1. Introduction
One of the most important steps in reducing neonatal morbidity is to prioritize noninvasive respiratory support and avoid invasive mechanical ventilation. However, the latter is used as rescue therapy in infants with severe respiratory distress. According to US Neonatal Research Network data for 2018, 84.3% of infants at <29 weeks of gestation received conventional mechanical ventilation support during their stay in the NICU [
1].
Rapid advances in ventilator technology have resulted in numerous basic and complex respiratory support modes. To understand how a mechanical ventilator works, one must be knowledgeable about the features of these specific modes, how they interact with an alert and spontaneously breathing infant, and mode definitions specific to the ventilator in the unit [
2,
3].
At present, tidal volume-targeted synchronized ventilation modes are most recommended for ventilating preterm infants. Tidal volume-targeted synchronized ventilation has been shown to provide shorter ventilatory support duration and lower rates of adverse effects such as pneumothorax, hypocarbia, severe intracranial hemorrhage, mortality, and bronchopulmonary dysplasia (BPD) [
4,
5,
6,
7].
Along with the increase in available ventilator types and modes, ventilator modes have become more complex, and the parameters that were previously entered and adjusted by the clinician are now automatically adjusted by the device. Bilevel volume guarantee (VG) and pressure-regulated volume control (PRVC) modes are examples of this adaptive target scheme [
2]. In an adaptive target scheme, the ventilator uses breath-to-breath feedback to continuously adjust the delivered pressure to achieve the target tidal volume (V
T) [
8,
9].
As experience and evidence regarding the use of new tidal volume-targeted devices and modes in neonatal intensive care units (NICUs) are limited, there is a need for safety and efficacy studies and comparative trials of these modes. The present study aimed to compare the bilevel VG and PRVC modes of the GE® Carescape R860 model ventilator and test the safety and feasibility of these two modes in preterm neonates.
2. Materials and Methods
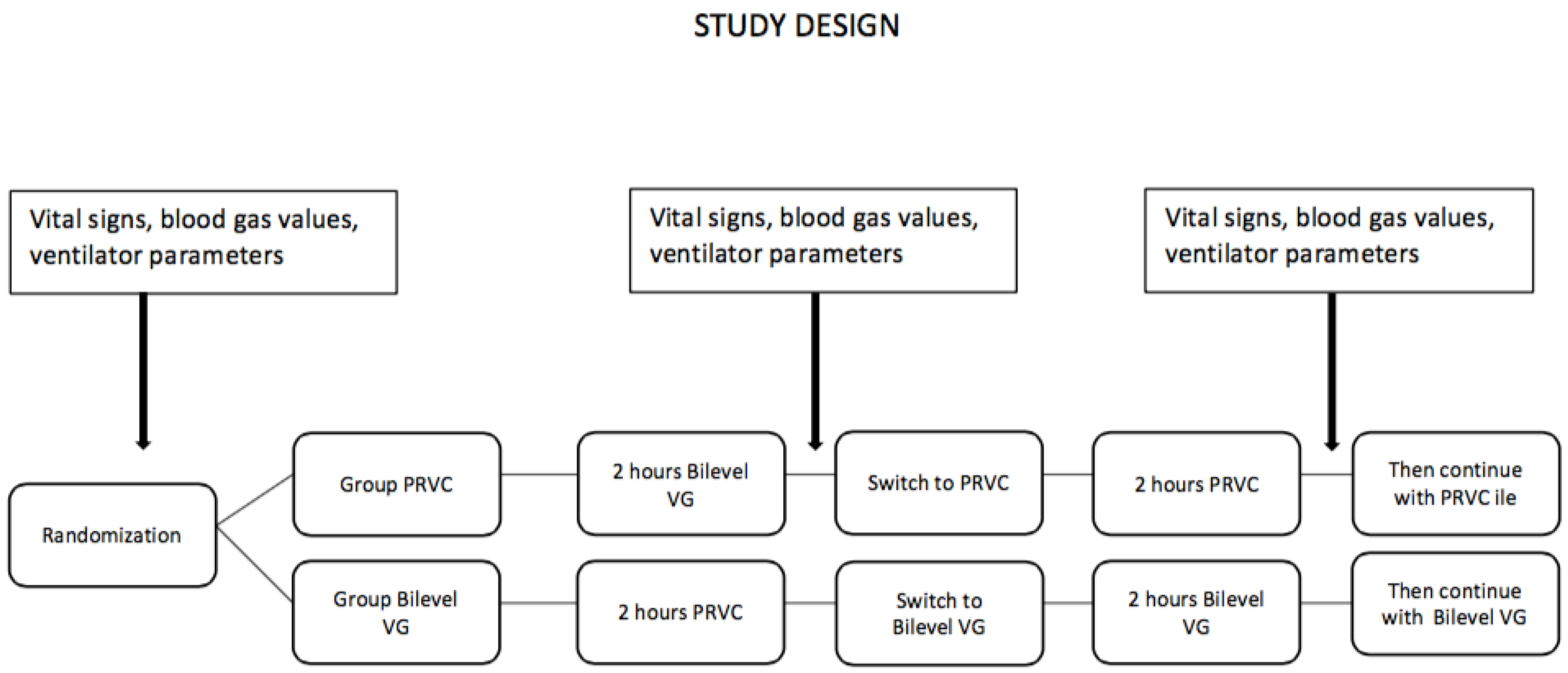
