Abstract
Choanal atresia is the most common congenital anatomical abnormality of the nasal cavities, manifested with a clinical picture of neonatal respiratory distress. The treatment requires interdisciplinary management based mainly on tertiary referral centre experiences. However, there is a lack of high-quality evidence in the available literature. Recommendations were prepared based on a systematic review of the supporting literature: on a website survey addressed to the participating authors consisting of 28 questions and on five live meetings. The initial response to the recommendations was determined at their presentation at the sectional meeting of the section for otorhinolaryngology of the Slovenian Medical Association. Then, reactions from the professional public were accepted until the recommendations were presented at the Expert Council for Otorhinolaryngology of the Slovenian Medical Association. A systematic literature review identified eight systematic reviews or meta-analyses and four randomized controlled clinical trials. Thirty-four recommendations for diagnosis, treatment and postoperative management were consolidated. The paper presents the proposal and first Slovenian recommendations for treating patients with choanal atresia. They are based on foreign medical institutions’ published literature and our clinical experience. They represent the basic requirements of diagnostics and may represent an essential guide in treatment.
1. Causes of Nasal Obstruction in an Infant
Normal nasal patency is essential for the breathing of a baby, who, in the first months of life, breathes mainly through the nose [1]. However, in the early period of a child’s life, breathing through the nose can be hindered due to congenital or acquired diseases of the nose or nasopharynx (Table 1). Congenital anatomical abnormalities of the nasal cavity (Table 1), including choanal atresia (CA), are rare causes of obstruction but often cause neonatal respiratory distress. In addition to congenital anatomical abnormalities, nasal obstruction can also be caused by congenital tumours, injuries, foreign bodies and inflammation. Inflammation of the nose and paranasal sinuses is the most common cause of nasal obstruction in infants but is mostly not directly life-threatening [2].

Table 1.
Causes of nasal obstruction in infants.
2. Epidemiology, Pathogenesis and Anatomical Characteristics of Choanal Atresia
Choanae are paired anatomical structures that are superiorly delimited by the inferior surface of the sphenoid body, medially by vomer, laterally by the medial plate of the pterygoid process and inferiorly by horizontal part of the palatine bone.
CA is the most common congenital anatomical abnormality of the nasal cavities [1]. It occurs in one in 5000–8000 neonates, twice as common in females, twice as often on the right and twice as often unilaterally (i.e., the rule of two) [4]. In 70%, CA is bony, and in 30%, it is bony-membranous since the atretic plate also consists of the membrane and the bone. Very rarely, CA is exclusively membranous [2]. The atretic plate may partially (i.e., stenosis) or completely (i.e., atresia) block the choana [4]. At least half of patients have syndromes associated with CA, most commonly CHARGE syndrome, characterized by coloboma (C), congenital heart defect (H), choanal atresia (A), mental retardation and growth disorder (R), developmental disorders of the genitals or urinary tract (G) and ear deformity (E) [5]. CA can occur as part of Treacher-Collins [2], Apert [2], fetal alcohol [2], Pfeiffer [2,6], Crouzon [4], Binder syndrome [2], ectrodactyly-ectodermal dysplasia clefting syndrome [7], hypoparathyroidism, deafness and renal dysplasia syndrome [8], mandibulofacial dysostosis [4], craniofacial clefts [9], etc.
CA results from disturbed embryogenesis in the fourth week of gestational age [2]. From the first description of the disease by the German physician Johann George Röderer in 1755 until today, the origin is explained with four theories: 1. persistence of the embryonic buccopharyngeal membrane, 2. persistence of the nasobuccal Hochstetter’s membrane, 3. improper migration of neural crest cells and 4. irregular growth of the mesoderm [10]. Disrupted embryogenesis is accompanied by medialization and ossification of the lateral nasal wall, especially the medial plates of pterygoid processes [2]. In addition, CA can occur due to vitamin A deficiency or taking thyrostatics during pregnancy [4].
3. The Clinical Picture of Choanal Atresia
The clinical picture of a patient with CA depends on the side and degree of impairment, associated craniofacial anomalies and systemic disease.
3.1. Bilateral Choanal Atresia
The position of the epiglottis above the soft palate and the tongue’s contact with the soft palate along its entire length a few months after birth prevents normal breathing through the mouth. The infant begins to learn to breathe through the mouth between the fourth and sixth week of age, allowing for normal feeding through the mouth [3]. At six months of age, mouth breathing is usually established [2]. As a result, bilateral CA due to nasal obstruction in the infant may occur as life-threatening respiratory distress immediately at birth, when the infant cannot breathe, or later as respiratory distress at first feeding.
The clinical picture of nasal obstruction consists of cyanosis during feeding and improvement of breathing during crying (i.e., cyclic cyanosis), episodes of apnea, snoring, nasal flutter, nasal congestion, nasal discharge, stertor, snoring, intercostal and jugulum retractions, hyponasal cry, aerophagia with abdominal tension, sleep disturbances and growth retardation [3]. In addition, in associated diseases of the head, neck or distant organs, when endotracheal intubation of the neonate is required immediately after birth, CA may first appear only after several failed extubation attempts [4].
Bilateral CA is associated in 34% with other upper respiratory tract abnormalities, which lead to respiratory distress. These include subglottic stenosis, laryngomalacia and tracheomalacia. In addition, in 21%, bilateral CA is accompanied by other congenital craniofacial abnormalities (Table 1) [5].
3.2. Unilateral Choanal Atresia
Unilateral CA rarely causes neonatal respiratory distress syndrome. It is most commonly expressed later than bilateral CA, with a clinical picture of chronic unilateral nasal discharge and unilateral obstruction, which may resemble chronic rhinosinusitis or a foreign body in the nasal cavity. As a result, unilateral CA is often diagnosed later in adulthood [4].
