Causes of Intensive Care Unit Admissions in Children with SARS-CoV-2: A Single-Centre Observational Study
Abstract
:1. Introduction
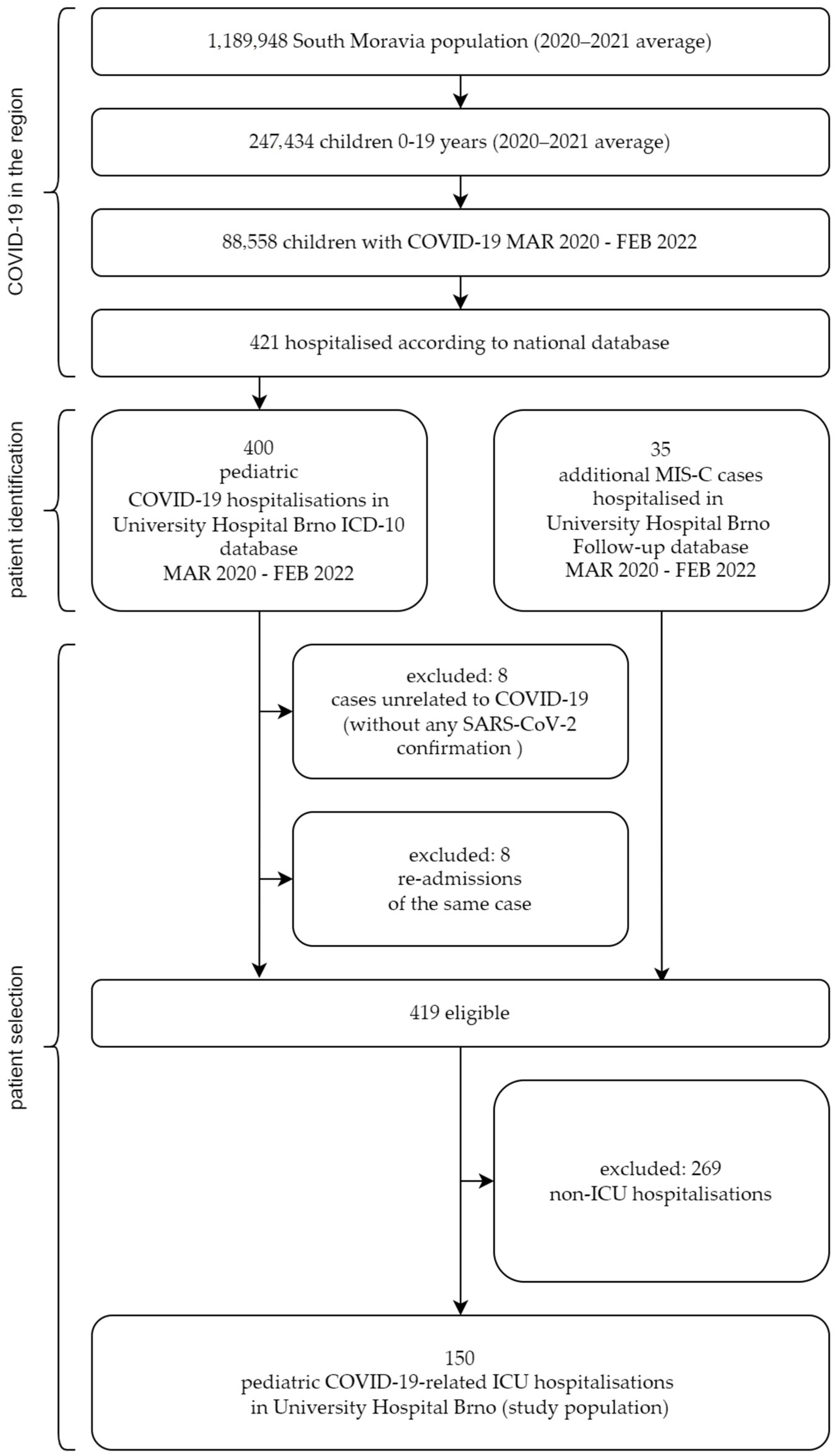
2. Materials and Methods
- Newly developed symptoms: flu-like symptoms (fever, headache, sore throat, myalgia), clinical symptoms of respiratory (rhinorrhea, cough, dyspnea) or gastrointestinal infection ((nausea, vomiting, abdominal pain, diarrhoea), neurological symptoms (loss of smell or taste, febrile seizures), rash [6];
- Direct detection of SARS-CoV-2 by antigen test or RT-PCR from a nasopharyngeal swab during current illness [7];
- No other pathogen was identified that could explain the above symptoms.