This study was conducted in the NICU of Ankara City Hospital between January 2020 and June 2021, serving as a referral for tertiary care. This study was designed as a prospective, randomized crossover study. Infants who were less than 30 weeks of gestational age, required intubation and mechanical ventilation in the first 24 h of life, received surfactant at least once, and were clinically stable at postnatal 24 h were included in the study. Patients whose families did not provide consent, who had major congenital anomalies at admission, or who had congenital heart disease were excluded. Patients who met the selection criteria were randomized after the first surfactant administration to crossover ventilation with bilevel VG and PRVC for 2 h each using the sealed envelope method with grouping by weight.
Group 1 (PRVC): Bilevel VG mode first, PRVC mode second, then continued with PRVC
Group 2 (bilevel VG): PRVC mode first, bilevel VG mode second, then continued with bilevel VG.
In block randomization, weight stratification categories were determined as 500–750 g, 751–1000 g, and 1001–1250 g. As blinding was not possible due to the nature of the study, the sealed envelope method was used to help ensure balance and reduce bias.
After group assignment, the infants were monitored with the designated ventilation mode. The following ventilator parameters were set in PRVC mode: FiO2, positive end-expiratory pressure (PEEP), pressure support (PS), VT, respiratory rate, inspiratory time (Tins), inspiratory pressure minimum (Pmin) and maximum (Pmax). In bilevel VG mode, FiO2, VT, PEEP, respiratory rate, Tins, and Pmax were set. VT was set to 5 mL/kg and the target blood gas values were pH 7.25–7.35, PaCO2 45–55 mmHg, and PaO2 50–70 mmHg.
2.1. Operating Principles of the Ventilation Modes
In SIMV PRVC mode, the ventilator delivers synchronized pressure-regulated, volume-controlled breaths at the set respiratory rate. For each mechanical breath, the ventilator adjusts the inspiration pressure to the lowest pressure needed to provide the target VT. All spontaneous efforts trigger pressure-assisted breaths.
The ventilator provides volume-controlled ventilation for 10 s or two breaths (whichever is longer when the mode is initiated) to determine the patient’s lung compliance. Inspiratory pressure is determined according to lung compliance and used for subsequent breaths.
When adjusting inspiratory pressure, the ventilator uses PEEP + Pmin as the lower limit and Pmax—5 cmH2O as the upper limit. The difference in inspiratory pressure between breaths does not exceed ±3 cmH2O. If a high airway pressure alarm is activated for the current breath, the target pressure for the next breath is reduced by 0.5 cmH2O.