4. Materials and Methods
When preparing the recommendations, we followed the procedure of Rosenfeld et al. (2013) for the preparation of clinical guidelines and recommendations in otorhinolaryngology—head and neck surgery [11] and general instructions for the preparation of clinical guidelines [12].
Instead of conference calls, five live meetings were held. Recommendations were written (Table 2), which were coordinated with the participating authors and institutions based on the supporting literature searched according to the procedure described in Table 3 and a website survey addressed to the participating authors consisting of 28 questions following the example of Moreddu et al. (2019) [13]. In addition, eight systematic reviews or meta-analyses [14,15,16,17,18,19,20,21] and four randomized controlled clinical trials [22,23,24,25] were identified.

Table 2.
Summary of Slovenian recommendations for the management of choanal atresia.

Table 3.
Three-step process of reviewing the supporting literature in preparing Slovenian recommendations for the treatment of choanal atresia.
For each recommendation, we determined the level of evidence and the evidence grade using the OCEBM Levels of Evidence (Table 4) [29]. In addition, we examined the risks and benefits of adopting each recommendation. Finally, we determined four levels of strength of recommendations (i.e., four types of text usage) following the example of the recommendations of the American Academy of Otolaryngology-Head and Neck Surgery (Table 4): strongly recommended, recommended, optional and strongly recommended against [11]:

Table 4.
Determination of recommendations’ strength.
∙ Strongly recommended means that the physician should follow the recommendation unless there is a clear and compelling argument against the recommendation.
∙ Recommended means that the physician should follow the recommendation but pay attention to new information and patient peculiarities.
∙ Optional means that the physician must be flexible in making decisions and accepting the recommendation. He must consider the possibility of taking different measures and measures adapted to the patient [11].
First, the evidence grade was determined for the included studies according to OCEBM [29], and then, the determination of strength with the benefit–harm ratio assessment by Rosenfeld et al. (2013) [11].
The preliminary response to the recommendations was determined at their presentation at the sectional meeting of the section for otorhinolaryngology of the Slovenian Medical Association on 19th December 2020. We accepted responses from the professional public until the recommendations were presented at the Expert Council for Otorhinolaryngology of the Slovenian Medical Association. This is the umbrella regulatory body for the field of otorhinolaryngology in Slovenia. It reviewed recommendations, suggested corrections and approved them on 10th May 2021.
5. Results
Thirty-four recommendations were written (Table 2) and coordinated based on the website survey and review of the literature (Table 3), which identified eight systematic reviews or meta-analyses [14,15,16,17,18,19,20,21] and four randomized controlled clinical trials [22,23,24,25].
5.1. Diagnosis of Choanal Atresia
5.1.1. Bilateral Choanal Atresia
At birth or in the early postpartum period, the clinical picture of respiratory obstruction should be checked for nasal patency to rule out bilateral CA [30] and other causes of nasal obstruction (Table 1).
Recommendation 1:

In the case of respiratory distress in a neonate, we recommend that the physician perform a test with a saline solution to confirm or rule out nasal obstruction—level of Evidence IIa, evidence grade C (Table 2) (Figure 1).

Figure 1.
Algorithm for neonatal respiratory distress syndrome due to nasal obstruction caused by bilateral choanal atresia. The paediatrician and otorhinolaryngologist play a leading role in extensive diagnostic management. In cooperation with the otorhinolaryngologist, the paediatrician also takes care of coordination in treating the child by other specialists and for imaging and diagnostic evaluation. The otorhinolaryngologist primarily provides management by other subspecialist otorhinolaryngologists. A positive saline and aspiration catheter test means that the saline or catheter does not pass through the nasal cavity into the pharynx.
A few drops of saline solution are instilled into both nostrils. If the saline solution pours forward from the nostrils, the test is positive on the side (or both) where the spill is observed. This makes CA likely. If the saline solution passes into the throat, the test is negative. Therefore, CA is not possible, but respiratory distress can be caused by other causes of nasal obstruction (Table 1).
Recommendation 2:
An aspiration catheter is used to assess the patency of the nasal cavities and the nasopharynx and, in some cases, eliminates the reversible cause of nasal obstruction (e.g., meconium plug, lanugo, vernix). If it is not possible to insert the aspiration catheter 1–2 cm beyond the nostril, the obstruction is most likely due to deviation of the nasal septum or thickening of the inferior nasal turbinate, and in the case of the obstruction 3–3.5 cm deep, it is most likely CA or choanal stenosis. In addition to the test with an aspiration catheter, there is also a test with the instillation of methylene blue in the nostril with the examination of the pharynx, a test with a cotton swab or a mirror to observe the airflow through the nostrils [4] and the use of a stethoscope with the funnel removed to listen to the airflow through the nostrils [31].
After establishing a free airway and the initial clinical examination, an extended diagnostic workup is required in the early postpartum period, which depends on the clinical presentation and risk factors for associated abnormalities.
Recommendation 3:

We strongly recommend that the otorhinolaryngological examination include examining the nose and face to rule out associated abnormalities (Table 1). This should be followed by anterior rhinoscopy and toilet, anemization, and epimucosal anaesthesia of the nasal cavities with the selected anaesthetic. An examination should then be performed with a flexible or rigid endoscope—level of evidence I, evidence grade A (Figure 2) (Table 2) [13].

Figure 2.
Endoscopic photograph of the left nasal cavity in a three-day-old neonate with bilateral bony choanal atresia. SER—sphenoethmoid recess; NP—nasal septum; MT—middle turbinate; IT—inferior turbinate; MPP—medial pterygoid plate; AP—atretic plate.