- An individual aged <21 years presenting with fever, laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalisation, with multisystem (>2) organ involvement (cardiac, renal, respiratory, haematologic, gastrointestinal, dermatologic, or neurological);
- No alternative plausible diagnoses;
- Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms [8].
- A patient was admitted for an exacerbation of a chronic underlying condition (lasting at least one month)oradmitted with another acute condition in which multiple causative agents have been identified and the problem, therefore, cannot be clearly attributed to a single one;
- Direct detection of SARS-CoV-2 by antigen test or RT-PCR from nasopharyngeal swab during a current illness.
- A patient admitted for a condition likely unrelated to ongoing SARS-CoV-2 infection: elective procedures and treatments, surgery, trauma, intoxication, genetic diseases, clearly defined localised bacterial infections (e.g., urinary tract, eye, etc.) or patients hospitalised for other conditions in which SARS-CoV-2 infection has been evaluated as nosocomial;
- Direct detection of SARS-CoV-2 by antigen test or RT-PCR from nasopharyngeal swab during current illness.
3. Results
3.1. Population
3.2. Demographics and Groups
3.3. Comparison of Groups
3.3.1. Severity
3.3.2. Length of Hospitalisation
3.3.3. Recovery
3.3.4. Laboratory Tests
3.3.5. Brain MRI
3.3.6. Chest X-ray
3.3.7. Cardiac Complications
3.3.8. Neurological Complications
3.3.9. Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, P.; Pittet, L.F.; Finn, A.; Pollard, A.J.; Curtis, N. Should Children Be Vaccinated against COVID-19? Arch. Dis. Child. 2022, 107, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J. Hospitalizations Associated with COVID-19 Among Children and Adolescents—COVID-NET, 14 States, March 1, 2020–August 14, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1255. [Google Scholar] [CrossRef]
- Marks, K.J.; Whitaker, M.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kirley, P.D.; Armistead, I.; McLafferty, S.; et al. Hospitalisations of Children and Adolescents with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, July 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 271–278. [Google Scholar] [CrossRef]
- Swann, O.V.; Holden, K.A.; Turtle, L.; Pollock, L.; Fairfield, C.J.; Drake, T.M.; Seth, S.; Egan, C.; Hardwick, H.E.; Halpin, S.; et al. Clinical Characteristics of Children and Young People Admitted to Hospital with COVID-19 in United Kingdom: Prospective Multicentre Observational Cohort Study. BMJ 2020, 370, m3249. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Whitaker, M.; O’Halloran, A.; Kambhampati, A.; Chai, S.J.; Reingold, A.; Armistead, I.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; et al. Hospitalisation Rates and Characteristics of Children Aged < 18 Years Hospitalised with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Irfan, O.; Muttalib, F.; Tang, K.; Jiang, L.; Lassi, Z.S.; Bhutta, Z. Clinical Characteristics, Treatment and Outcomes of Paediatric COVID-19: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2021, 106, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021, 325, 1074. [Google Scholar] [CrossRef]
- HAN Archive-00432|Health Alert Network (HAN). Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 30 October 2022).
