Improving Pharmacist-Led Pediatric Patient Education on Oral Chemotherapy at Home
Abstract
:1. Oral Chemotherapy Agents for At-Home Administration
2. Patient Education and Medication Counseling for Children
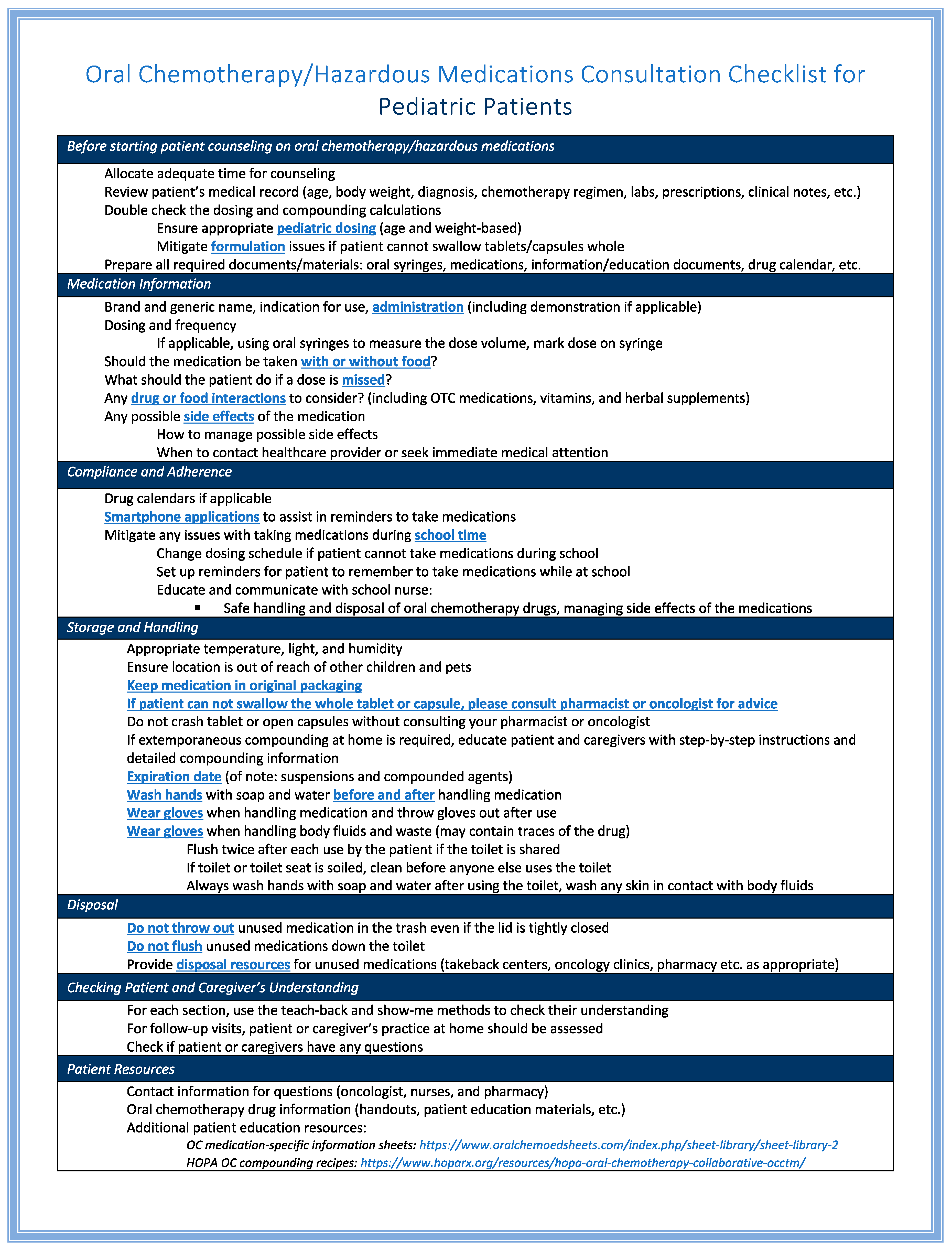
3. Essential Subjects for Oral Chemotherapy Counseling in Pediatric Patients
3.1. Safety Considerations (Administration, Handling, and Disposal)
3.2. Medication Adherence for Optimal Treatment Effectiveness
4. Optimizing Pharmacist-Led Education on OC in Pediatric Patients
4.1. Challenges and Barriers to Providing Patient Education on OC
4.2. Possible Solutions to Improve OC Education in Pediatric Patients
4.2.1. Bridging the Knowledge Gap on OC
4.2.2. Developing a Comprehensive Patient Counseling Checklist
4.2.3. Providing Informational Handouts with Oral Chemotherapy Prescriptions
4.2.4. Developing Smartphone or Online Applications to Assist in Patient Education
4.2.5. Maximizing Telepharmacy Potential for Patient Education
4.2.6. Utilizing Artificial Intelligence to Optimize Patient Education
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA. New Drug Therapy Approvals 2020. January 2021. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2020 (accessed on 18 August 2022).
