Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development
Abstract
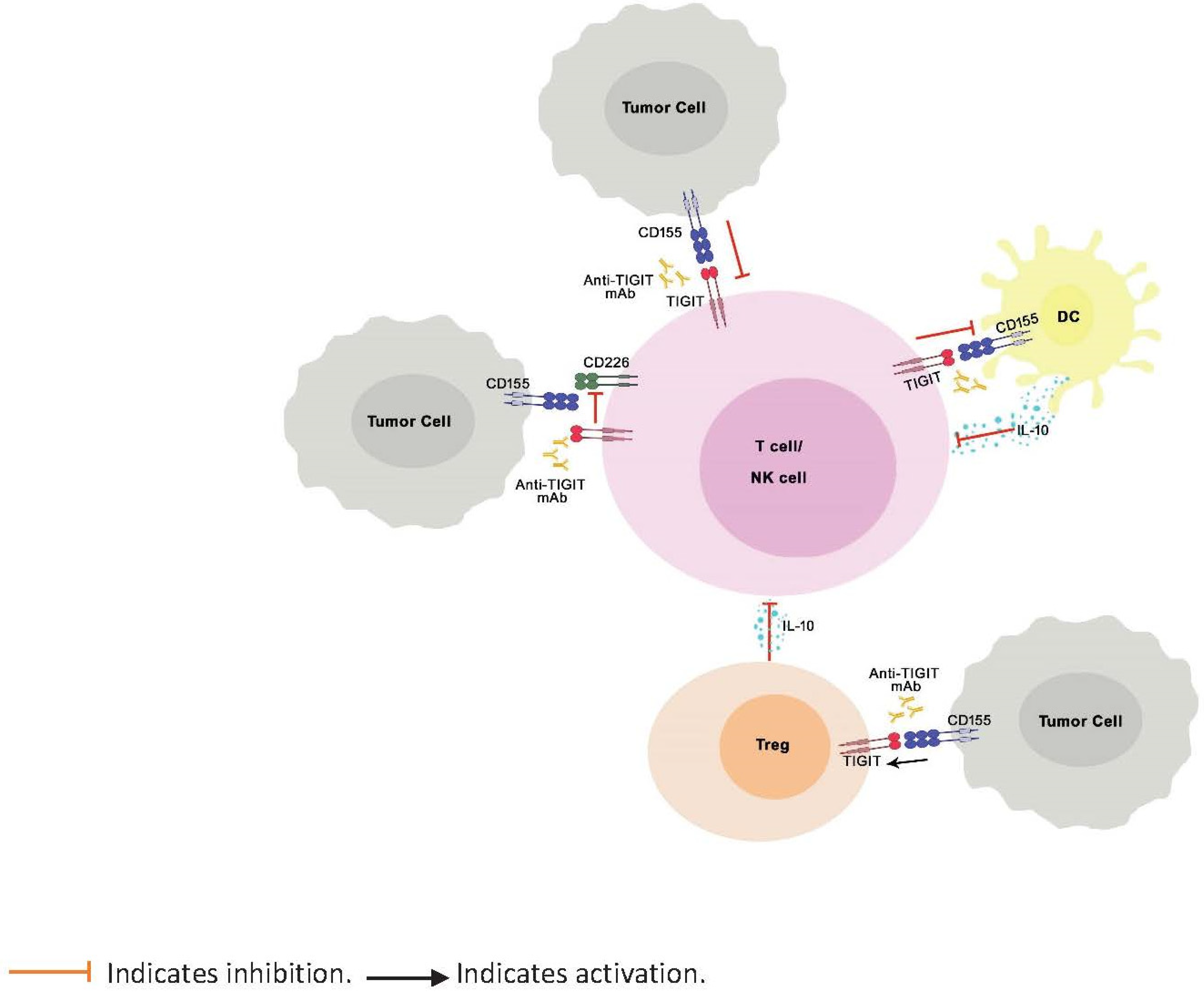
:1. Introduction
2. Tigit
2.1. Discovery
2.2. Expression
2.3. Ligands and Cells Expressing Ligands
2.4. Regulation of Immune Response
2.5. Target for Cancer Immunotherapy
3. Anti-Tigit Antibodies in Development
3.1. Factors Considered during Development
3.2. Origin
3.3. IgG Isotype and FcγR Binding
3.4. Dose
3.5. Safety
4. Clinical Status
5. Challenges
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Bhandaru, M. Interleukin-2. In Immunotherapy of Melanoma; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Rotte, A.; Bhandaru, M. Interferon-a2b. In Immunotherapy of Melanoma; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Bhandaru, M.; Rotte, A. Blockade of programmed cell death protein-1 pathway for the treatment of melanoma. J. Dermatol. Res. Ther. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Bhandaru, M.; Rotte, A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. Methods Mol. Biol. 2019, 1904, 83–108. [Google Scholar] [CrossRef]
- Rotte, A.; Bhandaru, M.; Zhou, Y.; McElwee, K.J. Immunotherapy of melanoma: Present options and future promises. Cancer Metastasis Rev. 2015, 34, 115–128. [Google Scholar] [CrossRef]
- Varade, J.; Magadan, S.; Gonzalez-Fernandez, A. Human immunology and immunotherapy: Main achievements and challenges. Cell Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Zhao, D.; Xie, B.; Yang, Y.; Yan, P.; Liang, S.N.; Lin, Q. Progress in immunotherapy for small cell lung cancer. World J. Clin. Oncol. 2020, 11, 370–377. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily—CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 2001, 410, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, T.; Taniwaki, M.; Ishida, Y.; Kawaichi, M.; Honjo, T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 1994, 23, 704–706. [Google Scholar] [CrossRef]
- Nishimura, H.; Honjo, T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001, 22, 265–268. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Lemaire, V.; Shemesh, C.; Rotte, A. Pharmacology-Based Ranking of Anti-Cancer Drugs to Guide Clinical Development of Cancer Immunotherapy Combinations; 2021.
- Tarhini, A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: The underlying mechanisms and clinical management. Scientifica 2013, 2013, 857519. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015, 125, 4053–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Harjunpaa, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step towards Successful Combination Immune Checkpoint Therapy in Cancer. Front Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef]
- Yeo, J.; Ko, M.; Lee, D.H.; Park, Y.; Jin, H.S. TIGIT/CD226 Axis Regulates Anti-Tumor Immunity. Pharmaceuticals 2021, 14, 200. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016, 12, e1005349. [Google Scholar] [CrossRef] [Green Version]
