Atopy and Elevation of IgE, IgG3, and IgG4 May Be Risk Factors for Post COVID-19 Condition in Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Extraction and Event Variables
2.3. Data Analysis
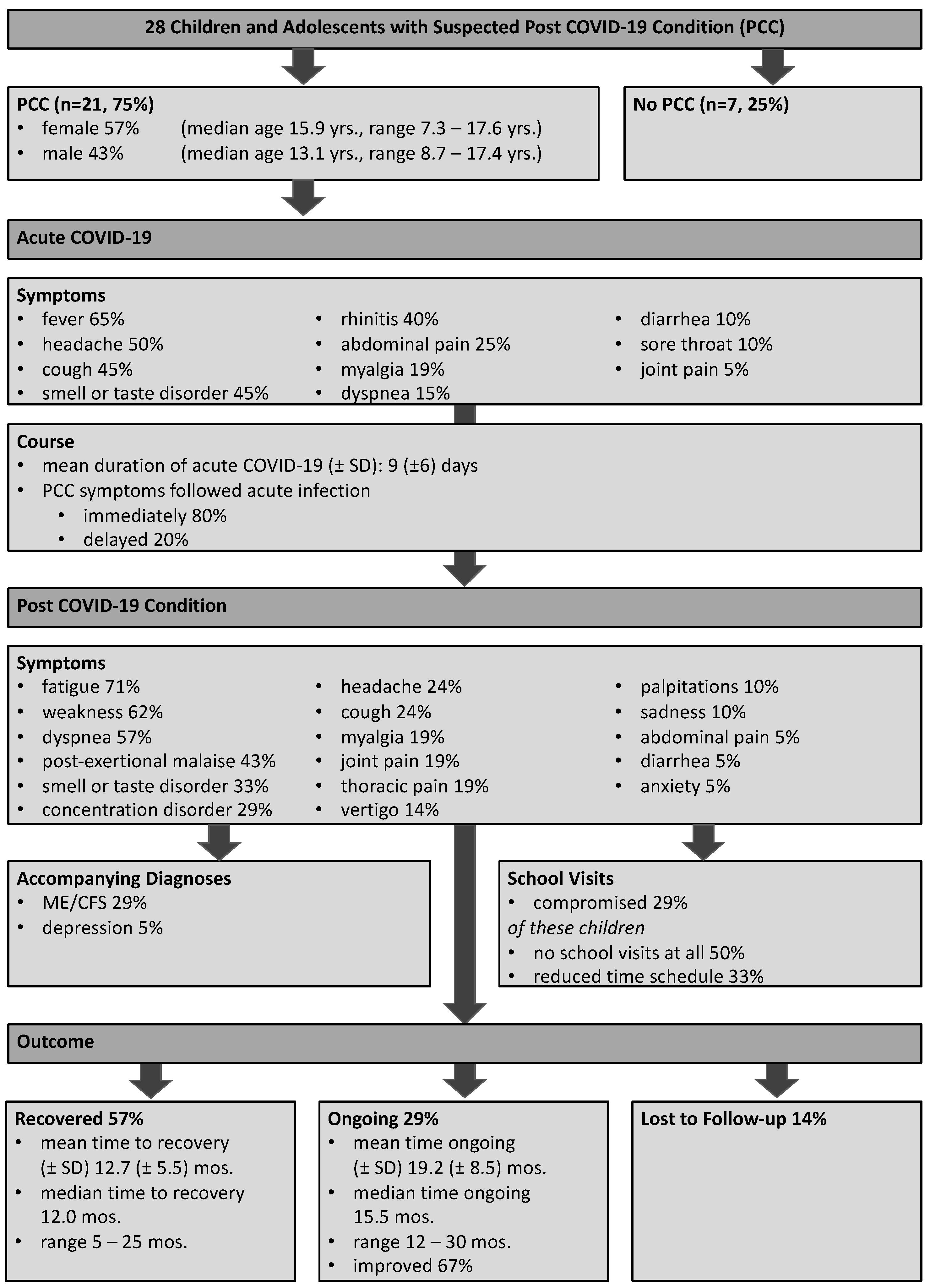
3. Results
3.1. Patient Cohort and Disease Characteristics
3.2. Acute COVID-19 Disease Course
3.3. Post COVID-19 Condition Disease Course
3.4. Pulmonary Function Tests for Dyspnea
3.5. Molecular Investigations for Organ Dysfunction
3.6. Immunological Diagnostics for Immune Dysregulation
4. Discussion
4.1. General Cohort Characteristics in Pediatric and Adolescent PCC
4.2. Neuropsychological Phenotypes
4.3. Discrepancy in Self-Reported Dyspnea and Unremarkable Pulmonary Function
4.4. Molecular Investigations
4.5. Potential Immune Dysregulation as a Risk Factor for Pediatric PCC
4.6. Recovery
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category | Sub-Category | Criteria |
|---|---|---|
| A. Exhaustion after exertion | Significant new and persistent physical/mental exhaustion | |
| Increased general feeling of sickness and/or pain after exertion | ||
| Significant exhaustion after exertion | ||
| Significantly delayed recovery periods (hours to days) | ||
| Low tolerance to physical/mental exertion, reduced activity level | ||
| B. Neurological disorders | B.1 Neurocognitive impairments | Difficulties in processing information (concentration, perceptual problems, confusion) |
| Impairment of short-term memory (word-finding, reading disorders, impaired working memory) | ||
| B.2. Pain | Significant muscle and/or joint pain | |
| Headaches (including migraines, ascending neck pain) | ||
| B.3. Sleep disturbances | Difficulty falling asleep/staying asleep, altered day-night rhythm | |
| Unrestful sleep, daytime sleepiness | ||
| B.4 Neurosensory, perceptual or motor disorders | Sensory/perceptual disorders (difficulty seeing, hearing, smelling, tasting, touching) | |
| Movement disorders (incoordination, muscle weakness, gait disturbance) | ||
| Sensory/perceptual disorders (difficulty seeing, hearing, smelling, tasting, touching) | ||
| Movement disorders (incoordination, muscle weakness, gait disturbance) | ||
| C. Immunological or autonomic disorders | Flu-like symptoms (painful lymph nodes, sore throat, feeling sick) | |
| Susceptibility to viral infections with prolonged recovery periods | ||
| Gastrointestinal disorders (nausea, diffuse pain, burning sensation, bloating) | ||
| Bladder dysfunction | ||
| New onset of intolerance to foods, medications, odors | ||
| D. Cardiorespiratory or temperature stability disorders | Cardiovascular (dizziness, palpitations and/or “blacking out” with changes in position; palpitations) | |