- Alwardt, M. Oral Oncolytics Will Require Health Care System to Adapt. OncologyLive 2020, 21, 68–69. [Google Scholar]
- Weingart, S.N.; Brown, E.; Bach, P.B.; Eng, K.; Johnson, S.A.; Kuzel, T.M.; Langbaum, T.S.; Leedy, R.D.; Muller, R.J.; Newcomer, L.N.; et al. NCCN Task Force Report: Oral chemotherapy. J. Natl. Compr. Cancer Netw. 2008, 6 (Suppl. S3), S1–S14. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- O’Neill, V.J.; Twelves, C.J. Oral cancer treatment: Developments in chemotherapy and beyond. Br. J. Cancer 2002, 87, 933–937. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Antiemesis; Version 2.2023; NCCN: Plymouth Meeting, PA, USA, 2023. [Google Scholar]
- Centers for Disease Control and Prevention: Child Health. 2023. Available online: https://www.cdc.gov/nchs/fastats/child-health.htm (accessed on 4 August 2023).
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef]
- Kasi, P.M.; Grothey, A. Chemotherapy Maintenance. Cancer J. 2016, 22, 199–204. [Google Scholar] [CrossRef]
- Close, P.; Burkey, E.; Kazak, A.; Danz, P.; Lange, B. A prospective, controlled evaluation of home chemotherapy for children with cancer. Pediatrics 1995, 95, 896–900. [Google Scholar] [CrossRef]
- Allen, H.C.; Garbe, M.C.; Lees, J.; Aziz, N.; Chaaban, H.; Miller, J.L.; Johnson, P.; DeLeon, S. Off-Label Medication use in Children, More Common Than We Think: A Systematic Review of the Literature. J. Okla. State Med. Assoc. 2018, 111, 776–783. [Google Scholar]
- Fernandez, E.; Perez, R.; Hernandez, A.; Tejada, P.; Arteta, M.; Ramos, J.T. Factors and Mechanisms for Pharmacokinetic Differences between Pediatric Population and Adults. Pharmaceutics 2011, 3, 53–72. [Google Scholar] [CrossRef]
- Ivanovska, V.; Rademaker, C.M.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, S.H.; Putri, D.C.A.; Virginia, D.M.; Gani, M.R.; Riswanto, F.D.O. Prevalence, Risk, and Challenges of Extemporaneous Preparation for Pediatric Patients in Developing Nations: A Review. Pharmaceutics 2023, 15, 840. [Google Scholar] [CrossRef] [PubMed]
- Litalien, C.; Berube, S.; Tuleu, C.; Gilpin, A.; Landry, E.K.; Valentin, M.; Strickley, R.; Turner, M.A. From paediatric formulations development to access: Advances made and remaining challenges. Br. J. Clin. Pharmacol. 2022, 88, 4349–4383. [Google Scholar] [CrossRef]
- Billstein-Leber, M.; Carrillo, C.J.D.; Cassano, A.T.; Moline, K.; Robertson, J.J. ASHP Guidelines on Preventing Medication Errors in Hospitals. Am. J. Health Syst. Pharm. 2018, 75, 1493–1517. [Google Scholar] [CrossRef] [PubMed]
- Institute For Safe Medication Practices. Death Due to Pharmacy Compounding Error Reinforces Need for Safety Focus; Institute For Safe Medication Practices: Plymouth Meeting, PA, USA, 2017. [Google Scholar]
