The Complexity of Hearing Aid Fitting: Children with Congenital Hearing Loss and Middle Ear Dysfunction
Abstract
1. Introduction
1.1. Early Detection of HL and HA Fitting
1.2. ME Effect on HA Fitting
2. Methods
2.1. Research Procedures
2.2. Participants
2.3. Clinical Instruments
2.4. Statistical Analysis
3. Results
3.1. Treatment for the ME Pathology during the HA Fitting
3.2. Referrals to an Otolaryngologist
3.3. Number of Fitting Sessions
3.4. Number of Hearing Tests
3.5. RECD Measurement
3.6. HA Amplification Gain
3.7. HA Fitting Process Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshinaga-Itano, Y.C.; Sedey, A.L.; Mason, C.A.; Wiggin, M.; Chung, I. Early intervention, parent talk, and pragmatic language in children with hearing loss. Pediatrics 2020, 146, S270–S277. [Google Scholar] [CrossRef] [PubMed]
- Tomblin, J.B.; Oleson, J.; Ambrose, S.E.; Walker, E.A.; Moeller, M.P. The influence of hearing aids on the speech and language development of children with hearing loss. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Fortnum, H.M.; Summerfield, A.Q.; Marshall, D.H.; Davis, A.C.; Bamford, J.M. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: Questionnaire-based ascertainment study. BMJ 2001, 323, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Prieve, B.; Dalzell, L.; Berg, A.; Bradley, M.; Cacace, A.; Campbell, D.; DeCristofaro, J.; Gravel, J.; Greenberg, E.; Gross, S.; et al. The New York State universal newborn hearing screening demonstration project: Outpatient outcome measures. Ear Hear. 2000, 21, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Infant Hearing. Year 2007 Position Statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007, 107, 898–921. [Google Scholar] [CrossRef]
- Slifer, K. Audiological evaluation of pediatric patients using evoked potentials. Perspect. Hear. Hear. Disord. Child. 2006, 16, 14–18. [Google Scholar] [CrossRef]
- Birkent, Ö.F.; Karlıdağ, T.; Başar, F.; Yalçın, Ş.; Kaygusuz, İ.; Keleş, E.; Akyiğit, A. Evaluation of the relationship between the air-bone gap prolonged ABR latencies in mixed-type hearing loss. J. Inter. Adv. Otol. 2017, 13, 88. [Google Scholar] [CrossRef]
- Paradise, J.L.; Rockette, H.E.; Colborn, D.K.; Bernard, B.S.; Smith, C.G.; Kurs-Lasky, M.; Janosky, J.E. Otitis media in 2253 Pittsburgh-area infants: Prevalence and risk factors during the first two years of life. Pediatrics 1997, 99, 318–333. [Google Scholar] [CrossRef]
- Karawani, H.; Matanis, W.; Na’ara, S.; Gordin, A. Middle ear effusion and newborn hearing screening. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 643–649. [Google Scholar] [CrossRef]
- Westerberg, B.D.; Morzaria, S.; Kozak, F.K.; Price, D. Medical management of middle ear disease in children less than 2 years of age with sensorineural hearing loss. J. Otolaryngol. 2005, 34, S64–S69. [Google Scholar]
- Illg, A.; Haack, M.; Lesinski-Schiedat, A.; Büchner, A.; Lenarz, T. Long-term outcomes, education, and occupational level in cochlear implant recipients who were implanted in childhood. Ear Hear. 2017, 38, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Punch, J.L.; Hitt, R.; Smith, S.W. Hearing loss quality of life. J. Commun. Disord. 2019, 78, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Priner, R.; Cranial, C.; Chayat, C.; Fraenkel, R.; Brand, D. Effect of auditory feedback on speech intelligibility of adults with cochlear implants. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- The Joint Committee on Infant Hearing. Year 2019 Position Statement: Principles and guidelines for early hearing detection and intervention programs. J. Early Hear. Detect. Interv. 2019, 4, 1–44. [Google Scholar] [CrossRef]
- Bagatto, M. Baby waves and hearing aids: Using ABR to fit hearing aids to infants. Hear. J. 2008, 61, 10–12. [Google Scholar] [CrossRef]