Recommendation 4:
Although CA is a clinical diagnosis, we strongly recommend that a 2–5 mm slice thickness CT scan of the facial structures, nose, paranasal sinuses and skull base be performed in every patient to confirm the diagnosis and plan treatment—level of evidence I, evidence grade A (Table 2) [4,13].
A CT can also identify other causes of nasal obstruction (Table 1) [4]. Before CT imaging, it is necessary to aspirate both nostrils, as mucus can be interpreted as soft tissue abnormalities, for example, encephalocele [32]. On CT, bony thickening of the medial plates of the pterygoid processes and thickening of the posterior part of the vomer is usually visible, narrowing or filling the lumen of the choana (Figure 3) [4]. At the same time, the same examination can also evaluate associated anatomical abnormalities, especially otological [13]. If the semicircular canal abnormalities are found simultaneously, CHARGE syndrome is very likely.

Figure 3.
CT of the nose and paranasal sinuses in the axial plane. Bilateral bony choanal atresia is seen in a three-day-old neonate. * indicates atretic plate.
Recommendation 5:
We strongly recommend that a head ultrasound be performed to rule out central nervous system involvement, which is common, especially in preterm newborns—level of Evidence I, evidence grade C (Table 2) [26].
An MRI of the head should only be performed in CA in selected cases [33].
Recommendation 6:
Bilateral CA often occurs in the context of other congenital syndromes, so clinical geneticist consultation is strongly recommended—level of Evidence I, evidence grade B (Table 2).
Recommendation 7:
From the point of view of the need for surgical care of a child with bilateral CA, we strongly recommend excluding congenital abnormalities that would put the child at risk during the procedure, especially heart disease, so we recommend an evaluation by a cardiologist—level of Evidence I, evidence grade B (Table 2).
Recommendation 8:
Even within otorhinolaryngology and in the postoperative period, we strongly recommend an audiovestibulological examination for a hearing evaluation and evaluation by a phoniatrician for additional swallowing problems—level of evidence I, evidence grade D (Table 2) [2,4,5,7,8,9].
A neurologist, urologist or ophthalmologist is included in the treatment if necessary (Figure 1) [13].
5.1.2. Unilateral Choanal Atresia
In the case of unilateral CA, the diagnosis is usually established later, as the clinical picture of unilateral CA is rarely manifested as respiratory distress. Therefore, diagnostics can be performed on an outpatient basis. The otorhinolaryngologist determines the patency of the nose using an aspiration catheter (appropriate size for the patient’s age), anterior rhinoscopy and nasal endoscopy. Despite everything, he must also pay special attention to possible associated diseases and refer the patient for further treatment.
Recommendation 9:
In the case of unilateral CA, we recommend a CT of the facial structures, nose, paranasal cavities and skull base be performed. With an unmistakable clinical picture and endoscopic status, this is indicated only before surgical treatment to avoid the unnecessary exposure of the child to ionizing radiation—level of Evidence IIa, evidence grade D (Table 2).
Recommendation 10:
In all cases of unilateral CA, we strongly recommend audiovestibulological evaluation, and for additional swallowing problems, treatment by a phoniatrician—level of evidence I, evidence grade D (Table 2).
5.2. Treatment
5.2.1. Bilateral Choanal Atresia
Airway Management
Before a thorough diagnosis and therapy, it is necessary to immediately ensure a free airway through the mouth by inserting a McGovern nipple in case of bilateral CA (Figure 1). Then, a nasogastric or feeding tube is inserted next to or through the nipple. An endotracheal intubation is required if a free airway cannot be established with a nipple [4]. In any case, the definitive treatment of the airway in a child who breathes on his own is to ensure the patency of the nose as soon as possible. Tracheotomy is considered only if long-term mechanical ventilation is expected in the infant, for example, with associated cardiac, pulmonary, neurological disease or multilevel respiratory obstruction. More specifically, the indications for tracheotomy were described by Walsh et al. (2018) [34]. If possible, tracheotomy is not recommended in cases where successful surgical treatment of CA can be performed. This paper does not provide an opinion or recommendations about tracheostomy.
Surgical Treatment
Recommendation 11:
We strongly recommend that bilateral CA be surgically treated between the 10th and 13th day of the neonate’s age, even in the case of prematurity—level of evidence I, evidence grade A (Table 2) [16,27].
Before surgical treatment, it is first necessary to carry out urgent diagnostic procedures to determine associated diseases and risk factors, especially cardiological treatment (Figure 1) [13]. Then, if the procedure under general anaesthesia is safe, the otorhinolaryngologist rhinosurgeon, will decide on the timing of the surgical treatment.
Even though the first description of surgical treatment of CA dates back to the mid-19th century, there is still no clear consensus on surgical techniques to date [4]. There are not enough systematic reviews and meta-analyses; only one systematic literature review was published in the Cochrane Library, in which it was found that out of 46 reviewed studies with descriptions of surgical techniques, none were suitable for the final analysis [14].
Surgical treatment can be divided into transnasal perforation, transpalatal resection, transnasal endoscopically assisted perforation and transnasal endoscopic choanoplasty (Figure 4). A stent can also be inserted regardless of the method to maintain choanal patency [4]. All surgical techniques are illustrated in Figure 5 and Figure 6.

Figure 4.
Types of surgical treatment methods of choanal atresia. Transnasal endoscopic choanoplasty is the gold standard with the best treatment outcome and lowest restenosis rate. Other surgical techniques present an alternative, e.g., transnasal endoscopically controlled perforation in cases where the manipulation with instruments is impossible due to the size of the nasal cavities, for example, in preterm infants. Transnasal perforation and transpalatinal resection represent the hierarchical bottom of surgical techniques and should not be performed.