In Bilevel VG mode, if the patient initiates a breath at the PEEP level, a pressure-supported breath is provided at the set PS. The ventilator switches between the PEEP and minimum pressure to maintain the target VT according to the set respiratory rate and Tins values. As in PRVC mode, the ventilator determines lung compliance by providing volume-controlled ventilation over 10 s or two breaths (whichever is longer when the mode is initiated) and this is used to determine inspiratory pressure for subsequent breaths. Inspiratory pressure limits and adjustments are also the same as in PRVC mode.
All patients were monitored with a GE
®, Chicago, IL, USA, Carescape R860 model ventilator. After randomization, initial ventilator settings were adjusted for each patient and recorded as the starting point. Respiratory rate, heart rate, and pulse oximetry (SpO
2 90–94%) values were continuously monitored in all patients. After the first 2 h of ventilation, the patients were switched to the other ventilator mode for 2 h. Ventilator parameters, vital signs, and blood gas values were evaluated at the beginning of the study, after the first ventilation mode, and after the second ventilation mode (
Figure 1). Infants with oxygen requirements greater than 35% received a second dose of surfactant 8–12 h after the first dose.
2.2. Definitions and Neonatal Outcomes
Gestational age was determined based on the mother’s last menstrual period, first-trimester prenatal ultrasound (US) data, or estimated postnatally using the New Ballard Score [
10]. Respiratory distress syndrome (RDS) was defined as early postnatal symptoms of respiratory distress accompanied by tachypnea, retraction, grunting, and cyanosis and supported by blood gas and chest X-ray findings [
11,
12,
13].
In accordance with unit protocols, surfactant therapy for RDS was administered prophylactically at NICU admission or as early rescue therapy in the first two hours according to the following criteria: as per the recommendations of the Turkish Neonatal Society, preterm infants at gestational age < 26 weeks that did not receive antenatal steroid therapy and preterm infants that required intubation in the delivery room received prophylactic surfactant; infants with signs of RDS findings and FiO2 requirement > 40% received early rescue surfactant therapy. All patients in the study received surfactant (poractant alfa 200 mg/kg) within the first 2 h of NICU admission and were monitored using GE®, Carescape R860 model ventilators.
Noninvasive ventilation techniques and less invasive surfactant administration methods are encouraged in all patients with a good respiratory drive however patients were intubated and mechanically ventilated if they fulfilled the following criteria. Hypoxemia despite FiO2 requirement ≥ 60%, respiratory acidosis (pH < 7.2) under PEEP ≥ 7 cm H2O, and recurrent apnea.
BPD in a preterm infant < 32 weeks of gestational age is defined as radiographically confirmed persistent parenchymal lung disease and is classified according to oxygen requirement at postmenstrual 36 weeks as follows [
14,
15]:
Mild BPD, nasal cannula oxygen < 2 L/min, FiO2 ≥ 21;
Moderate BPD, nasal cannula oxygen > 2 L/min or nCPAP or NIPPV, FiO2 ≥ 21;
Severe BPD, invasive PPV, FiO2 ≥ 21.
PDA refers to the nonclosure of the ductus arteriosus after the first 72 h of life. The diagnosis of hemodynamically significant PDA is made by demonstrating high-volume flow through the PDA on echocardiography. The first choice of medical treatment for PDA in our clinic is ibuprofen in two doses, 10 mg/kg/day followed by 5 mg/kg/day, administered 24 h apart [
16].
ROP screening is performed in all infants born at a gestational age of <34 weeks and a birth weight of <1700 g, as well as infants with a gestational age of ≥34 or a birth weight of >1700 g who received cardiopulmonary supportive treatment or is considered by the attending clinician to be at risk of ROP. The first ophthalmological examination is performed at postnatal 4 weeks. In our unit, the criteria specified by the multicenter ETROP (Early Treatment for Retinopathy of Prematurity) study group are used in the application of laser photocoagulation for ROP [
17,
18].
Proven sepsis is defined as the presence of clinical and laboratory findings consistent with sepsis and a demonstrated causative pathogen. Clinical sepsis is defined as the presence of clinical and laboratory findings consistent with sepsis for which a causative pathogen could not be demonstrated [
19,
20]. Intraventricular hemorrhage (IVH) is evaluated using bedside cranial ultrasound (US) and staged according to the Papile classification [
21].