- Mohr, J.J.; Lannon, C.M.; Thoma, K.A.; Woods, D.; Slora, E.J.; Wasserman, R.C.; Uhring, L. Learning from Errors in Ambulatory Pediatrics. In Advances in Patient Safety: From Research to Implementation (Volume 1: Research Findings); Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; NIH: Rockville, MD, USA, 2005. [Google Scholar]
- Weingart, S.N.; Zhang, L.; Sweeney, M.; Hassett, M. Chemotherapy medication errors. Lancet Oncol. 2018, 19, e191–e199. [Google Scholar] [CrossRef] [PubMed]
- Weingart, S.N.; Toro, J.; Spencer, J.; Duncombe, D.; Gross, A.; Bartel, S.; Miransky, J.; Partridge, A.; Shulman, L.N.; Connor, M. Medication errors involving oral chemotherapy. Cancer 2010, 116, 2455–2464. [Google Scholar] [CrossRef]
- Walsh, K.E.; Dodd, K.S.; Seetharaman, K.; Roblin, D.W.; Herrinton, L.J.; Von Worley, A.; Usmani, G.N.; Baer, D.; Gurwitz, J.H. Medication errors among adults and children with cancer in the outpatient setting. J. Clin. Oncol. 2009, 27, 891–896. [Google Scholar] [CrossRef]
- NIOSH. Managing Hazardous Drug Exposures: Information for Healthcare Settings; Publication No. 2023-130; U.S. Department of Health and Human Services: Washington, DC, USA; Centers for Disease Control and Prevention: Atlanta, GA, USA; National Institute for Occupational Safety and Health, DHHS (NIOSH): Washington, DC, USA, 2023. [Google Scholar]
- Connor, T.H. Hazardous anticancer drugs in health care: Environmental exposure assessment. Ann. N. Y. Acad. Sci. 2006, 1076, 615–623. [Google Scholar] [CrossRef]
- International Society of Oncology Pharmacy Practicioners Standards Committee. ISOPP standards of practice. Safe handling of cytotoxics. J. Oncol. Pharm. Pract. 2007, 13, 1–81. [Google Scholar] [CrossRef]
- Trovato, J.A.; Tuttle, L.A. Oral chemotherapy handling and storage practices among Veterans Affairs oncology patients and caregivers. J. Oncol. Pharm. Pract. 2014, 20, 88–92. [Google Scholar] [CrossRef]
- Alameda County: Safe Drug Disposal Ordinance. 24 July 2012. Available online: https://www.acgov.org/aceh/safedisposal/documents/SDDRevisedOrdinance_Final_02-02-5182016.pdf (accessed on 28 November 2021).
- County of San Mateo: Safe Medicine Disposal Ordinance. 28 May 2015. Available online: http://www.smchealth.org/general-information/safe-medicine-disposal-ordinance (accessed on 28 November 2021).
- County of Santa Clara: Disposal of County Residents’ Unwanted Pharmaceuticals. 23 June 2015. Available online: https://www.sccgov.org/sites/rwr/Documents/SafeDrugDisposalOrdinance.pdf (accessed on 28 November 2021).
- City of Santa Cruz: Safe Disposal of Drugs and Sharps. 28 June 2016. Available online: http://www.cityofsantacruz.com/home/showdocument?id=53808. (accessed on 28 November 2021).
- Marin County: Marin County Safe Drug Disposal Ordinance. 11 August 2015. Available online: https://www.municode.com/library/ca/marin_county/ordinances/code_of_ordinances?nodeId=73045526 (accessed on 28 November 2021).
