darker, curling, increased amount | Penis grows in length; testes continue to enlarge |
| IV | Areola elevated above the contour of the breast, forming a “double scoop” appearance | Coarse, curly, adult type | Penis grows in length/breadth; scrotum darkens, testes continue to enlarge |
| V | The areolar mound regresses into a single breast contour with areolar hyperpigmentation, papillae development, and nipple protrusion | Adult, extend to thighs | Adult shape/size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micangeli, G.; Paparella, R.; Tarani, F.; Menghi, M.; Ferraguti, G.; Carlomagno, F.; Spaziani, M.; Pucarelli, I.; Greco, A.; Fiore, M.; et al. Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy. Children 2023, 10, 1672. https://doi.org/10.3390/children10101672
Micangeli G, Paparella R, Tarani F, Menghi M, Ferraguti G, Carlomagno F, Spaziani M, Pucarelli I, Greco A, Fiore M, et al. Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy. Children. 2023; 10(10):1672. https://doi.org/10.3390/children10101672
Chicago/Turabian StyleMicangeli, Ginevra, Roberto Paparella, Francesca Tarani, Michela Menghi, Giampiero Ferraguti, Francesco Carlomagno, Matteo Spaziani, Ida Pucarelli, Antonio Greco, Marco Fiore, and et al. 2023. "Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy" Children 10, no. 10: 1672. https://doi.org/10.3390/children10101672
APA StyleMicangeli, G., Paparella, R., Tarani, F., Menghi, M., Ferraguti, G., Carlomagno, F., Spaziani, M., Pucarelli, I., Greco, A., Fiore, M., & Tarani, L. (2023). Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy. Children, 10(10), 1672. https://doi.org/10.3390/children10101672