- Reches, A.; Ophir, Y.; Stein, N.; Kol, I.; Isaacson, B.; Charpak Amikam, Y.; Elnekave, A.; Tsukerman, P.; Kucan Brlic, P.; Lenac, T.; et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Eberle, F.; Dubreuil, P.; Mattei, M.G.; Devilard, E.; Lopez, M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 1995, 159, 267–272. [Google Scholar] [CrossRef]
- Lopez, M.; Aoubala, M.; Jordier, F.; Isnardon, D.; Gomez, S.; Dubreuil, P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood 1998, 92, 4602–4611. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Horikawa, K.; Nakanishi, H.; Takahashi, K.; Miyahara, M.; Nishimura, M.; Tachibana, K.; Mizoguchi, A.; Takai, Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 2000, 275, 10291–10299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, D.; Jarry, A.; Baury, B.; Blanchardie, P.; Laboisse, C.; Lustenberger, P.; Denis, M.G. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 2001, 49, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevelacqua, V.; Bevelacqua, Y.; Candido, S.; Skarmoutsou, E.; Amoroso, A.; Guarneri, C.; Strazzanti, A.; Gangemi, P.; Mazzarino, M.C.; D’Amico, F.; et al. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget 2012, 3, 882–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, T.; Sato, S.; Kato, J.; Ito, Y.; Watanabe, T.; Tsuji, I.; Hori, A.; Kurokawa, T.; Kokubo, T. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol. Cancer 2013, 12, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escalante, N.K.; von Rossum, A.; Lee, M.; Choy, J.C. CD155 on human vascular endothelial cells attenuates the acquisition of effector functions in CD8 T cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Chambers, C.A.; Sullivan, T.J.; Allison, J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997, 7, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef]
- Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W.; Takai, Y. Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 2004, 15, 643–656. [Google Scholar] [CrossRef]
- Sakisaka, T.; Takai, Y. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 2004, 16, 513–521. [Google Scholar] [CrossRef]
- Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W.; Takai, Y. Roles of nectins in cell adhesion, signaling and polarization. Handb. Exp. Pharmacol. 2004, 343–372. [Google Scholar] [CrossRef]
- Fuchs, A.; Colonna, M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin. Cancer Biol. 2006, 16, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Stamm, H.; Oliveira-Ferrer, L.; Grossjohann, E.M.; Muschhammer, J.; Thaden, V.; Brauneck, F.; Kischel, R.; Muller, V.; Bokemeyer, C.; Fiedler, W.; et al. Targeting the TIGIT-PVR immune checkpoint axis as novel therapeutic option in breast cancer. Oncoimmunology 2019, 8, e1674605. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Liu, J.; Cui, J.; Ma, B.; Zhou, Q.; Yang, X.; Lu, Z.; Du, Y.; Su, C. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol. Med. Rep. 2019, 20, 3773–3781. [Google Scholar] [CrossRef] [Green Version]