| Respiratory (shortness of breath on light exertion, difficulty breathing, fatigue of auxiliary respiratory muscles) | ||
| Loss of thermostatic ability (body temperature adjustment disorder, sweating, feverish feeling, cold extremities) | ||
| Lack of tolerance for extreme temperatures |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Heinsohn, T.; Lange, B.; Vanella, P.; Rodiah, I.; Glockner, S.; Joachim, A.; Becker, D.; Brandle, T.; Dhein, S.; Ehehalt, S.; et al. Infection and transmission risks of COVID-19 in schools and their contribution to population infections in Germany: A retrospective observational study using nationwide and regional health and education agency notification data. PLoS Med. 2022, 19, e1003913. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Russell, S.J.; Croker, H.; Packer, J.; Ward, J.; Stansfield, C.; Mytton, O.; Bonell, C.; Booy, R. School closure and management practices during coronavirus outbreaks including COVID-19: A rapid systematic review. Lancet Child Adolesc. Health 2020, 4, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Allin, B.; Nugawela, M.D.; Rojas, N.; Dalrymple, E.; Pinto Pereira, S.; Soni, M.; Knight, M.; Cheung, E.Y.; Heyman, I.; et al. Long COVID (post-COVID-19 condition) in children: A modified Delphi process. Arch. Dis. Child. 2022, 107, 674–680. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Atchison, C.J.; Whitaker, M.; Donnelly, C.A.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Ashby, D.; Barclay, W.; Cooke, G.S.; Elliott, P.; et al. Characteristics and predictors of persistent symptoms post-COVID-19 in children and young people: A large community cross-sectional study in England. Arch. Dis. Child. 2023, 108, e12. [Google Scholar] [CrossRef]
- Kostev, K.; Smith, L.; Koyanagi, A.; Konrad, M.; Jacob, L. Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19. Pediatr. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Ganesh, R.; Grach, S.L.; Ghosh, A.K.; Bierle, D.M.; Salonen, B.R.; Collins, N.M.; Joshi, A.Y.; Boeder, N.D., Jr.; Anstine, C.V.; Mueller, M.R.; et al. The Female-Predominant Persistent Immune Dysregulation of the Post-COVID Syndrome. Mayo Clin. Proc. 2022, 97, 454–464. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated with Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Mogensen, I.; Ekstrom, S.; Hallberg, J.; Georgelis, A.; Melen, E.; Bergstrom, A.; Kull, I. Post COVID-19 symptoms are common, also among young adults in the general population. Sci. Rep. 2023, 13, 11300. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, S.F. Epidemiology and natural history of atopic diseases. Eur. Clin. Respir. J. 2015, 2, 24642. [Google Scholar] [CrossRef] [PubMed]
