Effect of the Use of Gnrh Analogs in Low-Grade Cerebral Glioma
Abstract
1. Introduction
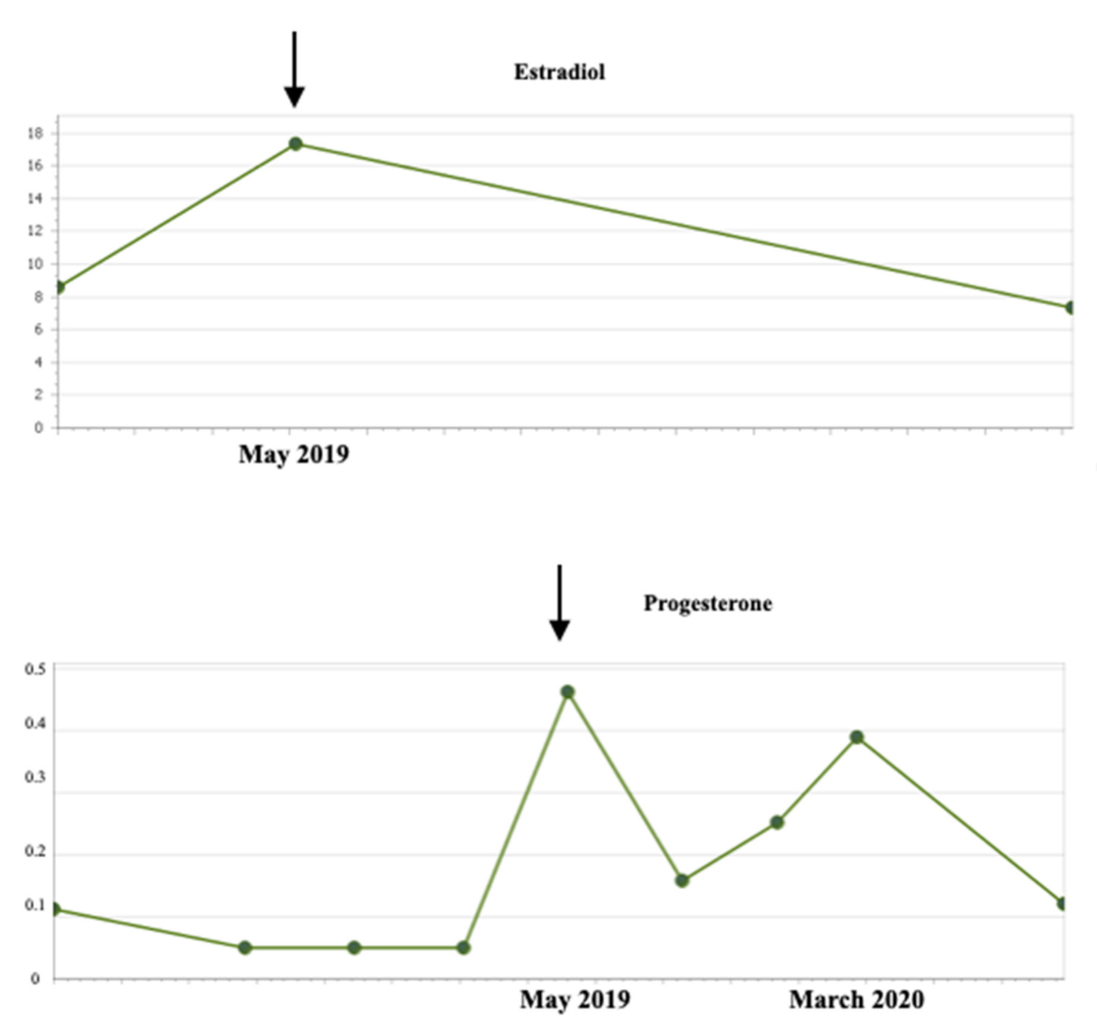
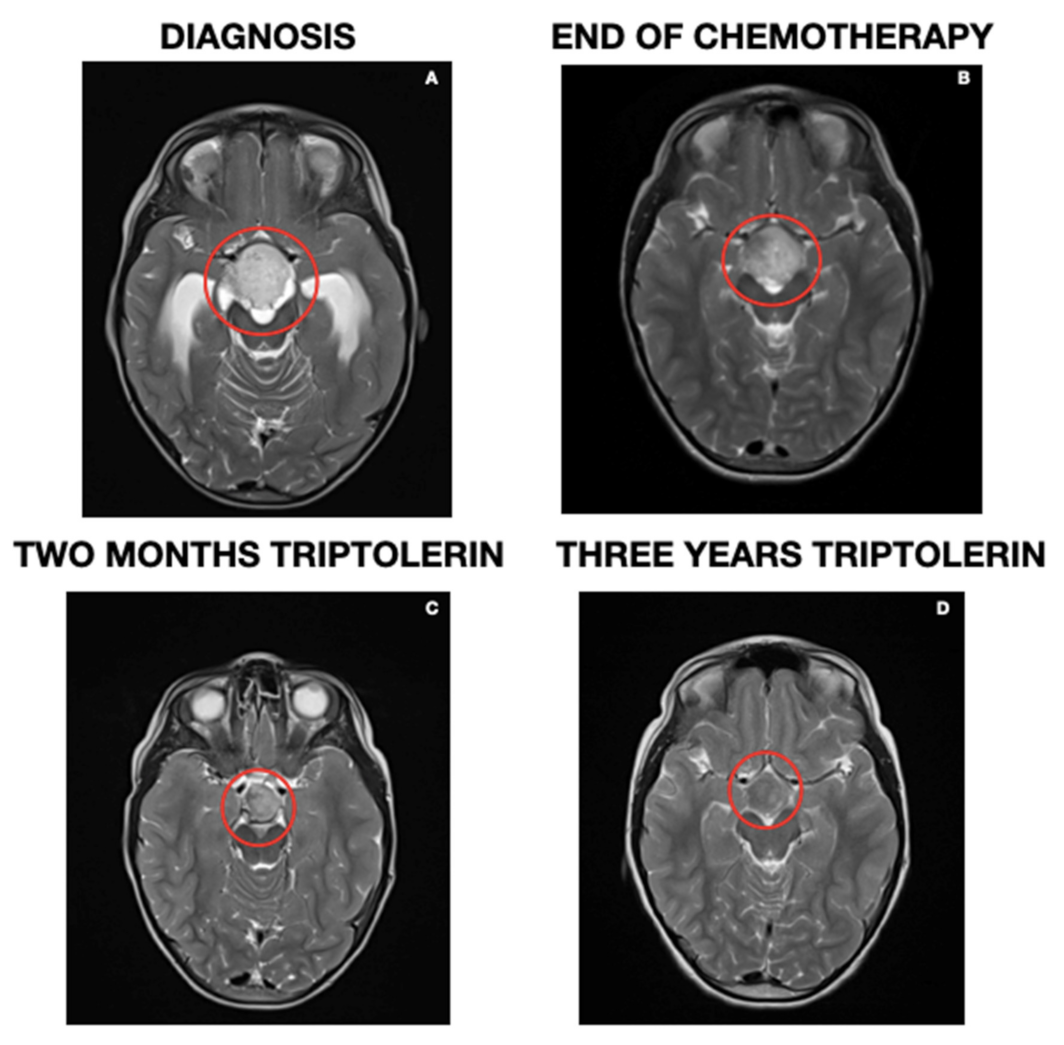
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cifras Básicas del Cáncer Infantil. Cáncer Infantil en España: Preguntas y datos. Registro Nacional de Tumores Infantiles RETI- SEHOP; España: RETI-SEHOP. Actualizado Octubre 2014. Available online: https://www.uv.es/rnti/pdfs/B1.05 (accessed on 19 September 2022).