- Vander Werff, K.R.; Prieve, B.A.; Georgantas, L.M. Infant air and bone conduction tone burst auditory brainstem responses for classification of hearing loss and the relationship to behavioral thresholds. Ear Hear. 2009, 30, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Norrix, L.W.; Velenovsky, D. Unraveling the mystery of auditory brainstem response corrections: The need for universal standards. J. Am. Acad. Audiol. 2017, 28, 950–960. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.W.; Kaminski, J.; Beauchaine, K.; Lenzen, N.; Simms, K.; Gorga, M.P. The impact of degree of hearing loss on auditory brainstem response predictions of behavioral thresholds. Ear Hear. 2015, 36, 309–319. [Google Scholar] [CrossRef]
- Bagatto, M.; Moodie, S.; Scollie, S.; Seewald, R.; Moodie, S.; Pumford, J.; Liu, K.P. Clinical protocols for hearing instrument fitting in the Desired Sensation Level method. Trends Amplif. 2005, 9, 199–226. [Google Scholar] [CrossRef]
- Gorga, M.P.; Johnson, T.A.; Kaminski, J.R.; Beauchaine, K.L.; Garner, C.A.; Neely, S.T. Using a combination of click- and tone burst-evoked auditory brain stem response measurements to estimate pure-tone thresholds. Ear Hear. 2006, 27, 60–74. [Google Scholar] [CrossRef]
- Quar, T.K.; Ching, T.Y.; Newall, P.; Sharma, M. Evaluation of real-world preferences and performance of hearing aids fitted according to the NAL-NL1 and DSL v5 procedures in children with moderately severe to profound hearing loss. Int. J. Audiol. 2013, 52, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.Y.; Johnson, E.E.; Hou, S.; Dillon, H.; Zhang, V.; Burns, L.; van Buynder, P.; Wong, A.; Flynn, C. A comparison of NAL and DSL prescriptive methods for pediatric hearing-aid fitting: Predicted speech intelligibility and loudness. Int. J. Audiol. 2013, 52, S29–S38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, V.K. Prevalence of otitis media with effussion in children with bilateral sensorineural hearing loss. Arch. Dis. Child. 1990, 65, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elhamd, K.E.A.; Moussa, A.E.; Soltan, M.A.E. Prevalence of middle ear pathologies in children with bilateral sensorineural hearing loss. Int. J. Ped. Otorhinolaryngol. 2006, 70, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Dhingan, D.G.; Jadav, B. A study of middle ear diseases in children with congenital bilateral severe to profound sensorineural hearing loss. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 4276–4280. [Google Scholar] [CrossRef] [PubMed]
- Fermo, S.; Frosolini, A.; Parrino, D.; Chiappetta, A.; Marioni, G.; de Filippis, C. Eustachian tube insufflation with thermal water: Effectiveness in the treatment of pediatric otitis media with effusion. Am. J. Otolaryngol. 2022, 43, 103504. [Google Scholar] [CrossRef] [PubMed]
- Venekamp, R.P.; Mick, P.; Schilder, A.G.; Nunez, D.A. Grommets (ventilation tubes) for recurrent acute otitis media in children. Cochrane Database Syst. Rev. 2018, 5, CD012017. [Google Scholar] [CrossRef] [PubMed]
- Maw, A.R.; Hall, A.J.; Pothier, D.D.G.; Steven, P.S.; Colin, D.S. The prevalence of tympanic membrane and related middle ear pathology in children. Otol. Neurotol. 2011, 32, 1256–1261. [Google Scholar] [CrossRef]
- Park, H.; Hong, S.N.; Kim, H.S.; Han, J.J.; Chung, J.; Seo, M.-W.; Oh, S.-H.; Chang, S.-O.; Lee, J.H. Determinants of conductive hearing loss in tympanic membrane perforation. Clin. Exp. Otorhinolaryngol. 2015, 8, 92–96. [Google Scholar] [CrossRef]
- American Academy of Audiology. Clinical Practice Guidelines Pediatric Amplification. 2013. Available online: http://galster.net/wp-content/uploads/2013/07/AAA-2013-Pediatric-Amp-Guidelines.pdf (accessed on 1 August 2022).