- Dafsari, H.S.; Ebrahimi-Fakhari, D.; Saffari, A.; Deneubourg, C.; Fanto, M.; Jungbluth, H. EPG5-Related Disorder. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Dafsari, H.S.; Pemberton, J.G.; Ferrer, E.A.; Yammine, T.; Farra, C.; Mohammadi, M.H.; Ghayoor Karimiani, E.; Hashemi, N.; Souaid, M.; Sabbagh, S.; et al. PI4K2A deficiency causes innate error in intracellular trafficking with developmental and epileptic-dyskinetic encephalopathy. Ann. Clin. Transl. Neurol. 2022, 9, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, G.; De Vittori, V.; De Nola, R.; Pignataro, E.; Anania, C.; De Castro, G.; Cinicola, B.; Gori, A.; Cicinelli, E.; Zicari, A.M. Updates on Children with Allergic Rhinitis and Asthma during the COVID-19 Outbreak. J. Clin. Med. 2021, 10, 2278. [Google Scholar] [CrossRef]
- Parisi, G.F.; Diaferio, L.; Brindisi, G.; Indolfi, C.; Umano, G.R.; Klain, A.; Marchese, G.; Ghiglioni, D.G.; Zicari, A.M.; Marseglia, G.L.; et al. Cross-Sectional Survey on Long Term Sequelae of Pediatric COVID-19 among Italian Pediatricians. Child. 2021, 8, 769. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Korner, R.W.; Weber, L.T. Prevalence of COVID-19 Among Children and Adolescents While Easing Lockdown Restrictions in Cologne, North Rhine-Westphalia, Germany. Klin. Padiatr. 2021, 233, 135–140. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Santa Cruz, A.; Mendes-Frias, A.; Azarias-da-Silva, M.; Andre, S.; Oliveira, A.I.; Pires, O.; Mendes, M.; Oliveira, B.; Braga, M.; Lopes, J.R.; et al. Post-acute sequelae of COVID-19 is characterized by diminished peripheral CD8(+)beta7 integrin(+) T cells and anti-SARS-CoV-2 IgA response. Nat. Commun. 2023, 14, 1772. [Google Scholar] [CrossRef]
- Irfan, O.; Muttalib, F.; Tang, K.; Jiang, L.; Lassi, Z.S.; Bhutta, Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 440–448. [Google Scholar] [CrossRef]
- Zheng, Y.B.; Zeng, N.; Yuan, K.; Tian, S.S.; Yang, Y.B.; Gao, N.; Chen, X.; Zhang, A.Y.; Kondratiuk, A.L.; Shi, P.P.; et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J. Infect. Public Health 2023, 16, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Morello, R.; Mariani, F.; Mastrantoni, L.; De Rose, C.; Zampino, G.; Munblit, D.; Sigfrid, L.; Valentini, P.; Buonsenso, D. Risk factors for post-COVID-19 condition (Long Covid) in children: A prospective cohort study. EClinicalMedicine 2023, 59, 101961. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.; Di Maio, J.; Weeraratne, T.; Kennedy, K.M.; Oliver, L.K.; Bouchard, M.; Malhotra, D.; Habashy, J.; Ding, J.; Bhopa, S.; et al. Resilience in adolescence during the COVID-19 crisis in Canada. BMC Public Health 2023, 23, 1097. [Google Scholar] [CrossRef] [PubMed]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C.; European Network on MC. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Heiss, R.; Tan, L.; Schmidt, S.; Regensburger, A.P.; Ewert, F.; Mammadova, D.; Buehler, A.; Vogel-Claussen, J.; Voskrebenzev, A.; Rauh, M.; et al. Pulmonary Dysfunction after Pediatric COVID-19. Radiology 2023, 306, e221250. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Duthoit, L.; Perez, T.; Felloni, P.; Faivre, J.B.; Fry, S.; Bautin, N.; Chenivesse, C.; Remy, J.; Duhamel, A. Assessment of pulmonary arterial circulation 3 months after hospitalization for SARS-CoV-2 pneumonia: Dual-energy CT (DECT) angiographic study in 55 patients. EClinicalMedicine 2021, 34, 100778. [Google Scholar] [CrossRef]
- George, P.M.; Reed, A.; Desai, S.R.; Devaraj, A.; Faiez, T.S.; Laverty, S.; Kanwal, A.; Esneau, C.; Liu, M.K.C.; Kamal, F.; et al. A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae. Sci. Transl. Med. 2022, 14, eabo5795. [Google Scholar] [CrossRef]
- Maniscalco, M.; Ambrosino, P.; Poto, R.; Fuschillo, S.; Poto, S.; Matera, M.G.; Cazzola, M. Can FeNO be a biomarker in the post-COVID-19 patients monitoring? Respir. Med. 2022, 193, 106745. [Google Scholar] [CrossRef]
- Kooner, H.K.; McIntosh, M.J.; Matheson, A.M.; Venegas, C.; Radadia, N.; Ho, T.; Haider, E.A.; Konyer, N.B.; Santyr, G.E.; Albert, M.S.; et al. (129)Xe MRI ventilation defects in ever-hospitalised and never-hospitalised people with post-acute COVID-19 syndrome. BMJ Open Respir. Res. 2022, 9, e001235. [Google Scholar] [CrossRef]
- Matheson, A.M.; McIntosh, M.J.; Kooner, H.K.; Abdelrazek, M.; Albert, M.S.; Dhaliwal, I.; Nicholson, J.M.; Ouriadov, A.; Svenningsen, S.; Parraga, G. Longitudinal follow-up of postacute COVID-19 syndrome: DL(CO), quality-of-life and MRI pulmonary gas-exchange abnormalities. Thorax 2023, 78, 418–421. [Google Scholar] [CrossRef]
- Turner, S.; Naidoo, C.A.; Usher, T.J.; Kruger, A.; Venter, C.; Laubscher, G.J.; Khan, M.A.; Kell, D.B.; Pretorius, E. Increased Levels of Inflammatory and Endothelial Biomarkers in Blood of Long COVID Patients Point to Thrombotic Endothelialitis. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Ruhe, J.; Giszas, B.; Schlosser, M.; Reuken, P.A.; Wolf, G.; Stallmach, A. Immune adsorption for the treatment of fatigue-dominant long-/post-COVID syndrome. Dtsch. Ärzteblatt Int. 2023, 120, 499–500. [Google Scholar] [CrossRef]
- Sukumaran, A.; Thomas, R.E.; Krishnan, R.A.; Thomas, T.; Thomas, R.; Vijayan, D.K.; Paul, J.K.; Vasudevan, D.M. Sequential Profiling of Anti-SARS-CoV-2 IgG Antibody in Post COVID-19 Patients. Indian. J. Clin. Biochem. 2022, 37, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.W.; Chua, G.T.; To, K.K.W.; Wong, J.S.C.; Tu, W.; Kwok, J.S.Y.; Wong, W.H.S.; Wang, X.; Zhang, Y.; Rosa Duque, J.S.; et al. Assessment of SARS-CoV-2 Immunity in Convalescent Children and Adolescents. Front. Immunol. 2021, 12, 797919. [Google Scholar] [CrossRef]