Data regarding the patients’ survival to discharge, any stage of BPD, PDA requiring medical treatment, any stage of ROP, grade 3–4 IVH, and clinical or proven sepsis was recorded from the patients’ records.
Follow-up ventilation management and other supportive treatments were provided by the attending neonatologist in accordance with unit protocols. In our unit, the feeding and care of preterm infants and the diagnosis and management of RDS, BPD, patent ductus arteriosus (PDA), and the retinopathy of prematurity (ROP) were carried out in accordance with Turkish Neonatology Society guidelines [
13,
14,
16,
17,
19,
22].
Written informed consent was obtained from the patients’ families. The study was approved by the Ankara City Hospital Ethics Committee (E1-19-198) in accordance with the Helsinki Declaration and prospectively registered at
clinicaltrials.gov (accessed on 12 May 2019) (NCT04191239).
2.3. Statistical Analysis
The data were analyzed using SPSS for Windows. Descriptive statistics are shown as frequency and percentage, mean and standard deviation, or median and range. A paired samples t-test was used for comparisons of repeated measures within subjects and the Mann–Whitney U test was used for comparisons between the two different groups. Statistical significance was accepted at p < 0.05.
3. Results
The study included a total of 28 patients, 14 in the PRVC group and 14 in the bilevel VG group. The mean birth weight was 876 g (range: 530–1170) and the mean gestational age was 26.4 weeks (range: 24–29). The characteristics of the patients are shown in
Table 1.
The patients’ peak inspiratory pressure (PIP2 and PIP3) was lower after ventilation in bilevel VG mode than in PRVC mode (13 vs. 14 cmH2O, respectively; paired samples t-test, p = 0.008). After 2 h of bilevel VG ventilation, the mean heart rate decreased from 149/min to 140/min (p = 0.001) and the oxygen saturation increased from 91% to 94% (p = 0.01). There were no significant differences in mean airway pressure, respiratory rate, or blood gas values.
As shown in
Table 2, there was no difference between the groups in terms of clinical outcomes. Both groups had similar rates of PDA, BPD, ROP, sepsis, and mortality. Two patients in the bilevel VG group and three patients in the PRVC group died. The cause of clinical deterioration in the nonsurviving infants was clinical sepsis, with positive culture in one patient. Grade 3–4 IVH was detected in four of the infants who died (two in each group).
4. Discussion
Mechanical ventilation is used when preterm infants have insufficient respiratory effort or in the presence of apnea or CO2 retention. CO2 excretion is determined by VT and the respiratory rate. Volume-targeted ventilation (VTV) strategies aim to provide a consistent VT. VTV has been used for many years in pediatric and adult patients, and tidal volume-targeted synchronized ventilation modes are currently the most recommended strategy for ventilating preterm infants.
The bilevel VG mode and PRVC mode of the GE® Carescape R860 model ventilator are two examples of adaptive target schemes for achieving target VT. This study aimed to compare and evaluate the safety and feasibility of these two modes for use in NICUs equipped with this ventilator model.
The literature includes very few studies of these modes and much less information about their use in neonates.
In a study investigating the effects of PRVC and VG ventilation modes on oxygenation parameters, airway pressures, and immunomodulation during one-lung ventilation (OLV) in 70 adult patients undergoing thoracic surgery, oxygenation parameters (PaO
2/FiO
2 and PaO
2) were significantly better in the PRVC group (
p = 0.004). The dead space volume/tidal volume (V
D/V
T) and inspiratory airway pressures were significantly lower in the PRVC group. In addition, all inflammatory parameters (alveolar and plasma interleukins and alveolar albumin levels and cell counts) were significantly lower in the PRVC group. Therefore, it was concluded that the PRVC mode had positive effects during OLV in thoracic surgery [
23].