- Octavius, G.S.; Wijaya, J.H.; Tan, A.O.; Muljono, M.P.; Chandra, S.; Juliansen, A. Autopsy Findings of Pediatric COVID-19: A Systematic Review. Egypt. J. Forensic Sci. 2022, 12, 32. [Google Scholar] [CrossRef]
- Klučka, J.; Klabusayová, E.; Kratochvíl, M.; Musilová, T.; Vafek, V.; Skříšovská, T.; Kosinová, M.; Havránková, P.; Štourač, P. Critically Ill Pediatric Patient and SARS-CoV-2 Infection. Children 2022, 9, 538. [Google Scholar] [CrossRef]
- Shekerdemian, L.S.; Mahmood, N.R.; Wolfe, K.K.; Riggs, B.J.; Ross, C.E.; McKiernan, C.A.; Heidemann, S.M.; Kleinman, L.C.; Sen, A.I.; Hall, M.W.; et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020, 174, 868. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Campbell, A.P.; Taylor, C.A.; Chai, S.J.; Kawasaki, B.; Meek, J.; Anderson, E.J.; Weigel, A.; Monroe, M.L.; Reeg, L.; et al. Risk Factors for Severe COVID-19 in Children. Pediatrics 2022, 149, e2021053418. [Google Scholar] [CrossRef] [PubMed]
- Bixler, D.; Miller, A.D.; Mattison, C.P.; Taylor, B.; Komatsu, K.; Peterson Pompa, X.; Moon, S.; Karmarkar, E.; Liu, C.Y.; Openshaw, J.J.; et al. SARS-CoV-2–Associated Deaths among Persons Aged <21 Years—United States, February 12–July 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and Multisystem Inflammatory Syndrome in Children and Adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Calò Carducci, F.I.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in Children and Adolescents in Europe: A Multinational, Multicentre Cohort Study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.S.; Whitaker, M.; Marks, K.J.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kawasaki, B.; Meek, J.; et al. Hospitalisations of Children Aged 5–11 Years with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- De Filippo, M.; Magri, P.; Bossi, G.; Brambilla, I.; Castagnoli, R.; Mascolo, A.; Votto, M.; Licari, A.; Marseglia, G.L. Clinical and Epidemiological Features of Pediatric Patients with COVID-19 in a Tertiary Pediatric Hospital. Acta Biomed. Atenei Parm. 2022, 93, e2022039. [Google Scholar] [CrossRef]
- Cloete, J.; Kruger, A.; Masha, M.; du Plessis, N.M.; Mawela, D.; Tshukudu, M.; Manyane, T.; Komane, L.; Venter, M.; Jassat, W.; et al. Paediatric Hospitalisations Due to COVID-19 during the First SARS-CoV-2 Omicron (B.1.1.529) Variant Wave in South Africa: A Multicentre Observational Study. Lancet Child Adolesc. Health 2022, 6, 294–302. [Google Scholar] [CrossRef]
- Whittaker, R.; Greve-Isdahl, M.; Bøås, H.; Suren, P.; Buanes, E.A.; Veneti, L. COVID-19 Hospitalization Among Children <18 Years by Variant Wave in Norway. Pediatrics 2022, 150, e2022057564. [Google Scholar] [CrossRef]
- Carlotti, A.P.d.C.P.; de Carvalho, W.B.; Johnston, C.; Rodriguez, I.S.; Delgado, A.F. COVID-19 Diagnostic and Management Protocol for Pediatric Patients. Clinics 2020, 75, e1894. [Google Scholar] [CrossRef]