- Hinsch, A.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; et al. Expression of the immune checkpoint receptor TIGIT in seminoma. Oncol. Lett. 2019, 18, 1497–1502. [Google Scholar] [CrossRef] [Green Version]
- Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Patterns of TIGIT Expression in Lymphatic Tissue, Inflammation, and Cancer. Dis. Markers 2019, 2019, 5160565. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer 2018, 18, 1209. [Google Scholar] [CrossRef] [PubMed]
- Au, Q.; Hanifi, A.; Parnell, E.; Kuo, J.; Leones, E.; Sahafi, F.; Pham, K.; Padmanabhan, R.K.; Hoe, N.; William, J. Characterization of TIGIT expression using MultiOmyxTM hyperplexed immunofluorescence assay in solid tumors [abstract]. In Proceedings of the American Association for Cancer Research Annual Meeting. Cancer Res. 2019, 79, AM2019–AM2497. [Google Scholar]
- Pal, S.K.; Vanderwalde, A.M.; Szeto, C.; Reddy, S.; Hamid, O. PD-L1 expression is strongly associated with TIGIT, FOXP3 and LAG3 across advanced cancers, but not OX40, TIM3 and IDO. Ann. Oncol. 2018, 29, VIII421–VIII422. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, J.; Chen, Y.; Cui, J.; Lei, Y.; Cui, Y.; Jiang, N.; Jiang, W.; Chen, L.; Chen, Y.; et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). Int. Immunopharmacol. 2020, 80, 106198. [Google Scholar] [CrossRef]
- Yu, H.; Koczara, C.; Lohinai, Z.; Badzio, A.; Czapiewski, P.; Döme, B.; Moldvay, J.; Fillinger, J.; Gao, D.; Ellison, K.; et al. Expression of the Immune Checkpoint Axis-PVR/TIGIT in Small Cell Lung Cancer [Abstract]. J. Thor. Oncol. 2018, 1, S974–S975. [Google Scholar] [CrossRef] [Green Version]
- Schorer, M.; Rakebrandt, N.; Lambert, K.; Hunziker, A.; Pallmer, K.; Oxenius, A.; Kipar, A.; Stertz, S.; Joller, N. TIGIT limits immune pathology during viral infections. Nat. Commun. 2020, 11, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 2018, 7, e1466769. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Harjunpaa, H.; Carrie, N.; Kassem, S.; Teo, T.; Miles, K.; Krumeich, S.; Weulersse, M.; Cuisinier, M.; Stannard, K.; et al. TIGIT immune checkpoint blockade restores CD8(+) T-cell immunity against multiple myeloma. Blood 2018, 132, 1689–1694. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Xu, Y.; Chen, Y.; Shan, S. TIGIT enhances CD4(+) regulatory T-cell response and mediates immune suppression in a murine ovarian cancer model. Cancer Med. 2020, 9, 3584–3591. [Google Scholar] [CrossRef] [Green Version]
- Roche/Genentech. Roche’s Novel Anti-Tigit Tiragolumab Granted Fda Breakthrough Therapy Designation in Combination with Tecentriq for Pd-L1-High Non-Small Cell Lung Cancer. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0000498.pub2 (accessed on 17 September 2021).