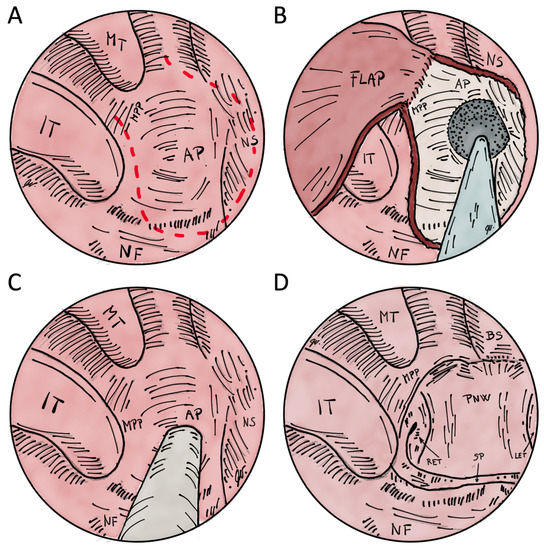
Figure 5.
Illustrations of transnasal endoscopic choanoplasty and perforation for the treatment of choanal atresia. (A)–red dashed line shows flap margins before resection of atretic plate in right nasal cavity. (B)–flap is elevated to drill the exposed bone of the atretic plate and posterior nasal septum resection. After the resection, the flap is layed on the exposed bone of medial pterygoid plate and base of the sphenoid. (C)–transnasal perforation technique is shown with or without the assistance of endoscope. There is no mucosal flap elevation. (D) after posterior nasal septum resection and drilling of atretic plate to the level of medial pterygoid plate, an unichoana is created. MT–middle turbinate; IT–inferior turbinate; AP–atretic plate; NS–nasal septum; NF–nasal floor; MPP–medial pterygoid plate; BS–base of the sphenoid sinus; RET–right Eustachian tube; LET–left Eustachian tube; PNW–posterior nasopharyngeal wall; SP–soft palate.
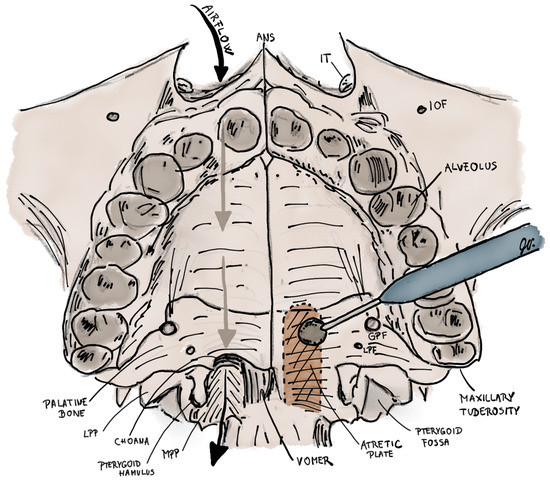
Figure 6.
Illustration of transpalatal resection for the treatment of left-sided choanal atresia. Right nasal cavity has a normal patency. First the palatal flap pedicled posteriorly on both greater palatine arteries is elevated to expose the bone of hard palate. Then, the atretic plate, which is located between vomer and medial pterygoid plate, is burred (coloured brown) up to the height to the base of sphenoid sinus, medially to the vomer and laterally to the level of medial pterygoid plate. ANS–anterior nasal spine; IT–inferior turbinate; IOF–infraorbital foramen; LPP–lateral pterygoid plate; MPP–medial pterygoid plate; GPF–greater palatine foramen; LPF–lesser palatine foramen.
Transnasal Endoscopic Choanoplasty
Transnasal endoscopic choanoplasty is considered the surgical technique of choice for the treatment of CA by most otorhinolaryngologists [4]. It is based on the endoscopic removal of the atretic area and the posterior part of the nasal septum, thereby creating a single choana (i.e., unichoana) (Figure 5A,B,D). Instruments for cold steel bone resection are primarily used, and, in the case of medialized medial pterygoid plates, a diamond drill is used [13] (Figure 7). The successful use of the CO2 laser [13,35] and balloon dilators [13,36] has also been described. Suppose it is impossible to manipulate the instrument through the same nostril; the endoscope is inserted in the contralateral nasal cavity. In that case, it is advised to first puncture the posterior part of the nasal septum under the control of the endoscope and then continue resectioning the atretic plate with the endoscope inserted in the contralateral nasal cavity. The mucosa must be preserved in the form of flaps, which are placed on the exposed bony walls of the neochoana at the end of the procedure, thereby preventing restenosis [4,20]. The success rate of transnasal endoscopic choanoplasty, determined by the occurrence of restenosis or the need for revision, is 65% according to a meta-analysis by Strychowsky et al. (2015) [18]. The possible risk factors for restenosis are associated congenital abnormalities, reflux of gastric contents in the nasopharynx and the age of the neonate <10 days since a lower age determines more unfavourable anatomical conditions that limit the visualization and extent of resection [4].
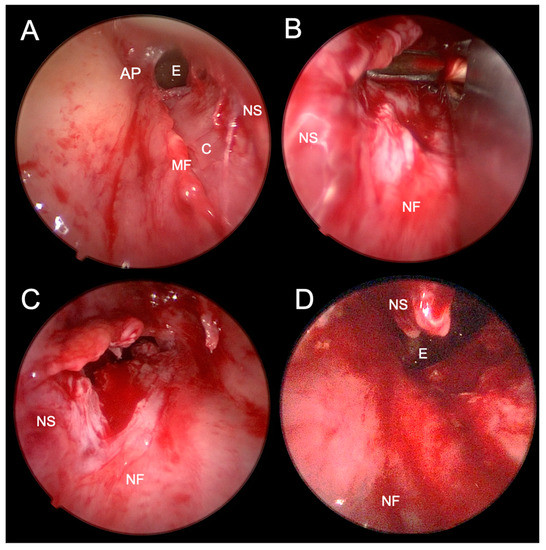
Figure 7.