- Ruiz, J.; Lesser, G.J. Low-grade gliomas. Curr. Treat Options Oncol. 2009, 10, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Figarella-Branger, D.; Appay, R.; Metais, A.; Tauziède-Espariat, A.; Colin, C.; Rousseau, A.; Varlet, P. La classification de l’OMS 2021 des tumeurs du système nerveux central. [The 2021 WHO classification of tumours of the central nervous system]. Ann. de Pathol. 2021, 42, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Chamdine, O.; Broniscer, A.; Wu, S.; Gajjar, A.; Qaddoumi, I. Metastatic Low-Grade Gliomas in Children: 20 Years’ Experience at St. Jude Children’s Research Hospital. Pediatr. Blood Cancer 2015, 63, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Baker, S.J.; West, A.N.; Fraser, M.M.; Proko, E.; Kocak, M.; Dalton, J.; Zambetti, G.P.; Ellison, D.W.; Kun, L.E.; et al. Clinical and Molecular Characteristics of Malignant Transformation of Low-Grade Glioma in Children. J. Clin. Oncol. 2007, 25, 682–689. [Google Scholar] [CrossRef]
- Krishnatry, R.; Zhukova, N.; Guerreiro Stucklin, A.S.; Pole, J.D.; Mistry, M.; Fried, I.; Ramaswamy, V.; Bartels, U.; Huang, A.; Laperriere, N.; et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer 2016, 122, 1261–1269. [Google Scholar] [CrossRef]
- Sievert, A.J.; Fisher, M.J. Pediatric Low-Grade Gliomas. J. Child Neurol. 2009, 24, 11. [Google Scholar] [CrossRef]
- Batistatou, A.; Stefanou, D.; Goussia, A.; Arkoumani, E.; Papavassiliou, A.G.; Agnantis, N.J. Estrogen receptor beta (ERbeta) is expressed in brain astrocytic tumors and declines with dedifferentiation of the neoplasm. J. Cancer Res. Clin. Oncol. 2004, 130, 405–410. [Google Scholar] [CrossRef]
- Batistatou, A.; Kyzas, P.A.; Goussia, A.; Arkoumani, E.; Voulgaris, S.; Polyzoidis, K.; Agnantis, N.J.; Stefanou, D. Estrogen receptor beta (ERβ) protein expression correlates with BAG-1 and prognosis in brain glial tumours. J. Neuro-Oncol. 2005, 77, 17–23. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Monteleone, P.; Gambacciani, M. Hormonal influence on the central nervous system. Maturitas 2002, 43, 11–17. [Google Scholar] [CrossRef]
- Tavares, C.B.; Gomes-Braga, F.D.C.S.A.; Costa-Silva, D.R.; Escórcio-Dourado, C.S.; Borges, U.S.; Conde, A.M.; Barros-Oliveira, M.D.C.; Sousa, E.B.; Barros, L.D.R.; Martins, L.M.; et al. Expression of estrogen and progesterone receptors in astrocytomas: A literature review. Clinics 2016, 71, 481–486. [Google Scholar] [CrossRef]
- Kabat, G.C.; Etgen, A.M.; Rohan, T.E. Do steroid hormones play a role in the etiology of glioma? Cancer Epidemiol. Biomark. Prev. 2010, 19, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Arroyo, I.; Hernández-Hernández, O.T.; Cabrera-Muñoz, E. Role of progesterone in human astrocytomas growth. Curr. Top. Med. Chem. 2011, 11, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Jakacki, R.; Goldman, S.; Hargrave, D.; Hawkins, C.; Shroff, M.; Hukin, J.; Bartels, U.; Foreman, N.; Kellie, S.; et al. Phase II Study of Weekly Vinblastine in Recurrent or Refractory Pediatric Low-Grade Glioma. J. Clin. Oncol. 2012, 30, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Spreafico, F.; Cefalo, G.; Riccardi, R.; Tesoro-Tess, J.D.; Gandola, L.; Riva, D.; Ruggiero, A.; Valentini, L.; Mazza, E.; et al. High Response Rate to Cisplatin/Etoposide Regimen in Childhood Low-Grade Glioma. J. Clin. Oncol. 2002, 20, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Poisson, M.; Pertuiset, B.F.; Hauw, J.J.; Philippon, J.; Buge, A.; Moguilewsky, M.; Philibert, D. Steroid hormone receptors in human meningiomas, gliomas and brain metastases. J. Neurooncol. 1983, 1, 179–189. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.M.; Garcia-Lopez, P. Estrogen Receptors as Molecular Targets of Endocrine Therapy for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 12404. [Google Scholar] [CrossRef]