- Ma, L.; Gai, J.; Qiao, P.; Li, Y.; Li, X.; Zhu, M.; Li, G.; Wan, Y. A novel bispecific nanobody with PD-L1/TIGIT dual immune checkpoint blockade. Biochem. Biophys. Res. Commun. 2020, 531, 144–151. [Google Scholar] [CrossRef]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 576–588. [Google Scholar] [CrossRef]
- Teillaud, J.L. Antibody-dependent Cellular Cytotoxicity (ADCC). In eLS; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Zahavi, D.; AlDeghaither, D.; O’Connell, A.; Weiner, L.M. Enhancing antibody-dependent cell-mediated cytotoxicity: A strategy for improving antibody-based immunotherapy. Antib. Ther. 2018, 1, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Merlano, M.C. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: Biological evidence and clinical perspectives. Ann. Transl. Med. 2019, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Temming, A.R.; de Taeye, S.W.; de Graaf, E.L.; de Neef, L.A.; Dekkers, G.; Bruggeman, C.W.; Koers, J.; Ligthart, P.; Nagelkerke, S.Q.; Zimring, J.C.; et al. Functional Attributes of Antibodies, Effector Cells, and Target Cells Affecting NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity. J. Immunol. 2019, 203, 3126–3135. [Google Scholar] [CrossRef]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.; Quek, J.K.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; O’Donovan, N.; McGowan, P.M.; O’Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann. Oncol. 2012, 23, 1788–1795. [Google Scholar] [CrossRef]
- Iannello, A.; Ahmad, A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005, 24, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Waight, J.D.; Chand, D.; Dietrich, S.; Gombos, R.; Horn, T.; Gonzalez, A.M.; Manrique, M.; Swiech, L.; Morin, B.; Brittsan, C.; et al. Selective FcgammaR Co-engagement on APCs Modulates the Activity of Therapeutic Antibodies Targeting T Cell Antigens. Cancer Cell 2018, 33, 1033–1047.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Wang, J.; Yu, Y.; Yu, X.; Hu, Y.; Ai, X.; Ma, Z.; Li, X.; Zhuang, W.; Liu, Y.; et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J. Thorac. Oncol. 2021, 38. [Google Scholar] [CrossRef]
- Osarogiagbon, R.U. Tislelizumab-A Promising New Option for Enhancing Chemotherapy Benefit in Treatment for Advanced Squamous Cell Lung Cancer. JAMA Oncol. 2021, 7, 717–719. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Yu, X.; Hu, Y.; Sun, Y.; Wang, Z.; Zhao, J.; Yu, Y.; Hu, C.; Yang, K.; et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 709–717. [Google Scholar] [CrossRef]
- Chand, D.; Waight, J.D.; Paltrinieri, E.; Dietrich, S.; Bushell, M.; Costa, M.; Gombos, R.; Wilson, N.S.; Buell, J.S.; Stein, R.B.; et al. FcgR co-engagement by anti-TIGIT monoclonal antibodies enhances T cell functionality and antitumor immune responses [abstract]. In Proceedings of the American Association for Cancer Research Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019; p. 2390. [Google Scholar]
- Anderson, A.E.; Lopez, A.; Udyavar, A.; Narasappa, N.; Lee, S.; DiRenzo, D.; Zhang, K.; Singh, H.; Zhao, S.; Gerrick, K.; et al. Characterization of AB154, a Humanized, Non-Depleting α-TIGIT Antibody Undergoing Clinical Evaluation in Subjects with Advanced Solid Tumors. In Proceedings of the SITC Annual Meeting, National Harbor, MD, USA, 6–10 November 2019. [Google Scholar]
- Tiragolumab Impresses in Multiple Trials. Cancer Discov. 2020, 10, 1086–1087. [CrossRef]