Endoscopic photographs of transnasal endoscopic choanoplasty of bilateral choanal atresia. (A)–after perforation of the atretic plate of the right nasal cavity and elevation of the mucoperichondrial flap of the nasal septum and atretic plate. (B)–resection of the posterior nasal septum with forceps through the left nasal cavity. (C)–after partial resection of the posterior nasal septum visible through the left nasal cavity. (D)–after a partially surgically formed unichoana, which connects both nasal cavities in the posterior nasal septum resection area. AP–atretic plate; E–epipharynx; C–cartilage; MF–mucosal flap; NS–nasal septum; NF–nasal floor.
Recommendation 12:
The insertion of stents, taking into account the advantages and disadvantages, can neither be recommended nor advised against since the success rate, regardless of stent insertion, is only 65% [18]. The advantages of using stents are a lower incidence of restenosis and satisfactory patency in the initial postoperative period. At the same time, the disadvantages are the need for more frequent treatment due to stent changes, irritation, erosion or ulceration of the nasal cavity, which can lead to the formation of adhesions and restenosis [4,21].
Recommendation 13:
If a stent is used, we recommend its removal within seven days to reduce the risk of complications [18] —level of Evidence IIa, evidence grade A (Table 2).
Recommendation 14:
In case of associated craniofacial abnormalities, we recommend using navigation, which can be CT and/or MR-guided—level of evidence IIa, evidence grade C (Table 2).
Navigation enables easier recognition of altered anatomical conditions and safer intervention in CA [4,9].
Transnasal Perforation
Transnasal perforation is an older surgical technique primarily performed blindly with a probe or dilator through the nostrils. Later, they started to simultaneously use a 120° endoscope or a mirror to examine the area of atresia. However, especially with the blind technique, there is a significant risk of complications arising from damage to the nasal septum, lateral nasal wall, nasal vault or clivus [4]. In addition, a high risk of restenosis has also been described [10].
Recommendation 15:
A stent can be a shortened endotracheal tube, an aspiration catheter or a similar medical device [25]. All should be changed regularly postoperatively (described below). In addition, the effective use of corticosteroid-eluting stents has also been described [37].
The use of mitomycin has been described in the prevention of restenosis. Still, it is not recommended due to its potential carcinogenicity and the lack of clinical efficacy data so far [13].
Transnasal Endoscopically Assisted Perforation
Recommendation 16:
Transnasal endoscopically assisted perforation with stent insertion is allowed as an option when transnasal endoscopic choanoplasty is not possible due to anatomical conditions—level of evidence IIb, evidence grade C (Table 2) [4,13].
The procedure is identical to the described blind perforation with additional endoscopic control. This reduces the possibility of unwanted damage to adjacent tissues (Figure 1 and Figure 5C).
In the case of prematurity, endoscopic transparency and the use of endoscopic instruments are limited, so transnasal endoscopic choanoplasty is often not possible. Then, transnasal endoscopically assisted perforation is the method of choice for treating bilateral CA. This procedure will most likely require a later (so-called revision) transnasal endoscopic choanoplasty when the child has grown, and the anatomical conditions allow this surgical treatment method.
There is no mucosal flap elevation and posterior nasal septum resection in transnasal perforation techniques (endoscopically assisted or unassisted).
Transpalatal Resection
In transpalatal resection, the mucous membrane of the hard palate is raised in a local flap, and the entire thickness of the bone in the area of bony atresia is drilled away (Figure 6). Despite the low incidence of restenosis, the complications of this operation are significant. These are malocclusion, palate necrosis, oronasal fistula, soft palate muscle dysfunction and velopharyngeal insufficiency. Therefore, this method is not recommended for children under six.
Recommendation 17:
In the primary treatment of CA, we recommend against transpalatal resection of CA—level of evidence III, evidence grade A (Table 2) [4,13].
5.2.2. Unilateral Choanal Atresia
The age at which unilateral CA is treated depends on the patient’s age at the time of the unequivocal diagnosis, which is based on the results of a systematic review by Murray et al. (2019).
Recommendation 18:
Even with the start of treatment after the third year of age, we expect the same results as before [13].
Recommendation 19:
Transnasal endoscopic choanoplasty is strongly recommended for unilateral CA due to the mostly good endoscopic visualization in older patients with unilateral CA. The extent of the resection should be large enough so that it is not necessary to use a stent—level of evidence I, evidence grade A (Table 2) [13].
5.3. Postoperative Management
Postoperative management depends on the CA’s location, the patient’s general health and the patient’s setting (i.e., outpatient or inpatient).
5.3.1. Bilateral Choanal Atresia
Postoperative treatment after surgical treatment of bilateral CA depends mainly on associated diseases, the gestational age of the neonate and the related need for treatment of other conditions. Children with CHARGE syndrome have a higher risk of postoperative complications and prolonged hospitalization [27]. The same is to be expected in premature infants. The intubated patient has an additional chance of complications due to extended postoperative mechanical ventilation and prolonged hospitalization [38].
Stented
Recommendation 20:
During this period, it is reasonable to increase the outer diameter of the stent so that the lumen of the neochoana increases significantly, such as twice the outer diameter of the endotracheal tube, as long as the anatomical conditions allow this increase (we did not reach the maximum possible dimensions). This is especially necessary after transnasal endoscopically guided perforation and transnasal perforation. After transnasal endoscopic choanoplasty, the insertion of stents is often not needed.
Recommendation 21:
We recommend that discharge from the hospital be planned for the second postoperative week or as soon as possible when the general state of health allows it and the patency of the stents is satisfactory with appropriate care—level of Evidence IIa, evidence grade D (Table 2).