- Zhou, M.; Sareddy, G.R.; Li, M.; Liu, J.; Luo, Y.; Venkata, P.P.; Viswanadhapalli, S.; Tekmal, R.R.; Brenner, A.; Vadlamudi, R.K. Estrogen receptor beta enhances chemotherapy response of GBM cells by down regulating DNA damage response pathways. Sci. Rep. 2019, 9, 6124. [Google Scholar] [CrossRef]
- Liu, J.; Sareddy, G.R.; Zhou, M.; Viswanadhapalli, S.; Li, X.; Lai, Z.; Tekmal, R.R.; Brenner, A.; Vadlamudi, R.K. Differential effects of estrogen receptor β isoforms on glioblastoma progression. Cancer Res. 2018, 78, 3176–3189. [Google Scholar] [CrossRef]
- Kumar, R.; Litwack, G. Structural and functional relationships of the steroid hormone receptors’ N-terminal transactivation domain. Steroids 2009, 74, 877–883. [Google Scholar] [CrossRef]
- Li, W.; Winters, A.; Poteet, E.; Ryou, M.-G.; Lin, S.; Hao, S.; Wu, Z.; Yuan, F.; Hatanpaa, K.J.; Simpkins, J.W.; et al. Involvement of estrogen receptor β5 in the progression of glioma. Brain Res. 2013, 1503, 97–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sareddy, G.R.; Nair, B.C.; Gonugunta, V.K.; Zhang, Q.G.; Brenner, A.; Brann, D.W.; Tekmal, R.R.; Vadlamudi, R.K. Therapeutic significance of estrogen receptor beta agonists in gliomas. Mol. Cancer Ther. 2012, 11, 1174–1182. [Google Scholar] [CrossRef]
- Dueñas Jiménez, J.M.; Candanedo Arellano, A.; Santerre, A.; Orozco Suárez, S.; Sandoval Sánchez, H.; Feria Romero, I.; López-Elizalde, R.; Alonso Venegas, M.; Netel, B.; de la Torre Valdovinos, B.; et al. Aromatase and estrogen receptor alpha mRNA expression as prognostic biomarkers in patients with astrocytomas. J. Neurooncol. 2014, 119, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.S.; Zhang, J.; Dashner, K.; Sar, M.; Black, P.M. Steroid Hormone Receptors in Astrocytic Neoplasms. Neurosurgery 1995, 37, 496–504. [Google Scholar] [CrossRef] [PubMed]
- González-Arenas, A.; Hansberg-Pastor, V.; Hernández-Hernández, O.T.; González-Garcia, T.K.; Henderson Villalpando, J.; Lemus-Hernández, D.; Cruz-Barrios, A.; Rivas-Suárez, M.; Camacho-Arroyo, I. Estradiol increases cell growth in human astrocytoma cell lines through ERa activation and its interaction with SRC-1 and SRC-3 coactivators. Biochim. Biophys. Acta 2012, 1823, 379–386. [Google Scholar] [CrossRef] [PubMed]
- González-Arenas, A.; Peña-Ortiz, M.A.; Hansberg-Pastor, V.; MarquinaSánchez, B.; Baranda-Ávila, N.; Nava-Castro, K.; Cabrera-Wrooman, A.; González-Jorge, J.; Camacho-Arroyo, I. PKCa and PKCd Activation Regulates Transcriptional Activity and Degradation of Progesterone Receptor in Human Astrocytoma Cells. Endocrinology 2015, 156, 101022. [Google Scholar] [CrossRef][Green Version]
- Lonard, D.M.; Smith, C.L. Molecular perspectives on selective estrogen receptor modulators (SERMs): Progress in understanding their tissue-specific agonist and antagonist actions. Steroids 2001, 67, 15–24. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E. The Effects of Progesterone on Glial Cell Line-derived Neurotrophic Factor Secretion from C6 Glioma Cells. Iran. J. Basic Med. Sci. 2012, 15, 1046–1052. [Google Scholar] [CrossRef]