- Frentzas, S.; Meniawy, T.; Kao, S.C.-H.; Wang, R.; Zuo, Y.; Zheng, H.; Tan, W. ADVANTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibody ociperlimab (BGB-A1217) in combination with tislelizumab in patients with advanced solid tumors. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Ahn, M.; Niu, J.; Kim, D.; Rasco, D.; Mileham, K.F.; Chung, H.C.; Vaishampayan, U.N.; Maurice-Dror, C.; Lo Russo, P.; Golan, T.; et al. 1400P—Vibostolimab, an anti-TIGIT antibody, as monotherapy and in combination with pembrolizumab in anti-PD-1/PD-L1-refractory NSCLC. Ann. Oncol. 2020, 31, S887. [Google Scholar] [CrossRef]
| Receptor/Ligand | Cells/Tissues |
|---|---|
| TIGIT | Resting CD4+CD25hi Treg cells, activated T cells, NK cells, NKT cells, memory T cells, and exhausted T cells |
| CD155 (PVR, nectin like protein-5) | DCs, T cells, B cells, macrophages, and cancer cells. Human vascular endothelial cells in response to IFN-γ |
| CD112 (PVRL-2, nectin-2) | Bone marrow, lung, pancreas, kidney, and some types of cancer |
| CD113 (PVRL-3, nectin-3 | Lung, liver, testis, kidney, placenta, and some types of cancer |
| Nectin-4 | Squamous epithelia, placenta, and some types of cancers |
| Study (First Author et al. [Reference]) | Finding |
|---|---|
| Yu et al. 2009 [35] Boles et al. 2008 [36] | Discovery of TIGIT TIGIT is expressed on activated T cells, NK cells |
| Joller et al. 2014 [48] | TIGIT is expressed on distinct subset of Tregs that specifically suppress Th1 and Th17 cells |
| Kurtulus et al. 2015 [29] | TIGIT is a marker for CD8+ T-cell exhaustion Tigit−/− mice bearing colon cancer (MC38) or melanoma (B16F10) have significantly lower tumor growth |
| Johnston et al. 2014 [37] | TIGIT expression correlates with PD-1 in human cancer Co-blockade of TIGIT and PD-1 resulted in synergistic CD8+-mediated rejection of tumors |
| Chew et al. 2016 [38] | TIGIT is a marker for T-cell exhaustion |
| Zhang et al. 2018 [47] | TIGIT is associated with NK cell exhaustion TIGIT blockade prevented NK cell exhaustion and resulted in NK cell–dependent tumor immunity |
| Guillerey et al. 2018 [68] | Multiple myeloma progression is associated with high TIGIT expression on CD8+ T cells Tigit−/− mice bearing myeloma tumors (Vk12653) have lower tumor growth and longer survival TIGIT blockers suppressed multiple myeloma growth in mice |
| Hung et al. 2018 [67] | TIGIT expression is higher in CD8+ T cells and Tregs in brain of glioblastoma tumor (GL261)-bearing mice Co-blockade of TIGIT and PD-1 improved survival in glioblastoma tumor-bearing mice |
| Generic Name | Type | FcγR Status | Company | Status |
|---|---|---|---|---|
| Tiragolumab (MTIG7192A) | Fully human IgG1 | Active | Genentech | Phase III |
| Ociperlimab (BGB-A1217) | Humanized IgG1 | Active | BeiGene USA, Inc | Phase III |
| Vibostolimab (MK-7684) | Fully human IgG1 | Active | Merck & Co Inc | Phase II |
| Domvanalimab (AB-154) | Fully human IgG1 | Inactive | Arcus Biosciences Inc | Phase II |
| BMS-986207 | Fully human IgG1 | Inactive | Bristol-Myers Squibb Co | Phase II |
| EOS-448 | Fully human IgG1 | Active | iTeos Therapeutics SA | Phase II |
| ASP-8374 | Fully human IgG4 | Inactive | Astellas Pharma Inc | Phase I |
| COM-902 | Mouse/cyno cross-reactive fully human IgG1 antibody | NA | Compugen Ltd. | Phase I |
| Etigilimab | Fully human IgG1 | Active | Mereo Biopharma Group Plc | Phase I |
| IBI-939 | NA | NA | Innovent Biologics Inc | IND Filed |
| AGEN-1307 | Fully human IgG1 | Active and enhanced | Agenus Inc | Preclinical |
| CASC-674 | Fully human IgG2a | Inactive | Seattle Genetics Inc | Preclinical |
| Anti-PVR Antibody (NB-6253) | NA | NA | Northern Biologics Inc | Preclinical |
| PH-804 | NA | NA | Phio Pharmaceuticals Corp | Preclinical |
| TIGIT-PD-L1 dual | NA | NA | Aurigene Discovery Technologies Ltd. | Preclinical |
| Drug | Phase | Dose and Regimen | Comment | Reference |
|---|---|---|---|---|