We monitor the child’s breathing patterns until discharge. In addition to normal breathing, the main goal of treatment for bilateral CA is independent feeding without breaks as soon as possible after surgical treatment.
Recommendation 22:
Stent replacement on an outpatient basis in the second week is optional—level of evidence IIb, evidence grade D (Table 2).
Recommendation 23:
In the third week, it is optional to replace the stent twice, and from the fourth week onwards, only once more—level of evidence IIb, evidence grade D (Table 2).
Recommendation 24:
We strongly recommend removing the stent in the coming weeks and rechecking the patency the week after—level of Evidence I, evidence grade D (Table 2).
The replacement of stents in the postoperative period can be postponed as long as their patency is satisfactory. Instead of changing stents, we can perform regular dilations, especially after transnasal endoscopically controlled perforation and transnasal perforation, for example, with a balloon dilator, which has already been used in the treatment of bilateral CA [36].
Recommendation 25:
Photo documentation of the lumen is recommended—level of evidence IIa, evidence grade D (Table 2).
The intervals between check-ups are gradually being prolonged. Otherwise, restenosis after one year is rare [13].
Recommendation 26:
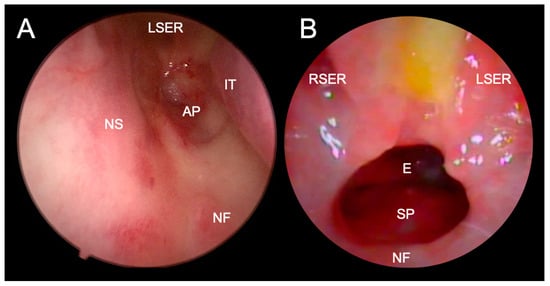
Follow-up is strongly recommended for at least two years after the procedure or until the end of growth to detect restenosis—level of evidence I, evidence grade C (Figure 1 and Figure 8) (Table 2).

Figure 8.
Endoscopic visualization of complete restenosis after transnasal perforation (A) and good patency of unichoana four weeks after transnasal endoscopic choanoplasty (B) of bilateral choanal atresia. A trace of milk and mucus is visible between the two sphenoethmoid recesses in Figure B, suggesting a significant influence of gastric contents reflux on the operative wound healing. LSER - left sphenoethmoid recess; RSER–right sphenoethmoid recess; IT–inferior turbinate; AP–atretic plate; NS–nasal septum; NF–nasal floor; E–epipharynx; SP–soft palate.
Upon discharge from the hospital, it is necessary to include the child’s parents in the treatment process. They should be taught how to clean the nose effectively and reinsert the stent, and clear instructions should be given in the event of the infant’s respiratory distress. In the case of breathing problems, poor nasal patency or discharge, a repeat examination by an otorhinolaryngologist is necessary to assess choanal patency accurately. In the postoperative period, we plan as few examinations as possible in the hospital.
Recommendation 27:
Revision transnasal endoscopic surgery is recommended in case of breathing problems, poor nasal patency or discharge and more than 50% reduction of the choanal lumen—level of evidence IIa, evidence grade A (Table 2).
CT or other radiological examinations are not routinely indicated in postoperative management, even in restenosis [13].
Recommendation 28:
During the postoperative treatment of the infant, the daily use of proton pump inhibitors is recommended for the first two months (e.g., per os esomeprazole 0.5 mg/kg day for infants or 10 mg/day for children over one year of age)—level of evidence IIa, evidence grade C [25].
Recommendation 29:
During the postoperative management of the infant, the instillation of saline solution (e.g., 3 drops, 5 times a day in each nostril) and nasal glucocorticoid drops with low systemic absorption (e.g., fluticasone, one time a day, one drop in each nostril) are strongly recommended for the first two months—level of evidence I, evidence grade C [13,28].
The drops should be instilled next to the stent and not into it.
Recommendation 30:
During the first two months of postoperative treatment of the infant, antibiotic drops are recommended exceptionally in the first postoperative week or later when noticeable purulent discharge appears—level of Evidence IIa, evidence grade D (Table 2).
In the case of systemic signs of infection, identification of the source of infection and systemic treatment are indicated.
Non-Stented
In the postoperative treatment of patients after transnasal endoscopic choanoplasty of bilateral CA without inserted stents, the infant can already start eating food by mouth on the first day, as soon as the effects of general anaesthesia (anaesthetics and narcotics) wear off after a few hours. We monitor the child’s breathing patterns, which must be appropriate in all life circumstances (sleeping and feeding). Otherwise, it is necessary to determine the cause of breathing problems, which may be local or an associated, unrecognized pathology.
Recommendation 31:
The first postoperative examination with the assessment of nasal breathing with the mouth closed and feeding by mouth without pauses for breathing is recommended the day after surgery (first postoperative day)—level of evidence Iia, evidence grade D.
Recommendation 32:
We allow the possibility of discharge from the hospital on the second postoperative day—level of evidence Iib, evidence grade D.
Recommendation 33:
Photo documentation enables comparison between inspections. Instructions to parents and recommendations regarding the use of drops and revision surgery are the same as for patients with inserted stents [13].
5.3.2. Unilateral Choanal Atresia
Recommendation 34:
With unilateral CA, the risk of respiratory distress is very low, so we recommend discharge on the first postoperative day—level of evidence IIa, evidence grade D (Table 2).
We perform the first examination on this day, especially the nasal cavity toilet. We are planning the subsequent outpatient check-up in one week. In the following weeks, the otorhinolaryngologist decides on the frequency of check-ups. Parental instructions and recommendations regarding the use of drops, follow-up and revision surgery are the same as for patients with stents.