- Wong, S.F.; Bounthavong, M.; Nguyen, C.P.; Chen, T. Outcome Assessments and Cost Avoidance of an Oral Chemotherapy Management Clinic. J. Natl. Compr. Cancer Netw. 2016, 14, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Amoyal, N.; Nisotel, L.; Fishbein, J.N.; MacDonald, J.; Stagl, J.; Lennes, I.; Temel, J.S.; Safren, S.A.; Pirl, W.F. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist 2016, 21, 354–376. [Google Scholar] [CrossRef] [PubMed]
- El-Rachidi, S.; LaRochelle, J.M.; Morgan, J.A. Pharmacists and Pediatric Medication Adherence: Bridging the Gap. Hosp. Pharm. 2017, 52, 124–131. [Google Scholar] [CrossRef]
- Hamilton, B.A. Oncology Care Model: Supporting Oral Chemotherapy Adherence; Brandeis University: Waltham, MA, USA, 2017. [Google Scholar]
- Johnson, L.A. Factors influencing oral adherence: Qualitative metasummary and triangulation with quantitative evidence. Clin. J. Oncol. Nurs. 2015, 19, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Winnick, S.; Lucas, D.O.; Hartman, A.L.; Toll, D. How do you improve compliance? Pediatrics 2005, 115, e718–e724. [Google Scholar] [CrossRef] [PubMed]
- McGrady, M.E.; Pai, A.L.H. A Systematic Review of Rates, Outcomes, and Predictors of Medication Non-Adherence among Adolescents and Young Adults with Cancer. J. Adolesc. Young Adult Oncol. 2019, 8, 485–494. [Google Scholar] [CrossRef] [PubMed]
- El Behadli Gonzalez, A.F. Culturally Informed Motivational Interviewing to Improve Oral Chemotherapy Adherence for Pediatric Acute Lymphoblastic Leukemia Patients and Their Caregivers: A Feasibility, Acceptability, and Preliminary Efficacy Trial; UT Southwestern Graduate School of Biomedical Sciences: Dallas, TX, USA, 2019. [Google Scholar]
- Dawson, L.A. What factors affect adherence to medicines? Arch. Dis. Child. Educ. Pract. Ed. 2019, 104, 49–52. [Google Scholar] [CrossRef]
- Keuler, N.; Bouwer, A.; Coetzee, R. Pharmacists’ Approach to Optimise Safe Medication Use in Paediatric Patients. Pharmacy 2021, 9, 180. [Google Scholar] [CrossRef]
- Santer, M.; Ring, N.; Yardley, L.; Geraghty, A.W.; Wyke, S. Treatment non-adherence in pediatric long-term medical conditions: Systematic review and synthesis of qualitative studies of caregivers’ views. BMC Pediatr. 2014, 14, 63. [Google Scholar] [CrossRef]
- Gutman, C.K.; Cousins, L.; Gritton, J.; Klein, E.J.; Brown, J.C.; Scannell, J.; Lion, K.C. Professional Interpreter Use and Discharge Communication in the Pediatric Emergency Department. Acad. Pediatr. 2018, 18, 935–943. [Google Scholar] [CrossRef]
- Klassen, A.; Raina, P.; Reineking, S.; Dix, D.; Pritchard, S.; O’Donnell, M. Developing a literature base to understand the caregiving experience of parents of children with cancer: A systematic review of factors related to parental health and well-being. Support. Care Cancer 2007, 15, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Dreyer, B.P.; van Schaick, L.; Foltin, G.L.; Dinglas, C.; Mendelsohn, A.L. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch. Pediatr. Adolesc. Med. 2008, 162, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Lemer, C.; Bates, D.W.; Yoon, C.; Keohane, C.; Fitzmaurice, G.; Kaushal, R. The role of advice in medication administration errors in the pediatric ambulatory setting. J. Patient Saf. 2009, 5, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.V.; Young, S.; Phillips, L.; Clark, D. Patient attitudes regarding the role of the pharmacist and interest in expanded pharmacist services. Can. Pharm. J. 2014, 147, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Colombo, L.R.P.; Aguiar, P.M.; Lima, T.M.; Storpirtis, S. The effects of pharmacist interventions on adult outpatients with cancer: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 414–424. [Google Scholar] [CrossRef]
- Darling, J.O.; Raheem, F.; Carter, K.C.; Ledbetter, E.; Lowe, J.F.; Lowe, C. Evaluation of a Pharmacist Led Oral Chemotherapy Clinic: A Pilot Program in the Gastrointestinal Oncology Clinic at an Academic Medical Center. Pharmacy 2020, 8, 46. [Google Scholar] [CrossRef]
- Kerr, J.R.M.; Mancini, R.S.; Wright, A.L.; Modlin, J.L. Pharmacist-Led Oral Oncology Programs. Hematol./Oncol. Pharm. Assoc. 2020, 17, 3–29. [Google Scholar]
- Hematology/Oncology Pharmacy Association. The Role of Hematology/Oncology Pharmacists. Available online: https://www.hoparx.org/documents/78/HOPA_About_Hem_Onc_Pharmacist_Issue_Brief_FINAL1.pdf (accessed on 18 August 2022).