| Tiragolumab | Phase III Multiple Solid tumors | 2 mg to 1200 mg Q3W RP2D: 600 mg Q3W | 100% receptor occupancy seen at ≥30 mg and clinical activity observed at doses 400 mg to 600 mg. 600 mg Q3W was proposed as dose for Ph2 study. | Bendell et al. AACR 2020 |
| Ociperlimab | Phase III | 50 mg to 900 mg RP2D: 900 mg Q3W | 100% receptor occupancy was observed at 50 mg, and linear PK was observed through 900 mg. | NCT04746924 |
| Domvanalimab (AB-154) | Phase I NSCLC | 0.5 mg/kg; 1 mg/kg & 3 mg/kg Q2W | 100% receptor occupancy seen at 3 mg/kg | Anderson et al. SITC 2019 p260 |
| Vibostolimab (MK-7684) | Phase I Multiple Solid tumors | 2.1 mg to 700 mg Q3W RP2D: 200 mg Q3W | ORR 19% in combination with pembrolizumab Vibostolimab well tolerated as monotherapy and in combination with 200 mg pembrolizumab | Golan et al. SITC 2018 |
| BMS-986207 | Phase I/II | Not disclosed | No details | NCT02913313 |
| EOS-448 | Phase I | 0.1 mg/kg, 1 mg/kg and 10 mg/kg | Receptor occupancy increased with dose. Nearly 100% occupancy was seen at 10 mg/kg dose. Dose-limiting toxicity was not seen. | Nguyen et al. AACR 2020 |
| ASP-8374 | Phase I Solid tumors | Not disclosed | Details not available | NCT03260322 |
| COM-902 | Phase I Solid tumors | 7 doses to be tested for dose limiting toxicity. Q3W regimen | Data not available. Study posted in April 2020. | NCT04354246 |
| Etigilimab | Phase I | 0.3 mg/kg to 20 mg/kg Q2W | Safely administered up to 20 mg/kg. Stable disease was seen in 7/18 patients across all doses | Sharma et al. SITC 2018 |
| Drug; Sponsor | Clinical Trial Identifier; Phase | Study Title | Status as of August 2021 |
|---|---|---|---|
| BMS-986207 Multiple Myeloma Research Consortium | NCT04150965; Phase I, II | Immuno-Oncology Drugs Elotuzumab, Anti-LAG-3, and Anti-TIGIT | Recruiting |
| BMS-986207; Compugen | NCT04570839 Phase I, II | COM701 in Combination With BMS-986207 and Nivolumab in Subjects With Advanced Solid Tumors. | Recruiting |
| IBI939; Innovent Biologics | NCT04353830; Phase I | A Study Evaluating the Safety, Tolerability, and Initial Efficacy of Recombinant Human Anti-T-cell Immunoreceptor With Ig and ITIM Domains (TIGIT) Monoclonal Antibody Injection (IBI939) in Subjects With Advanced Malignant Tumors | Recruiting |
| Ociperlimab; BeiGene | NCT04047862; Phase I | Study of BGB-A1217 in Combination With Tislelizumab in Advanced Solid Tumors | Recruiting |
| Ociperlimab; BeiGene | NCT04693234; Phase II | AdvanTIG-202: Anti-PD-1 Monoclonal Antibody Tislelizumab (BGB-A317) Combined With or Without Anti-TIGIT Monoclonal Antibody Ociperlimab (BGB-A1217) in Participants With Previously Treated Recurrent or Metastatic Cervical Cancer | Recruiting |
| Ociperlimab; BeiGene | NCT04732494; Phase II | AdvanTIG-203: Anti-PD-1 Monoclonal Antibody Tislelizumab (BGB-A317) Combined With or Without Anti-TIGIT Monoclonal Antibody Ociperlimab (BGB-A1217) in Participants With Recurrent or Metastatic Esophageal Squamous Cell Carcinoma | Recruiting |
| Ociperlimab; BeiGene | NCT04746924; Phase III | A Study of Ociperlimab With Tislelizumab Compared to Pembrolizumab in Participants With Untreated Lung Cancer | Recruiting |
| Ociperlimab; BeiGene | NCT04952597; Phase II | Study of Ociperlimab Plus Tislelizumab Plus Chemoradiotherapy in Participants With Untreated Limited-Stage Small Cell Lung Cancer | Recruiting |
| COM902; Compugen | NCT04354246; Phase I | COM902 (A TIGIT Inhibitor) in Subjects With Advanced Malignancies | Recruting |
| M6223; EMD Serono Research & Development Institute, Inc | NCT04457778; Phase I | First in Human Study of M6223 in Participants With Metastatic or Locally Advanced Solid Unresectable Tumors | Recruting |
| Tiragolumab; Genentech | NCT03563716 Phase II | A Study of MTIG7192A in Combination With Atezolizumab in Chemotherapy-Naïve Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer | Active, Not Recruiting |