6. Conclusions
The paper presents the first Slovenian recommendations for treating patients with choanal atresia. They are based on foreign medical institutions’ published literature and our clinical experience. They represent the basic requirements of diagnostics and are a possible essential guide in treatment, which, however, must be adapted according to the current situation. Therefore, a thorough review of each recommendation is necessary before implementation.
However, these recommendations focus on the otorhinolaryngological management of choanal atresia, which should be considered. Moreover, only a small number of systematic reviews and meta-analyses are included in this study.
In further decades of experience and technology development, recommendations can be expected to improve due to changes in treatment, especially transnasal endoscopic surgical techniques, the use of stents and other methods of preventing restenosis. Therefore, it makes sense to create a register of patients with choanal atresia and other congenital anomalies of the craniofacial area, upper respiratory tract and gastrointestinal tract for prospective data collection.
Author Contributions
Conceptualization, D.V. and J.U.; methodology, D.V.; software, D.V.; validation, D.V.; formal analysis, D.V.; investigation, D.V.; resources, D.V., J.U. and S.B.; data curation, D.V.; writing—original draft preparation, D.V. and J.U.; writing—review and editing, D.V., J.U., S.B., I.B., L.B., M.G., Č.I., K.J., B.L. and T.S.K.; visualization, D.V. and J.U.; supervision, D.V., J.U. and S.B.; project administration, D.V. and J.U..; funding acquisition, D.V., J.U. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajan, R.; Tunkel, D.E. Choanal Atresia and Other Neonatal Nasal Anomalies. Clin. Perinatol. 2018, 45, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Roehm, C.E.; Lawrason, A.; Valdez, T.A. Nasal Obstruction in Newborn. In Encyclopedia of Otolaryngology, Head and Neck Surgery; Kountakis, S.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1746–1757. [Google Scholar]
- Gnagi, S.H.; Schraff, S.A. Nasal Obstruction in Newborns. Pediatr. Clin. 2013, 60, 903–922. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.M. Current Updates on Choanal Atresia. Front. Pediatr. 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.R.; Harmon, P.; Shirley, W.P.; Hill, J.S.; Woolley, A.L.; Wiatrak, B.J. Operative Management of Choanal Atresia: A 15-Year Experience. JAMA Otolaryngol. –Head Neck Surg. 2013, 139, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Amiji, I.; Kalezi, Z.E.; Abdulshakoor, A.; Tarimo, J.F.; Leiya, R.; Zuechner, A.; Naburi, H.E.; Massawe, A.; Manji, K.P. Pfeiffer Syndrome Type 2; A Case Report of Cranio-Orbitofaciostenosis with Bilateral Choanal Atresia at Muhimbili National Hospital, Tanzania. Clin. Case Rep. 2020, 8, 1613–1617. [Google Scholar] [CrossRef]
- Childs, A.J.; Mabin, D.C.; Turnpenny, P.D. Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome Presenting with Bilateral Choanal Atresia and Rectal Stenosis. Am. J. Med. Genet. Part A 2020, 182, 1939–1943. [Google Scholar] [CrossRef]
- Kita, M.; Kuwata, Y.; Usui, T. Familial Congenital Choanal Atresia with GATA3 Associated Hypoparathyroidism-Deafness-Renal Dysplasia Syndrome Unidentified on Auditory Brainstem Response. Auris Nasus Larynx 2019, 46, 808–812. [Google Scholar] [CrossRef]
- Sung, J.Y.; Cho, K.-S.; Bae, Y.C.; Bae, S.H. Image-Guided Navigation Surgery for Bilateral Choanal Atresia with a Tessier Number 3 Facial Cleft in an Adult. Arch. Craniofacial Surg. 2020, 21, 64–68. [Google Scholar] [CrossRef]
- Hengerer, A.S.; Brickman, T.M.; Jeyakumar, A. Choanal Atresia: Embryologic Analysis and Evolution of Treatment, a 30-Year Experience. Laryngoscope 2008, 118, 862–866. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Shiffman, R.N.; Robertson, P. Clinical Practice Guideline Development Manual, Third Edition: A Quality-Driven Approach for Translating Evidence into Action. Otolaryngol. –Head Neck Surg. 2013, 148, S1–S55. [Google Scholar] [CrossRef]
- Gersak, K.; Fras, Z.; Rems, M. Do We Know What Makes a Good Clinical Guideline? Zdr. Vestn. 2016, 85, 6–14. [Google Scholar] [CrossRef]
- Moreddu, E.; Rizzi, M.; Adil, E.; Balakrishnan, K.; Chan, K.; Cheng, A.; Daniel, S.J.; de Alarcon, A.; Hart, C.; Hartnick, C.; et al. International Pediatric Otolaryngology Group (IPOG) Consensus Recommendations: Diagnosis, Pre-Operative, Operative and Post-Operative Pediatric Choanal Atresia Care. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Cedin, A.C.; Atallah, Á.N.; Andriolo, R.B.; Cruz, O.L.; Pignatari, S. Surgery for Congenital Choanal Atresia. Cochrane Database Syst. Rev. 2012, CD008993. [Google Scholar] [CrossRef] [PubMed]
- El-Begermy, M.M.; Samir, M.M.; Fawaz, S.A.; Elkelany, W.G. Effect of the Type of Surgery, Use of Intraoperative Topical Mitomycin C or Stenting on the Outcome of Choanal Atresia Repair: A Systematic Review and Meta-Analysis. Egypt. J. Otolaryngol. 2016, 32, 255–263. [Google Scholar] [CrossRef]