- Ontario Ministry of Children and Youth Services. Protocol for the Provision of Amplification. 2014. Available online: https://www.uwo.ca/nca/pdfs/clinical_protocols/IHP_Amplification%20Protocol_2019.01.pdf (accessed on 1 August 2022).
- Brookhouser, P.E.; Worthington, D.W.; Kelly, W.J. Middle ear disease in young children with sensorineural hearing loss. Laryngoscope 1993, 103, 371–378. [Google Scholar] [CrossRef]
- Muñoz, K.; Olson, W.A.; Twohig, M.P.; Preston, E.; Blaiser, K.; White, K.R. Pediatric hearing aid use: Parent-reported challenges. Ear Hear. 2015, 36, 279–287. [Google Scholar] [CrossRef]
- Liming, B.J.; Carter, J.; Cheng, A.; Choo, D.; Curotta, J.; Carvalho, D.; Germiller, J.A.; Hone, S.; Kenna, M.A.; Loundon, N. International Pediatric Otolaryngology Group (IPOG) consensus recommendations: Hearing loss in the pediatric patient. Int. J. Ped. Otorhinolaryngol. 2016, 90, 251–258. [Google Scholar] [CrossRef]
- Stool, S.E.; Berg, A.O. Otitis Media with Effusion in Young Children: Clinical Practice Guideline; DIANE Publishing: Collingdale, PA, USA, 1998. [Google Scholar]