| Tiragolumab; Genentech | NCT04294810; Phase III | A Study of Tiragolumab in Combination With Atezolizumab Compared With Placebo in Combination With Atezolizumab in Patients With Previously Untreated Locally Advanced Unresectable or Metastatic PD-L1-Selected Non-Small Cell Lung Cancer (SKYSCRAPER-01) | Recruiting |
| Tiragolumab; Genentech | NCT04256421; Phase III | A Study of Atezolizumab Plus Carboplatin and Etoposide With or Without Tiragolumab in Patients With Untreated Extensive-Stage Small Cell Lung Cancer (SKYSCRAPER-02) | Recruiting |
| Tiragolumab; Hoffmann-La Roche | NCT03281369; Phase Ib, II | A Study of Multiple Immunotherapy-Based Treatment Combinations in Patients With Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction Cancer (G/GEJ) or Esophageal Cancer (Morpheus-Gastric and Esophageal Cancer) | Recruiting |
| Tiragolumab; Hoffmann-La Roche | NCT04543617; Phase III | A Study of Atezolizumab With or Without Tiragolumab in Participants With Unresectable Esophageal Squamous Cell Carcinoma Whose Cancers Have Not Progressed Following Definitive Concurrent Chemoradiotherapy (SKYSCRAPER-07) | Recruiting |
| AB154; Arcus Biosciences | NCT03628677; Phase I | A Study to Evaluate the Safety and Tolerability of AB154 in Participants With Advanced Malignancies | Recruiting |
| AB154; Yale University | NCT04656535; Early Phase I | AB154 Combined With AB122 for Recurrent Glioblastoma | Recruiting |
| Vibostolimab; Merck | NCT02964013; Phase I | Study of Vibostolimab Alone and in Combination With Pembrolizumab in Advanced Solid Tumors (MK-7684-001) | Recruiting |
| Vibostolimab; Merck | NCT04165070; Phase II | Substudy 1: Efficacy and Safety Study of Pembrolizumab (MK-3475) Plus Chemotherapy When Used With Investigational Agents in Treatment-Naïve Participants With Advanced NonSsmall Cell Lung Cancer (NSCLC) (MK-3475-01A/KEYNOTE-01A) | Recruiting |
| Vibostolimab; Merck | NCT04305041; Phase I, II | Substudy 02A: Safety and Efficacy of Pembrolizumab in Combination With Investigational Agents in Participants With Programmed Cell-Death 1 (PD-1) Refractory Melanoma (MK-3475-02A) | Recruiting |
| Vibostolimab; Merck | NCT04305054; Phase II | Substudy 02B: Safety and Efficacy of Pembrolizumab in Combination With Investigational Agents or Pembrolizumab Alone in Participants With First-Line (1L) Advanced Melanoma (MK-3475-02B) | Recruiting |
| Vibostolimab; Merck | NCT04303169; Phase II | Substudy 02C: Safety and Efficacy of Pembrolizumab in Combination With Investigational Agents or Pembrolizumab Alone in Participants With Stage III Melanoma Who Are Candidates for Neoadjuvant Therapy (MK-3475-02C) | Recruiting |
| ASP8374; Astellas Pharma | NCT03260322; Phase I | A Multiple-dose Study of ASP8374, an Immune Checkpoint Inhibitor, as a Single Agent and in Combination With Pembrolizumab in Subjects With Advanced Solid Tumors | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotte, A.; Sahasranaman, S.; Budha, N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines 2021, 9, 1277. https://doi.org/10.3390/biomedicines9091277
Rotte A, Sahasranaman S, Budha N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines. 2021; 9(9):1277. https://doi.org/10.3390/biomedicines9091277
Chicago/Turabian StyleRotte, Anand, Srikumar Sahasranaman, and Nageshwar Budha. 2021. "Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development" Biomedicines 9, no. 9: 1277. https://doi.org/10.3390/biomedicines9091277
APA StyleRotte, A., Sahasranaman, S., & Budha, N. (2021). Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines, 9(9), 1277. https://doi.org/10.3390/biomedicines9091277