Another study compared PCV and PRCV with VCV modes as lung-protective ventilation modes in 14 patients with acute lung injury or ARDS and examined whether PCV and PRVC reduced the work of breathing (WOB) more than VCV. A target V
T of 6.4 ± 0.5 mL/kg was specified during VCV and PRVC. During PCV, the inspiration pressure was adjusted as needed to achieve the same V
T. The authors reported that PCV and PRVC provided no advantage over high-flow VCV in reducing WOB during lung-protective ventilation [
24].
A study including 39 older intubated patients with chronic obstructive pulmonary disease compared PRVC and SIMV-VC modes and evaluated the efficacy of PRVC mode and its prevention of pulmonary barotrauma. Assessment of respiratory mechanics, arterial blood gas analysis, and vital signs at 2–4 h and at 48 h showed that PRVC resulted in rapid improvement in arterial blood gas parameters while maintaining low peak inspiratory pressure [
25].
Kıhtır et al. evaluated 61 children (median age 12) with acute respiratory failure ventilated with PRVC or pressure-control (PC) modes. PC ventilation mode was used on 40 (65.6%) patients and PRVC mode was used on 21 (34.4%) patients. Forty-four patients (72.1%) had hypercapnic respiratory failure and twenty-eight patients (45.9%) had hypoxemic respiratory failure. Although the PC ventilation mode was preferred more frequently in hypoxemic respiratory failure, no difference was found in terms of MV duration, length of stay in the pediatric intensive care unit, and mortality between the two respiratory modes. It was stated that PRVC mode can be used as a safe option for units that do not have sufficient experience in using PC ventilation mode [
26].
Another study compared PRVC and SIMV modes in 212 preterm infants under 1250 g who received mechanical ventilation in the first 6 h of life. There was no significant difference between the SIMV and PRVC groups in terms of infants alive and extubated on day 14 (41% vs. 37%), the duration of mechanical ventilation in survivors (median 24 vs. 33 days), or the proportion of infants not needing supplemental oxygen at postmenstrual 36 weeks (57% vs. 63%). Mode change due to unsuccessful ventilation was more frequent in the SIMV group (33%) than in the PRVC group (20%), but the inclusion of this unfavorable outcome did not change the overall result. The authors concluded that PRVC ventilation had no effect on the extubation time compared to SIMV [
27].
In a study comparing PRVC and IMV modes in 60 neonates under 2500 g who required mechanical ventilation, the duration of ventilation and the incidence of BPD were found to be similar. The incidence of IVH grade 3 or higher was lower in the PRVC group (
p < 0.005). In the subgroup analysis of infants under 1000 g, the PRVC group had shorter ventilation duration and a lower incidence of hypotension (
p < 0.005). It was reported that PRVC ventilation can be used safely in newborns and may contribute to a lower complication rate [
28].
Volume-targeted, volume-guaranteed ventilation is currently the most valid ventilation mode for preterm infants [
4]. Some devices using VG are specially designed for neonates. VG provides better results in preterm infants in terms of BPD, PCO
2 values, and air leaks. In our practice, we prefer VG and volume-targeted modes. We planned this study both because GE-brand ventilators have not been evaluated in neonatal and preterm infants in the medical literature and because these devices are available and being used in some hospitals.
In recent trials, valuable information regarding HFO ventilation in preterm infants has been provided. Although there is information that HFO can reduce BPD when used as the first mode in premature babies, more studies need to be designed on this subject [
29,
30].
To our knowledge, this is the first study comparing these two modes driven by the GE® Carescape R860 model ventilator in very preterm infants. Moreover, the infants were diagnosed and treated by experienced staff with strictly adapted guidelines.
The small number of patients is the main limitation of this study. Our results do not indicate that one of these modes is superior or more useful than other modes. However, devices and modes not previously used in neonatal studies were used as treatment options in very small preterm infants and were shown to be safe and applicable in this group. Another limitation may be our selection criteria because the infants included in the study are preterm infants with surfactant-treated RDS. The limited gestational age and the underlying cause may have altered the results. Larger studies including infants with different gestational ages and conditions that lead to intubation and invasive mechanical ventilation are required.