- Hong, S.H.; Cho, Y.S.; Chung, W.H.; Koh, S.J.; Seo, I.S.; Woo, H.C. Changes in external ear resonance after ventilation tube (Grommet) insertion in children with otitis media with effusion. Int. J. Ped. Otorhinolaryngol. 2001, 58, 147–152. [Google Scholar] [CrossRef]

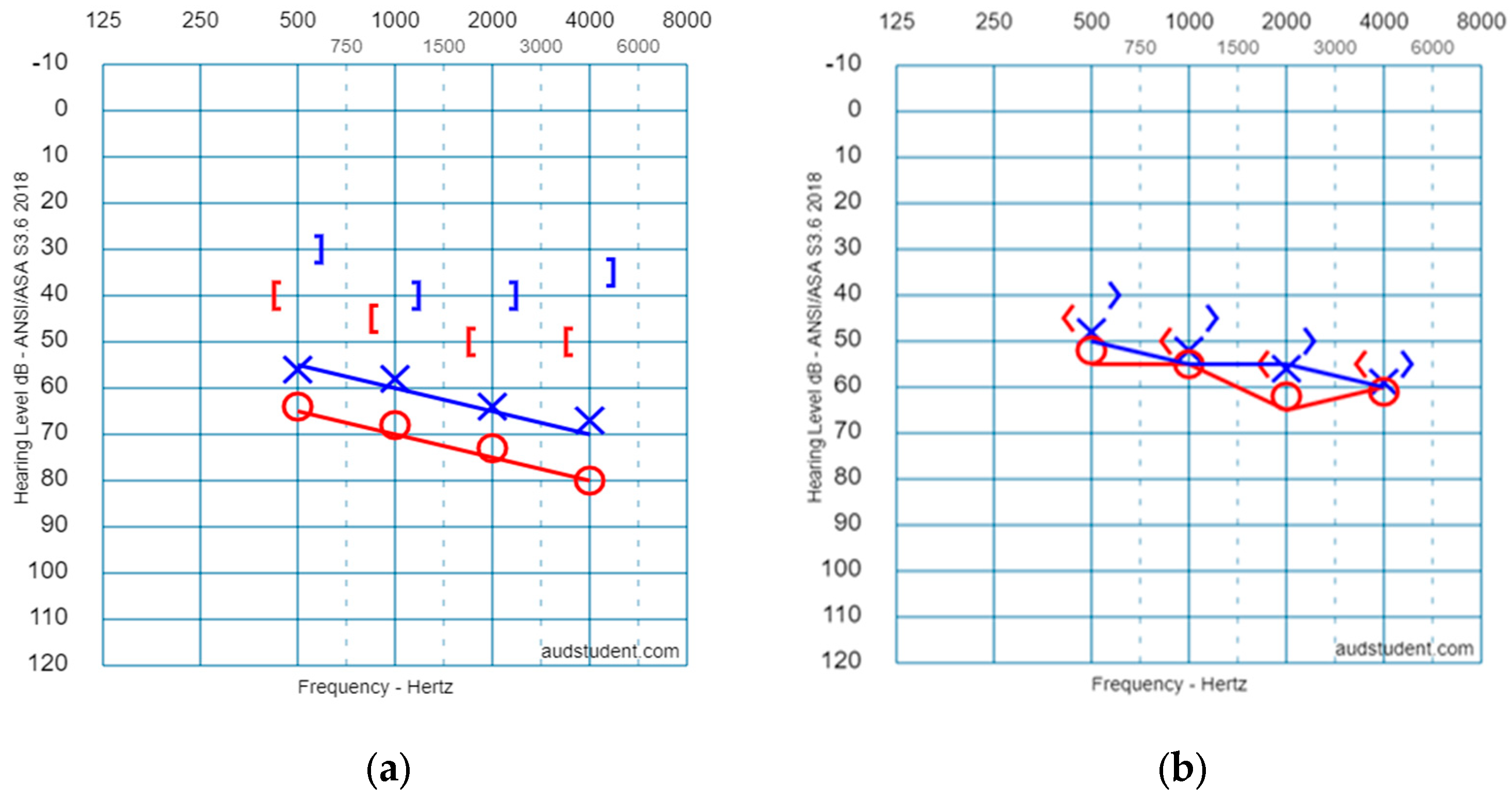

| Group | Freq. | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | Group | Freq. | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz |
|---|---|---|---|---|---|---|---|---|---|---|---|
| /Ear | /Ear | ||||||||||
| Study group (Mixed HL) | AC Right (17 ears) | 62.9 (13.7) | 66.9 (14.4) | 71.9 (13.1) | 78.1 (15.5) | Control group (SNHL) | AC Right (9 ears) | 52.5 (20.5) | 55.6 (21.6) | 62.1 (23.2) | 61.3 (23.3) |
| BC Right (14 ears) | 41.7 (13.7) | 43.4 (9.5) | 51.1 (12.7) | 51.2 (15.4) | BC Right (6 ears) | 42.5 (19.2) | 51.0 (19.5) | 57.0 (12.2) | 57.0 (17.2) | ||
| AC Left (13 ears) | 55.6 (19.2) | 58.3 (15.5) | 63.9 (14.6) | 66.0 (16.8) | AC Left (7 ears) | 47.9 (6.9) | 52.1 (6.8) | 55.7 (10.8) | 59.3 (12.5) | ||
| BC Left (7 ears) | 33.6 (8.5) | 40.8 (6.6) | 46.4 (9.4) | 43.6 (4.6) | BC Left (7 ears) | 40.0 (13.2) | 45.0 (12.9) | 50.7 (10.3) | 55.7 (14.0) |
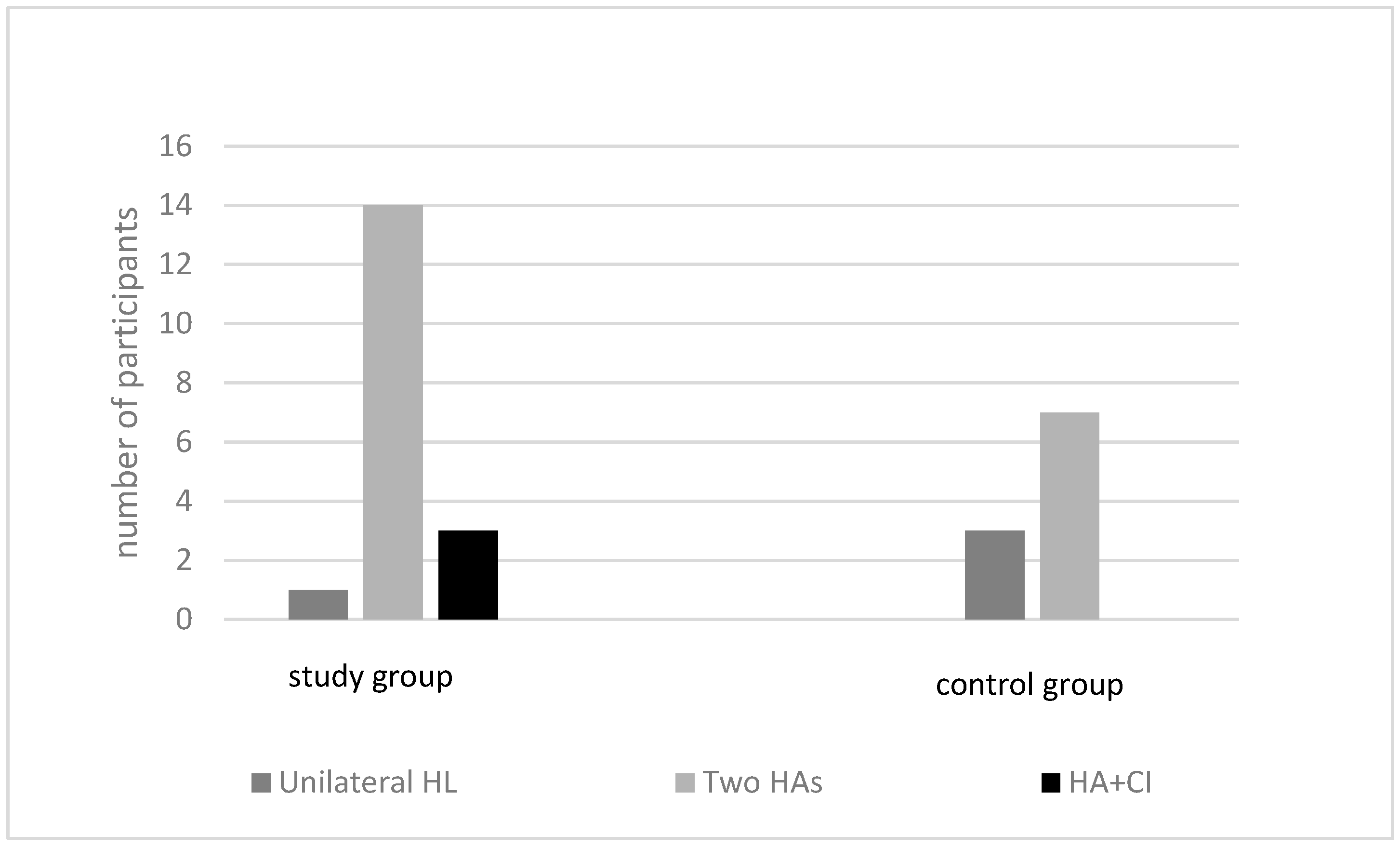