- Sobolewska-Pilarczyk, M.; Pokorska-Śpiewak, M.; Stachowiak, A.; Marczyńska, M.; Talarek, E.; Ołdakowska, A.; Kucharek, I.; Sybilski, A.; Mania, A.; Figlerowicz, M.; et al. COVID-19 Infections in Infants. Sci. Rep. 2022, 12, 7765. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Pittet, L.F.; Curtis, N. How Common Is Long COVID in Children and Adolescents? Pediatr. Infect. Dis. J. 2021, 40, e482–e487. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Ulyte, A.; Puhan, M.A.; Kriemler, S. Long-Term Symptoms After SARS-CoV-2 Infection in Children and Adolescents. JAMA 2021, 326, 869. [Google Scholar] [CrossRef] [PubMed]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Kläser, K.; Kerfoot, E.; Chen, L.; et al. Illness Duration and Symptom Profile in Symptomatic UK School-Aged Children Tested for SARS-CoV-2. Lancet Child Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.A.; Misra, N.; Ganigara, M.; Epstein, S.; Rajan, S.; Acharya, S.S.; Hayes, D.A.; Kearney, M.B.; Romano, A.; Friedman, R.A.; et al. Six Month Follow-up of Patients with Multi-System Inflammatory Syndrome in Children. Pediatrics 2021, 148, e2021050973. [Google Scholar] [CrossRef]
- Octavius, G.S.; Tan, R.; Pratama, T.A.; Budiputri, C.L.; Meliani, F.; Heriyanto, R.S.; Muljadi, R.; Juliansen, A. Cardiac Manifestations and Diagnostic Imaging in Pediatric Inflammatory Multisystem Syndrome Temporally Associated with COVID-19: A Systematic Review. Med. J. Indones. 2022, 31, 20–37. [Google Scholar] [CrossRef]
- Putri, N.D.; Prawira, Y.; Tartila, T.; Jasin, M.R.; Puspitasari, H.A.; Puspaningtyas, N.W.; Indawati, W.; Karyanti, M.R.; Setyanto, D.B.; Prayitno, A.; et al. Clinical Features of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 in Indonesia. J. Trop. Pediatr. 2022, 68, fmac025. [Google Scholar] [CrossRef]
- O’Loughlin, L.; Alvarez Toledo, N.; Budrie, L.; Waechter, R.; Rayner, J. A Systematic Review of Severe Neurological Manifestations in Pediatric Patients with Coexisting SARS-CoV-2 Infection. Neurol. Int. 2021, 13, 410–427. [Google Scholar] [CrossRef]
| Study Population | COVID | MIS-C | WORSENING | ISOLATION | |
|---|---|---|---|---|---|
| Cases | 150 | 32.70% (49/150) | 30.0% (45/150) | 14.7% (22/150) | 22.7% (34/150) |
| Sex, male | 66.7% (100/150) | 66.3% (33/49) | 75.6% (34/45) | 59.1% (13/22) | 58.8% (20/34) |
| Age, years (IQR) | 8.6 (3.5–13.3) | 6.1 (1.3–14.5) | 10.1 (7.4–11.7) | 7.0 (3.0–14.5) | 9.8 (3.5–14.3) |
| COVID | MIS-C | WORSENING | ISOLATION | p-Value | |
|---|---|---|---|---|---|
| Severity | p < 0.001 B | ||||
| Asymptomatic | 0% | 0% | 36.4% (8/22) | 52.9% (18/34) | |
| Mild | 59.2% (29/49) | 82.2% (37/45) | 63.6% (14/22) | 44.1% (15/34) | |
| Medium | 22.4% (11/49) | 2.2% (1/45) | 0% | 2.9% (1/34) | |
| Severe | 6.1% (3/49) | 4.4% (2/45) | 0% | 0% | |
| Critical | 12.2% (6/49) | 11.1% (5/45) | 0% | 0% | |
| Hospital stay, days (IQR) ICU stay, days (IQR) | 6.0 (4.0–11.0) 5.0 (3.0–10.0) | 11.0 (9.0–13.0) 9.0 (7.0–11.0) | 4.5 (3.0–10.0) 4.5 (3.0–10.0) | 5.5 (2.0–10.0) 3.5 (2.0–7.0) | p < 0.001 C p < 0.001 C |
| Recovery | p = 0.09 B | ||||
| Complete Short sequelae Long sequelae | 69.4% (34/49) 16.3% (8/49) 14.3% (7/49) | 80.0% (36/45) 15.6% (7/45) 4.4% (2/45) | 95.5% (21/22) 4.5% (1/22) 0% | 85.3% (29/34) 14.7% (5/34) 0% |
| COVID | MIS-C | WORSENING | ISOLATION | p-Value | |
|---|---|---|---|---|---|
| Laboratory tests | |||||
| CRP, mg/L (IQR) | 12.2 (5.4–73.6) | 180.2 (106.5–230.6) | 8.1 (1.0–53.5) | 3.8 (1.3–76.3) | p < 0.001 C |
| Fibrinogen, g/L (IQR) | 4.0 (2.7–5.7) | 5.9 (5.0–7.3) | 2.7 (2.2–3.7) | 3.4 (2.5–4.1) | p < 0.001 C |