- Di Chiara, C.; Cantarutti, A.; Costenaro, P.; Dona, D.; Bonfante, F.; Cosma, C.; Ferrarese, M.; Cozzani, S.; Petrara, M.R.; Carmona, F.; et al. Long-term Immune Response to SARS-CoV-2 Infection Among Children and Adults after Mild Infection. JAMA Netw. Open 2022, 5, e2221616. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, B.; Wang, X.; Zhang, L. The humoral response and antibodies against SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Dobano, C.; Ramirez-Morros, A.; Alonso, S.; Rubio, R.; Ruiz-Olalla, G.; Vidal-Alaball, J.; Macia, D.; Catalina, Q.M.; Vidal, M.; Casanovas, A.F.; et al. Sustained seropositivity up to 20.5 months after COVID-19. BMC Med. 2022, 20, 379. [Google Scholar] [CrossRef]
- Fuentes-Villalobos, F.; Garrido, J.L.; Medina, M.A.; Zambrano, N.; Ross, N.; Bravo, F.; Gaete-Argel, A.; Oyarzun-Arrau, A.; Amanat, F.; Soto-Rifo, R.; et al. Sustained Antibody-Dependent NK Cell Functions in Mild COVID-19 Outpatients during Convalescence. Front. Immunol. 2022, 13, 796481. [Google Scholar] [CrossRef]
- Thamm, R.; Poethko-Muller, C.; Huther, A.; Thamm, M. Allergic diseases in children and adolescents in Germany. Results of the cross-sectional KiGGS Wave 2 study and trends. J. Health Monit. 2018, 3, 3–16. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2022, 59, 2. [Google Scholar] [CrossRef]
- Bergantini, L.; Baldassarri, M.; d’Alessandro, M.; Brunelli, G.; Fabbri, G.; Zguro, K.; Degl’Innocenti, A.; Fallerini, C.; Bargagli, E.; Renieri, A. Ultra-rare RTEL1 gene variants associate with acute severity of COVID-19 and evolution to pulmonary fibrosis as a specific long COVID disorder. Respir. Res. 2023, 24, 158. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID after Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Kober, C.; Manni, S.; Wolff, S.; Barnes, T.; Mukherjee, S.; Vogel, T.; Hoenig, L.; Vogel, P.; Hahn, A.; Gerlach, M.; et al. IgG3 and IgM Identified as Key to SARS-CoV-2 Neutralization in Convalescent Plasma Pools. PLoS ONE 2022, 17, e0262162. [Google Scholar] [CrossRef]
- Bianchini, R.; Karagiannis, S.N.; Jordakieva, G.; Jensen-Jarolim, E. The Role of IgG4 in the Fine Tuning of Tolerance in IgE-Mediated Allergy and Cancer. Int. J. Mol. Sci. 2020, 21, 5017. [Google Scholar] [CrossRef] [PubMed]