- Power, L.A.; Coyne, J.W. ASHP Guidelines on Handling Hazardous Drugs. Am. J. Health Syst. Pharm. 2018, 75, 1996–2031. [Google Scholar] [CrossRef]
- Yang, S.; Patel, P.; Corcoran, A.; Dobberpuhl, E.; Isidro, S.; Le, D.; Klassen, A.; Rho, J.; Tran, D.; Beuttler, R.; et al. Gaps in Patient Education on Safe Handling and Disposal of Oral Chemotherapy Drugs: A Pilot Prospective Cohort Survey Study. J. Contemp. Pharm. Pract. 2022, 70, 23–33. [Google Scholar]
- O’Bryant, C.L.; Crandell, B.C. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J. Am. Pharm. Assoc. 2008, 48, 632–639. [Google Scholar] [CrossRef]
- Patel, S.K.; Kelm, M.J.; Bush, P.W.; Lee, H.J.; Ball, A.M. Prevalence and risk factors of burnout in community pharmacists. J. Am. Pharm. Assoc. 2021, 61, 145–150. [Google Scholar] [CrossRef] [PubMed]
- American Pharmacists Association. Pharmacist Burnout Hits Breaking Point, Impacting Patient Safety. Available online: https://pharmacist.com/APhA-Press-Releases/apha-pharmacist-burnout-hits-breaking-point-impacting-patient-safety (accessed on 8 July 2022).
- Department of Health and Human Services; Centers for Disease Control and Prevention; National Institute for Occupational Safety and Health. NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2016. Available online: https://www.cdc.gov/niosh/docs/2016-161/default.html (accessed on 4 July 2022).
- Hematology/Oncology Pharmacy Association. Guidelines, Standards, and Summaries. Available online: https://www.hoparx.org/resources/guidelines-standards-summaries (accessed on 18 August 2022).
- Neuss, M.N.; Polovich, M.; McNiff, K.; Esper, P.; Gilmore, T.R.; LeFebvre, K.B.; Schulmeister, L.; Jacobson, J.O. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. J. Oncol. Pract. 2013, 9, 5s–13s. [Google Scholar] [CrossRef]
- Goodin, S.; Griffith, N.; Chen, B.; Chuk, K.; Daouphars, M.; Doreau, C.; Patel, R.A.; Schwartz, R.; Tames, M.J.; Terkola, R.; et al. Safe handling of oral chemotherapeutic agents in clinical practice: Recommendations from an international pharmacy panel. J. Oncol. Pract. 2011, 7, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.E.; Chambers, C.R.; Vaida, A.J. Oncology medication safety: A 3D status report 2008. J. Oncol. Pharm. Pract. 2008, 14, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, K.; Scott, S.; Minard, L.V.; Lambourne, T. Optimizing patient education of oncology medications: A quantitative analysis of the patient perspective. J. Oncol. Pharm. Pract. 2019, 25, 1445–1455. [Google Scholar] [CrossRef]
- Siden, R.; Modlin, J.; Lee-Gabel, L.; Redic, K.A. Handout for research subjects receiving investigational oral chemotherapy. Am. J. Health Syst. Pharm. 2019, 76, 2009–2012. [Google Scholar] [CrossRef]
- Badawy, S.M.; Thompson, A.A.; Liem, R.I. Technology Access and Smartphone App Preferences for Medication Adherence in Adolescents and Young Adults with Sickle Cell Disease. Pediatr. Blood Cancer 2016, 63, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, H.; Asadi, F.; Mehrvar, A.; Nazemi, E.; Emami, H. Smartphone apps to help children and adolescents with cancer and their families: A scoping review. Acta Oncol. 2019, 58, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.M.; Alwan, L.; Pitney, C.; Taketa, C.; Indorf, A.; Held, L.; Lee, K.S.; Son, M.; Chi, M.; Diamantides, E.; et al. Establishing clinical pharmacist telehealth services during the COVID-19 pandemic. Am. J. Health Syst. Pharm. 2020, 77, 1403–1408. [Google Scholar] [CrossRef]