5. Conclusions
In conclusion, both the PRVC and bilevel VG modes of GE ventilators can be used safely in preterm infants, and although the difference may not be very pronounced, bilevel VG mode was associated with more favorable early clinical findings. Studies including more patients and comparing them with other modes will clarify and provide further evidence on this subject.
Author Contributions
Methodology, Ş.I. and F.E.C.; Formal analysis, F.E.C.; Investigation, H.G.K.K.; Resources, Ş.I., G.K.Ş. and Ö.E.; Data curation, Ö.E.; Writing—original draft, Ş.I.; Writing—review & editing, F.E.C. and H.G.K.K.; Visualization, G.K.Ş. and H.G.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ankara City Hospital Ethics Committee (E1-19-198, applied 24 December 2019, approved 3 March 2021).
Informed Consent Statement
Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bell, E.F.; Hintz, S.R.; Hansen, N.I.; Bann, C.M.; Wyckoff, M.H.; DeMauro, S.B.; Walsh, M.C.; Vohr, B.R.; Stoll, B.J.; Carlo, W.A.; et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA 2022, 327, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.P.; Karotkin, E.H.; Keszler, M.; Suresh, G.K. Assisted Ventilation of the Neonate; Overview of Assisted Ventilation; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; p. 140. ISBN 978-0-323-39006-4. [Google Scholar]
- Keszler, M. State of the art in conventional mechanical ventilation. J. Perinatol. 2009, 29, 262–275. [Google Scholar] [CrossRef]
- Klingenberg, C.; Wheeler, K.I.; McCallion, N.; Morley, C.J.; Davis, P.G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst. Rev. 2017, 10, CD003666. [Google Scholar] [CrossRef] [PubMed]
- Duman, N.; Tuzun, F.; Sutcuoglu, S.; Yesilirmak, C.D.; Kumral, A.; Ozkan, H. Impact of volume guarantee on synchronized ventilation in preterm infants: A randomized controlled trial. Intensive Care Med. 2012, 38, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Scopesi, F.; Calevo, M.; Rolfe, P.; Arioni, C.; Traggiai, C.; Risso, F.; Serra, G. Volume targeted ventilation (volume guarantee) in the weaning phase of premature newborn infants. Pediatr. Pulmonol. 2007, 42, 864–870. [Google Scholar] [CrossRef]
- Lista, G.; Colnaghi, M.; Castoldi, F.; Condò, V.; Reali, R.; Compagnoni, G.; Mosca, F. Impact of targeted-volume ventilation on lung inflammatory response in preterm infants with respiratory distress syndrome (RDS). Pediatr. Pulmonol. 2004, 37, 510–514. [Google Scholar] [CrossRef]
- Branson, R.D.; Robert, L.; Chatburn, R.L. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir. Care 2007, 52, 478–485. [Google Scholar]
- Chatburn, R.L. Classification of ventilator modes: Update and proposal for implementation. Respir. Care 2007, 52, 301–323. [Google Scholar]
- Ballard, J.; Khoury, J.; Wedig, K.; Wang, L.; Eilers-Walsman, B.; Lipp, R. New Ballard Score, expanded to include extremely premature infants. J. Pediatr. 1991, 119, 417–423. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Pareek, P.; Deshpande, S.; Suryawanshi, P.; Sah, L.K.; Chetan, C.; Maheshwari, R.; More, K. Less invasive surfactant administration (LISA) vs. intubation surfactant extubation (InSurE) in preterm infants with respiratory distress syndrome: A pilot randomized controlled trial. J. Trop. Pediatr. 2021, 67, fmab086. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, H.; Erdeve, O.; Kutman, H.G.K. Turkish Neonatal Society guideline on the management of respiratory distress syndrome and surfactant treatment. Turk. Arch. Pediatr. 2018, 53 (Suppl. S1), S45–S54. [Google Scholar] [CrossRef] [PubMed]