- Murray, S.; Luo, L.; Quimby, A.; Barrowman, N.; Vaccani, J.-P.; Caulley, L. Immediate versus Delayed Surgery in Congenital Choanal Atresia: A Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2019, 119, 47–53. [Google Scholar] [CrossRef]
- Bartel, R.; Levorato, M.; Adroher, M.; Cardelus, S.; Diaz, A.; Lacima, J.; Vazquez, C.; Veneri, A.; Wienberg, P.; Claveria, M.A.; et al. Performance of Endoscopic Repair with Endonasal Flaps for Congenital Choanal Atresia. A Systematic Review. Acta Otorrinolaringol. 2021, 72, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Strychowsky, J.E.; Kawai, K.; Moritz, E.; Rahbar, R.; Adil, E.A. To Stent or Not to Stent? A Meta-Analysis of Endonasal Congenital Bilateral Choanal Atresia Repair. Laryngoscope 2016, 126, 218–227. [Google Scholar] [CrossRef]
- Durmaz, A.; Tosun, F.; Yldrm, N.; Sahan, M.; Kvrakdal, C.; Gerek, M. Transnasal Endoscopic Repair of Choanal Atresia: Results of 13 Cases and Meta-Analysis. J. Craniofacial Surg. 2008, 19, 1270–1274. [Google Scholar] [CrossRef]
- Alsubaie, H.M.; Almosa, W.H.; Al-Qahtani, A.S.; Margalani, O. Choanal Atresia Repair with Stents and Flaps: A Systematic Review Article. Allergy Rhinol. 2021, 12, 21526567211058052. [Google Scholar] [CrossRef]
- Gundle, L.; Ojha, S.; Hendry, J.; Rosen, H. Stenting versus Stentless Repair for Bilateral Choanal Atresia: A Systematic Review of the Literature. Int. J. Pediatr. Otorhinolaryngol. 2021, 151, 110926. [Google Scholar] [CrossRef]
- Tomoum, M.O.; Askar, M.H.; Mandour, M.F.; Amer, M.A.; Saafan, M.E. Stentless Mirrored L-Shaped Septonasal Flap versus Stented Flapless Technique for Endoscopic Endonasal Repair of Bilateral Congenital Choanal Atresia: A Prospective Randomised Controlled Study. J. Laryngol. Otol. 2018, 132, 329–335. [Google Scholar] [CrossRef]
- Saafan, M.E. Endoscopic Management of Congenital Bilateral Posterior Choanal Atresia: Value of Using Stents. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Al-Ammar, A. Effect of Use of Mitomycin C on the Outcome of Choanal Atresia Repair. Saudi Med. J. 2007, 28, 1537–1540. [Google Scholar] [PubMed]
- ElSalmawy, A.; Abdelfattah, G.; Farag, T.; Farahat, A. Role of Proton Pump Inhibitor in Healing after Choanal Atresia Repair: A Randomized Control Trial. Egypt. J. Otolaryngol. 2022, 38, 44. [Google Scholar] [CrossRef]
- Hand, I.L.; Shellhaas, R.A.; Milla, S.S. Routine Neuroimaging of the Preterm Brain. Pediatrics 2020, 146, e2020029082. [Google Scholar] [CrossRef]
- Marston, A.P.; Patel, T.; Nguyen, S.A.; White, D.R. Short-Term Risk Factor Profile of Pediatric Choanal Atresia Repair Using ACS-NSQIP National Database. Ann. Otol. Rhinol. Laryngol. 2019, 128, 855–861. [Google Scholar] [CrossRef]
- Zoizner-Agar, G.; Rotsides, J.M.; Shao, Q.; Rickert, S.; Ward, R.; Greifer, M.; April, M. Proton Pump Inhibitor Administration in Neonates and Infants. Lack of Consensus—An ASPO Survey. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110200. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence Working Group* Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 18 March 2021).
- WHO Reproductive Health Library. WHO Recommendations on Newborn Health: Guidelines Approved by the WHO Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mason, K.; Royan, D.; Daya, H.; Pepper, C.; Tweedie, D. Use of a Modified Stethoscope to Assess Paediatric Nasal Airflow in Suspected Choanal Atresia, Nasal Stents or Nasopharyngeal Airways. Clin. Otolaryngol. 2020, 45, 654–655. [Google Scholar] [CrossRef]
- Elsheikh, E.; El-Anwar, M.W. False Computed Tomography Findings in Bilateral Choanal Atresia. Int. Arch. Otorhinolaryngol. 2016, 20, 163–165. [Google Scholar] [CrossRef][Green Version]
- Wineland, A.; Menezes, M.D.; Shimony, J.S.; Shinawi, M.S.; Hullar, T.E.; Hirose, K. Prevalence of Semicircular Canal Hypoplasia in Patients with CHARGE Syndrome: 3C Syndrome. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 168–177. [Google Scholar] [CrossRef]
- Walsh, J.; Rastatter, J. Neonatal Tracheostomy. Clin. Perinatol. 2018, 45, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Stavrakas, M.; Ray, J. Lasers in Rhinology—An Update. Ear Nose Throat J. 2020, 100, 77S–82S. [Google Scholar] [CrossRef] [PubMed]
- De Vincentiis, G.C.; Panatta, M.L.; De Corso, E.; Marini, G.; Bianchi, A.; Giuliani, M.; Sitzia, E.; Tucci, F.M. Endoscopic Treatment of Choanal Atresia and Use of Balloon Dilation: Our Experience. Acta Otorhinolaryngol. Ital. 2020, 40, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L.J.; Smith, M.M.; de Alarcon, A.; Epperson, M.; Born, H.; Hart, C.K. Use of Steroid-Eluting Stents after Endoscopic Repair of Choanal Atresia: A Case Series with Review. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1003–1010. [Google Scholar] [CrossRef]
- Patel, V.A.; Ramadan, J.; Carr, M.M. Congenital Choanal Atresia Repair: An Analysis of Adverse Perioperative Events. Otolaryngol. Head Neck Surg. 2018, 159, 920–926. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).