- Meltendorf, S.; Vogel, K.; Thurm, C.; Pratsch, F.; Reinhold, A.; Farber, J.; Heuft, H.G.; Kaasch, A.J.; Hachenberg, T.; Weinzierl, S.; et al. IL-13 determines specific IgE responses and SARS-CoV-2 immunity after mild COVID-19 and novel mRNA vaccination. Eur. J. Immunol. 2022, 52, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Van der Pouw Kraan, T.C.; Van der Zee, J.S.; Boeije, L.C.; De Groot, E.R.; Stapel, S.O.; Aarden, L.A. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin. Exp. Immunol. 1998, 111, 129–135. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schafer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K. Post-COVID mRNA-vaccine IgG4 shift: Worrisome? mSphere 2023, 8, e00085-23. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.; Walter, A.J.; Glück, V.; Magnus, C.L.; Glück, T.; Schuster, P.; Blaas, S.; Montanari, I.; Koller, M.; Mohr, A.; et al. IgG4 serum levels are not elevated in cases of Post-COVID syndrome. bioRxiv 2023. [CrossRef]
| Category | Parameter | Unit | Mean | SD |
|---|---|---|---|---|
| Electrolytes | Sodium | mmol/L | 138.7 | 1.77 |
| Potassium | mmol/L | 3.87 | 0.33 | |
| Chloride | mmol/L | 103.0 | 9.5 | |
| Calcium | mmol/L | 2.39 | 0.097 | |
| Phosphate | mmol/L | 1.35 | 0.23 | |
| Liver Function | Alanine aminotransferase | U/L | 24.14 | 5.2 |
| Aspartate aminotransferase | U/L | 18.67 | 7.66 | |
| γ-Glutamyltransferase | U/L | 17.57 | 8.93 | |
| Glutamate dehydrogenase | U/L | 1.37 | 1.13 | |
| Cholinesterase | kU/L | 8.08 | 2.21 | |
| Bilirubin | mg/dL | 0.41 | 0.36 | |
| Skeletal and Cardiac Muscle | Creatine kinase | U/L | 107.1 | 47.58 |
| Creatine kinase-MB | U/L | 14.95 | 4.55 | |
| Troponin T-high sensitivity | µg/L | 0.0019 | 0.0044 | |
| NT-proBNP | ng/L | 47.6 | 45.84 | |
| Kidney Function | Total protein | g/L | 74.33 | 4.61 |
| Albumin | g/L | 47.43 | 3.3 | |
| Creatinine | mg/dL | 0.63 | 0.17 | |
| Blood urea nitrogen | mg/dL | 26.14 | 5.28 | |
| Uric acid | mg/dL | 4.46 | 0.86 | |
| Thyroid Function | Thyroid-stimulating hormone | mU/L | 2.173 | 1.06 |
| Free thyroxine (T4) | ng/L | 12.42 | 1.64 | |
| Coagulation | International normalized ratio | 1.043 | 0.10 | |
| Activated partial thromboplastin time | s | 26.1 | 2.61 | |
| Fibrinogen | g/L | 2.59 | 0.45 | |
| D-dimer | mg/L | 0.23 | 0.27 | |
| Others | Blood sugar | mg/dL | 86.8 | 9.5 |
| Lactate dehydrogenase | U/L | 203 | 34.34 | |
| Iron | µmoL/L | 14.58 | 7.12 | |
| Ferritin | µg/L | 40.95 | 18.63 | |
| Transferrin | g/L | 2.8 | 0.5 | |
| Transferrin saturation | % | 21.62 | 11.74 | |
| Lipase | U/L | 27.89 | 19.53 | |
| Inflammation | C-reactive Protein | mg/L | 0.62 | 0.9 |
| Erythrocyte sedimentation rate | mm/h | 4.47 | 2.39 |
| Category | Parameter | Unit | Mean | SD |
|---|---|---|---|---|
| Immunoglobulins | IgG | g/L | 10.66 | 2.86 |
| IgG1 | mg/L | 6014 | 2015 | |
| IgG2 | mg/L | 2865 | 1530 | |
| IgG3 | mg/L | 863 | 1130 | |
| IgG4 | mg/L | 538 | 453 | |
| IgA | g/L | 1.24 | 0.64 | |
| IgM | g/L | 1.11 | 0.57 | |
| IgE | kU/L | 174.2 | 204.3 | |
| SARS-CoV-2 Spike RBD IgG | BAU/mL | 1233 | 1707 | |