| Troponin, ng/L (IQR) | 19.0 (3.0–43.0) | 23.5 (6.0–47.0) | 4.0 (0.0–17.0) | - | p = 0.210 C |
| NTproBNP, ng/L (IQR) | 365.5 (18.5–11,099.0) | 2646.0 (888.0–8843.0) | 303.5 (10.7–865.5) | - | p = 0.025 C |
| D-dimer, ng/mL FEU (IQR) | 1.6 (0.7–3.5) | 3.8 (2.2–5.6) | 0.7 (0.3–2.3) | 0.8 (0.3–2.3) | p < 0.001 C |
| COVID IgG anti-S positive | 12.8% (6/49) | 86.7% (39/45) | 13.6% (3/22) | 17.6% (6/34) | p < 0.001 B |
| COVID IgG anti-N positive | 4.1% (2/49) | 77.3% (34/45) | 9.1% (2/22) | 14.7% (5/34) | p < 0.001 B |
| Imaging | |||||
| Brain MRI pathology | 6.3% (3/49) | 2.3% (1/49) | 0% | 0% | p = 0.207 B |
| X-ray bronchitis Pneumonia Pleural effusion | 14.3% (7/49) 24.5% (12/49) 10.2% (5/49) | 44.4% (20/45) 4.4% (2/45) 8.9% (4/45) | 9.1% (2/22) 4.5% (1/22) 0% | 9.1% (3/34) 0% 3% (1/34) | p < 0.001 B |
| Complementary examinations | |||||
| Cardiac complications | 10.2% (5/49) | 55.6% (25/45) | 13.6% (3/22) | 0% | p < 0.001 B |
| Neurological complications | 10.2% (5/49) | 6.7% (3/45) | 9.1% (2/22) | 5.9% (2/34) | p = 0.885 B |
| COVID | MIS-C | WORSENING | ISOLATION | p-Value | |
|---|---|---|---|---|---|
| Antibiotics | 53.1 % (26/49) | 100.0 % (45/45) | 50% (11/22) | 52.9% (18/34) | p < 0.001 A |
| Steroids | 49.0% (24/49) | 93.3% (42/45) | 18.1% (4/22) | 15.2% (5/34) | p < 0.001 B |
| Anticoagulation therapy | 24.5% (12/49) | 93.3% (42/45) | 9.1% (2/22) | 8.8% (3/34) | p < 0.001 A |
| IVIG | 6.1% (3/49) | 86.7% (39/45) | 4.5% (1/22) | 5.9% (2/34) | p < 0.001 A |
| Monoclonal antibodies | 6.1% (3/49) | 6.7% (3/45) | 9.1% (2/22) | 2.9% (1/34) | p = 0.767 B |
| Vasopressors | 8.2% (4/49) | 15.6% (7/45) | 0% | 2.9% (1/34) | p = 0.116 B |
| Oxygen therapy | 36.7% (18/49) | 11.1% (5/45) | 0% | 2.9% (1/34) | p < 0.001 B |
| Ventilation | 10.2% (5/49) | 2.2% (1/45) | 0% | 0% | p = 0.101 B |
| Ventilation length, days (IQR) ECMO | 14.0 (7.0–18.0) 0% | 6.0 (6.0–6.0) 0% | 0.0 0% | 0.0 0% | p = 0.380 C - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homola, L.; Klučka, J.; Fabián, D.; Štourač, P.; Šikula, J.; Vávrová, E.; Jeřábková, B.; Sihlovec, M.; Musil, V.; Španělová, K.; et al. Causes of Intensive Care Unit Admissions in Children with SARS-CoV-2: A Single-Centre Observational Study. Children 2023, 10, 75. https://doi.org/10.3390/children10010075
Homola L, Klučka J, Fabián D, Štourač P, Šikula J, Vávrová E, Jeřábková B, Sihlovec M, Musil V, Španělová K, et al. Causes of Intensive Care Unit Admissions in Children with SARS-CoV-2: A Single-Centre Observational Study. Children. 2023; 10(1):75. https://doi.org/10.3390/children10010075
Chicago/Turabian StyleHomola, Lukáš, Jozef Klučka, Dominik Fabián, Petr Štourač, Josef Šikula, Eva Vávrová, Barbora Jeřábková, Martin Sihlovec, Václav Musil, Klára Španělová, and et al. 2023. "Causes of Intensive Care Unit Admissions in Children with SARS-CoV-2: A Single-Centre Observational Study" Children 10, no. 1: 75. https://doi.org/10.3390/children10010075
APA StyleHomola, L., Klučka, J., Fabián, D., Štourač, P., Šikula, J., Vávrová, E., Jeřábková, B., Sihlovec, M., Musil, V., Španělová, K., Mužlayová, P., & Danhofer, P. (2023). Causes of Intensive Care Unit Admissions in Children with SARS-CoV-2: A Single-Centre Observational Study. Children, 10(1), 75. https://doi.org/10.3390/children10010075