- Arsan, S.; Korkmaz, A.; Oguz, S. Turkish Neonatal Society guideline on prevention and management of bronchopulmonary dysplasia. Turk. Arch. Pediatr. 2018, 53 (Suppl. S1), S138–S150. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Köksal, N.; Aygün, C.; Uras, N. Turkish Neonatal Society guideline on the management of patent ductus arteriosus in preterm infants. Turk. Arch. Pediatr. 2018, 53 (Suppl. S1), S76–S87. [Google Scholar] [CrossRef]
- Koc, E.; Bas, A.Y.; Ozdek, S.; Ovali, F.; Basmak, H. Turkish Neonatal and Turkish Ophthalmology Societies consensus guideline on the retinopathy of prematurity. Turk. Arch. Pediatr. 2018, 53 (Suppl. S1), S151–S160. [Google Scholar] [CrossRef]
- Good, W.V.; Hardy, R.J.; Dobson, V.; Palmer, E.A.; Phelps, D.L.; Tung, B.; Redford, M.; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch. Ophthalmol. 2010, 128, 663–671. [Google Scholar] [CrossRef]
- Satar, M.; Arisoy, A.E.; Celik, I.H. Turkish Neonatal Society guideline on neonatal infections—Diagnosis and treatment. Turk. Arch. Pediatr. 2018, 53 (Suppl. S1), S88–S100. [Google Scholar] [CrossRef]
- Celik, I.H.; Hanna, M.; Canpolat, F.E.; Pammi, M. Diagnosis of neonatal sepsis: The past, present and future. Pediatr. Res. 2022, 91, 337–350. [Google Scholar] [CrossRef]
- Papile, L.-A.; Munsick-Bruno, G.; Schaefer, A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J. Pediatr. 1983, 103, 273–277. [Google Scholar] [CrossRef]
- Kultursay, N.; Bilgen, H.; Turkyilmaz, C. Turkish Neonatal Society guideline on enteral feeding of the preterm infant. Turk. Arch. Pediatr. 2018, 53 (Suppl. S1), S109–S118. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.; Ammar, A.; Kasemy, Z. Comparison Between Pressure Regulated Volume-Controlled (PRVC) and Volume-Controlled (VC) Ventilation on Oxygenation Parameters, Airway Pressures and Immune-Modulation During Thoracic Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H.; Campbell, A.R.; Dicker, R.A.; Katz, J.A.; Mackersie, R.C. Work of breathing during lung-protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: A comparison between volume and pressure-regulated breathing modes. Respir. Care 2005, 50, 1623–1631. [Google Scholar]
- Chang, S.C.; Shi, J.D.; Fu, C.P.; Wu, X.; Li, S. A comparison of synchronized intermittent mandatory ventilation and pressure-regulated volume control ventilation in elderly patients with acute exacerbations of COPD and respiratory failure. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Kihtir, H.S.; Akcay, N.; Sevketoglu, E. Pressure-regulated volume control and pressure-control ventilation modes in pediatric acute respiratory failure. Turk. J. Anaesthesiol. Reanim. 2022, 50, 18–23. [Google Scholar] [CrossRef]
- D’angio, C.T.; Chess, P.R.; Kovacs, S.J.; Sinkin, R.A.; Phelps, D.L.; Kendig, J.W.; Myers, G.J.; Reubens, L.; Ryan, R.M. Pressure-regulated volume control ventilation vs. synchronized intermittent mandatory ventilation for very low-birth-weight infants: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2005, 159, 868–875. [Google Scholar] [CrossRef]
- Piotrowski, A.; Sobala, W.; Kawczyński, P. Patient-initiated, pressure-regulated, volume-controlled ventilation compared with intermittent mandatory ventilation in neonates: A prospective, randomised study. Intensive Care Med. 1997, 23, 975–981. [Google Scholar] [CrossRef]
- Cools, F.; Offringa, M.; Askie, L.M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2015, 2015, CD000104. [Google Scholar] [CrossRef]
- Ackermann, B.W.; Klotz, D.; Hentschel, R.; Thome, U.H.; van Kaam, A.H. High-frequency ventilation in preterm infants and neonates. Pediatr. Res. 2023, 93, 1810–1818. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).