| Etiology | Study Group, n = 18 (Children with MEE) | Control Group, n = 10 (Children without MEE) |
|---|---|---|
| Genetic | 8 | 5 |
| Cytomegalovirus | 1 | 1 |
| Syndrome | 1 | 0 |
| Birth complication | 3 | 1 |
| Unknown | 3 | 3 |
| General developmental delay | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priner, R.; Brand, D. The Complexity of Hearing Aid Fitting: Children with Congenital Hearing Loss and Middle Ear Dysfunction. Children 2023, 10, 1630. https://doi.org/10.3390/children10101630
Priner R, Brand D. The Complexity of Hearing Aid Fitting: Children with Congenital Hearing Loss and Middle Ear Dysfunction. Children. 2023; 10(10):1630. https://doi.org/10.3390/children10101630
Chicago/Turabian StylePriner, Ronit, and Devora Brand. 2023. "The Complexity of Hearing Aid Fitting: Children with Congenital Hearing Loss and Middle Ear Dysfunction" Children 10, no. 10: 1630. https://doi.org/10.3390/children10101630
APA StylePriner, R., & Brand, D. (2023). The Complexity of Hearing Aid Fitting: Children with Congenital Hearing Loss and Middle Ear Dysfunction. Children, 10(10), 1630. https://doi.org/10.3390/children10101630