- Tzanetakos, G.; Ullrich, F.; Meuller, K. Telepharmacy Rules and Statutes: A 50-State Survey. Rural Policy Brief 2017, 4, 1–4. [Google Scholar]
- Unni, E.J.; Patel, K.; Beazer, I.R.; Hung, M. Telepharmacy during COVID-19: A Scoping Review. Pharmacy 2021, 9, 183. [Google Scholar] [CrossRef]
- Scott Kruse, C.; Karem, P.; Shifflett, K.; Vegi, L.; Ravi, K.; Brooks, M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J. Telemed. Telecare 2018, 24, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.; Orooji, A.; Sheikhtaheri, A. Teleoncology for children with cancer: A scoping review on applications and outcomes. Int. J. Med. Inform. 2020, 139, 104118. [Google Scholar] [CrossRef] [PubMed]
- Utidjian, L.; Abramson, E. Pediatric Telehealth: Opportunities and Challenges. Pediatr. Clin. N. Am. 2016, 63, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Andras, I.; Mazzone, E.; van Leeuwen, F.W.B.; De Naeyer, G.; van Oosterom, M.N.; Beato, S.; Buckle, T.; O’Sullivan, S.; van Leeuwen, P.J.; Beulens, A.; et al. Artificial intelligence and robotics: A combination that is changing the operating room. World J. Urol. 2020, 38, 2359–2366. [Google Scholar] [CrossRef]
- Nelson, S.D.; Walsh, C.G.; Olsen, C.A.; McLaughlin, A.J.; LeGrand, J.R.; Schutz, N.; Lasko, T.A. Demystifying artificial intelligence in pharmacy. Am. J. Health Syst. Pharm. 2020, 77, 1556–1570. [Google Scholar] [CrossRef]
- Alanazi, M.F.; Shahein, M.I.; Alsharif, H.M.; Alotaibi, S.M.; Alanazi, A.O.; Alanazi, A.O.; Alharbe, U.A.; Almfalh, H.S.S.; Amirthalingam, P.; Hamdan, A.M.; et al. Impact of automated drug dispensing system on patient safety. Pharm. Pract. 2022, 20, 2744. [Google Scholar] [CrossRef]
- Ahtiainen, H.K.; Kallio, M.M.; Airaksinen, M.; Holmstrom, A.R. Safety, time and cost evaluation of automated and semi-automated drug distribution systems in hospitals: A systematic review. Eur. J. Hosp. Pharm. 2020, 27, 253–262. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Nguyen, C.M.; Willins, K.; Wang, E.Y.; Magedman, G.; Yang, S. Improving Pharmacist-Led Pediatric Patient Education on Oral Chemotherapy at Home. Children 2023, 10, 1656. https://doi.org/10.3390/children10101656
Patel A, Nguyen CM, Willins K, Wang EY, Magedman G, Yang S. Improving Pharmacist-Led Pediatric Patient Education on Oral Chemotherapy at Home. Children. 2023; 10(10):1656. https://doi.org/10.3390/children10101656
Chicago/Turabian StylePatel, Anika, Christopher M. Nguyen, Kristin Willins, Elsabella Y. Wang, Grace Magedman, and Sun Yang. 2023. "Improving Pharmacist-Led Pediatric Patient Education on Oral Chemotherapy at Home" Children 10, no. 10: 1656. https://doi.org/10.3390/children10101656
APA StylePatel, A., Nguyen, C. M., Willins, K., Wang, E. Y., Magedman, G., & Yang, S. (2023). Improving Pharmacist-Led Pediatric Patient Education on Oral Chemotherapy at Home. Children, 10(10), 1656. https://doi.org/10.3390/children10101656