- Cabrera-Muñoz, E.; González-Arenas, A.; Saqui-Salces, M.; Camacho, J.; Larrea, F.; García-Becerra, R.; Camacho-Arroyo, I. Regulation of progesterone receptor isoforms content in human astrocytoma cell lines. J. Steroid Biochem. Mol. Biol. 2009, 113, 80–84. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Zhang, K.; Bian, C.; Zhao, Y.; Zhang, J. Expression of estrogen receptors, androgen receptor and steroid receptor coactivator-3 is negatively correlated to the differentiation of astrocytic tumors. Cancer Epidemiol. 2014, 38, 291–297. [Google Scholar] [CrossRef]
- González-Agüero, G.; Ondarza, R.; Gamboa-Domínguez, A.; Cerbón, M.A.; Camacho-Arroyo, I. Progesterone receptor isoforms expression pattern in human astrocytomas. Brain Res. Bull. 2001, 56, 43–48. [Google Scholar] [CrossRef]
- Rosal, A.M.; Da Silva, B.B. Evaluation of estrogen and progesterone receptors in non-neoplasic breast tissue of women of reproductive age explosed to tamoxifen and raloxifene: A randomized, double-blind study. Breast Cancer Res. Treat. 2011, 125, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Central Brain Tumor Registry of the United States: Statistical Report: Primary Brain Tumors in the United States, 1995–1999. Available online: http://www.cbtrus.org/2002/2002report.pdf (accessed on 18 September 2022).
- Hsu, D.W.; Efird, J.T.; Hedley-Whyte, E.T. Progesterone and estrogen receptors in meningiomas: Prognostic considerations. J. Neurosurg. 1997, 86, 113. [Google Scholar] [CrossRef] [PubMed]
- Germán-Castelán, L.; Manjarrez-Marmolejo, J.; González-Arenas, A.; González-Morán, M.G.; Camacho-Arroyo, I. Progesterone induces the growth and infiltration of human astrocytoma cells implanted in the cerebral cortex of the rat. BioMed Res Int. 2014, 2014, 393174. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.; Yousuf, S.; Stein, D.G. Anti-tumor effects of progesterone in human glioblastoma multiforme: Role of PI3K/Akt/mTOR signaling. J. Steroid Biochem. Mol. Biol. 2015, 146, 62–73. [Google Scholar] [CrossRef]
- Rozen, W.M.; Joseph, S.; Lo, P.A. Spontaneous Regression of Low-Grade Gliomas in Pediatric Patients without Neurofibromatosis. Pediatr. Neurosurg. 2008, 44, 324–328. [Google Scholar] [CrossRef]
- Parsa, C.F.; Hoyt, C.S.; Lesser, R.L.; Weinstein, J.M.; Strother, C.M.; Muci-Mendoza, R.; Ramella, M.; Manor, R.S.; Fletcher, W.A.; Repka, M.X.; et al. Spontaneous regressions of optic gliomas: Thirteen cases documented by serial neuroimaging. Arch. Ophthalmol. 2001, 119, 516–529. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lucio Delgado, A.; Villegas Rubio, J.A.; Riaño-Galán, I.; Pérez Gordón, J. Effect of the Use of Gnrh Analogs in Low-Grade Cerebral Glioma. Children 2023, 10, 115. https://doi.org/10.3390/children10010115
de Lucio Delgado A, Villegas Rubio JA, Riaño-Galán I, Pérez Gordón J. Effect of the Use of Gnrh Analogs in Low-Grade Cerebral Glioma. Children. 2023; 10(1):115. https://doi.org/10.3390/children10010115
Chicago/Turabian Stylede Lucio Delgado, Ana, Jose Antonio Villegas Rubio, Isolina Riaño-Galán, and Juan Pérez Gordón. 2023. "Effect of the Use of Gnrh Analogs in Low-Grade Cerebral Glioma" Children 10, no. 1: 115. https://doi.org/10.3390/children10010115
APA Stylede Lucio Delgado, A., Villegas Rubio, J. A., Riaño-Galán, I., & Pérez Gordón, J. (2023). Effect of the Use of Gnrh Analogs in Low-Grade Cerebral Glioma. Children, 10(1), 115. https://doi.org/10.3390/children10010115