| Complete Blood Count | Leukocytes | 109/L | 6.85 | 2.11 |
| Erythrocytes | 1012/L | 4.92 | 0.44 | |
| Hemoglobin | g/dL | 13.72 | 1.07 | |
| Hematocrit | % | 40.48 | 3.03 | |
| Mean corpuscular volume | fl | 82.57 | 2.48 | |
| Mean corpuscular hemoglobin | pg | 28.05 | 1.28 | |
| Mean corpuscular hemoglobin concentration | g/dL | 82.57 | 2.48 | |
| Red cell distribution width | % | 12.49 | 0.58 | |
| Mean thrombocyte volume | fl | 9.7 | 0.95 | |
| Normoblasts, rel.; abs. | %; 109/L | 0; 0 | 0; 0 | |
| Neutrophils, rel.; abs. | %; 109/L | 49.27; 3.35 | 11.2; 1.36 | |
| Immature granulocytes, rel.; abs. | %; 109/L | 0.26; 0.017 | 0.08; 0.011 | |
| Lymphocytes, rel.; abs. | %; 109/L | 39.82; 2.76 | 11.04; 1.40 | |
| Monocytes, rel.; abs. | %; 109/L | 7.39; 0.48 | 2.49; 0.17 | |
| Eosinophils, rel.; abs. | %; 109/L | 2.31; 0.16 | 1.35; 0.12 | |
| Basophils, rel.; abs. | %; 109/L | 0.60; 0.043 | 0.26; 0.027 | |
| Lymphocyte Subsets (FACS) | Lymphocytes, rel.; abs. | %; 109/L | 39.26; 2.7 | 8.81; 1.16 |
| T lymphocytes CD3+, rel.; abs. | %; 109/L | 71.76; 1.9 | 7.71; 0.73 | |
| T lymphocytes CD3+HLA-DR+, rel.; abs. | %; 109/L | 8.12; 0.14 | 6.8; 0.12 | |
| T lymphocytes CD4+, rel.; abs. | %; 109/L | 42.83; 1.12 | 8.14; 0.46 | |
| T lymphocytes CD8+, rel.; abs. | %; 109/L | 23.40; 0.63 | 4.67; 0.28 | |
| T lymphocytes CD8+CD38+, rel.; abs. | %; 109/L | 66.06; 0.37 | 10.53; 0.16 | |
| CD4/CD8 ratio | 1.98 | 0.7 | ||
| B lymphocytes CD19+, rel.; abs. | %; 109/L | 16.85; 0.49 | 5.76; 0.37 | |
| NK cells CD16+CD56+, rel.; abs. | %; 109/L | 9.87; 0.27 | 5.1; 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Körner, R.W.; Bansemir, O.Y.; Franke, R.; Sturm, J.; Dafsari, H.S. Atopy and Elevation of IgE, IgG3, and IgG4 May Be Risk Factors for Post COVID-19 Condition in Children and Adolescents. Children 2023, 10, 1598. https://doi.org/10.3390/children10101598
Körner RW, Bansemir OY, Franke R, Sturm J, Dafsari HS. Atopy and Elevation of IgE, IgG3, and IgG4 May Be Risk Factors for Post COVID-19 Condition in Children and Adolescents. Children. 2023; 10(10):1598. https://doi.org/10.3390/children10101598
Chicago/Turabian StyleKörner, Robert Walter, Ole Yannick Bansemir, Rosa Franke, Julius Sturm, and Hormos Salimi Dafsari. 2023. "Atopy and Elevation of IgE, IgG3, and IgG4 May Be Risk Factors for Post COVID-19 Condition in Children and Adolescents" Children 10, no. 10: 1598. https://doi.org/10.3390/children10101598
APA StyleKörner, R. W., Bansemir, O. Y., Franke, R., Sturm, J., & Dafsari, H. S. (2023). Atopy and Elevation of IgE, IgG3, and IgG4 May Be Risk Factors for Post COVID-19 Condition in Children and Adolescents. Children, 10(10), 1598. https://doi.org/10.3390/children10101598






