Cancer as a Dysfunctional Immune Disorder: Pro-Tumor TH1-like Immune Response and Anti-Tumor THαβ Immune Response Based on the Complete Updated Framework of Host Immunological Pathways
Abstract
:1. Introduction
2. Main Texts
2.1. Overview of Host Immunological Reactions
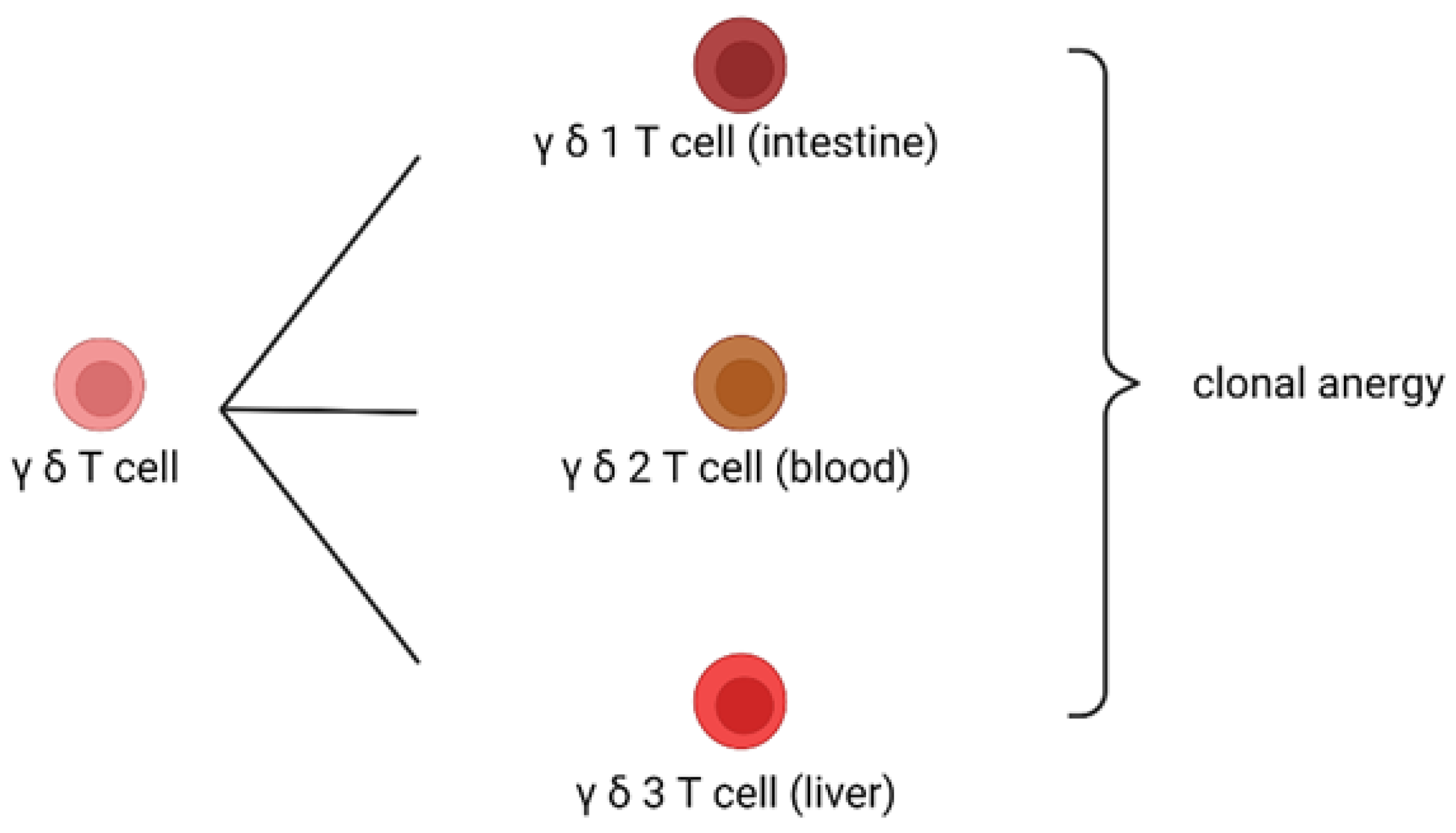
2.2. Mechanism of Clonal Anergy
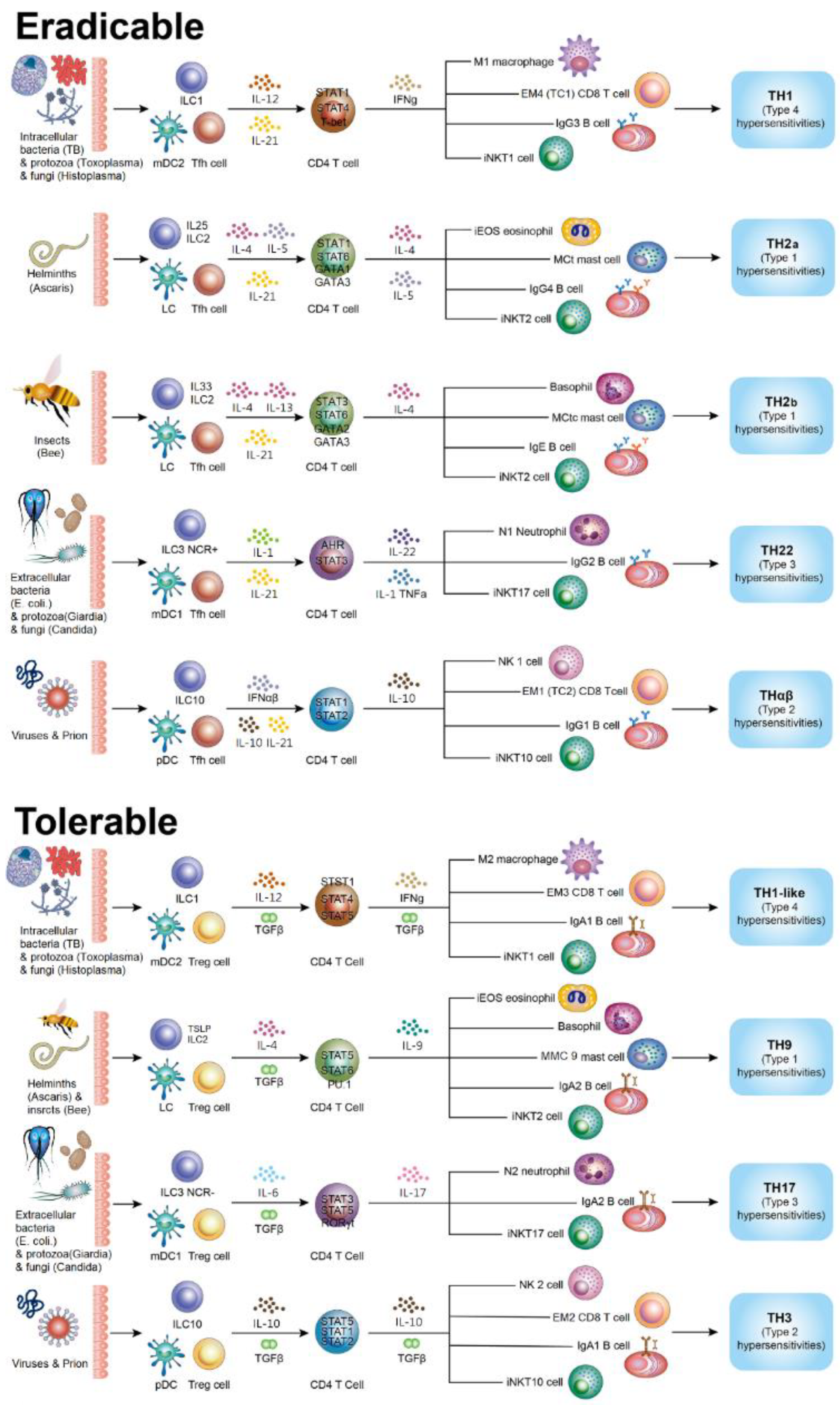
2.3. Triggering of Eradicable Host Immune Reactions
2.4. Triggering of Tolerable Host Immune Reactions
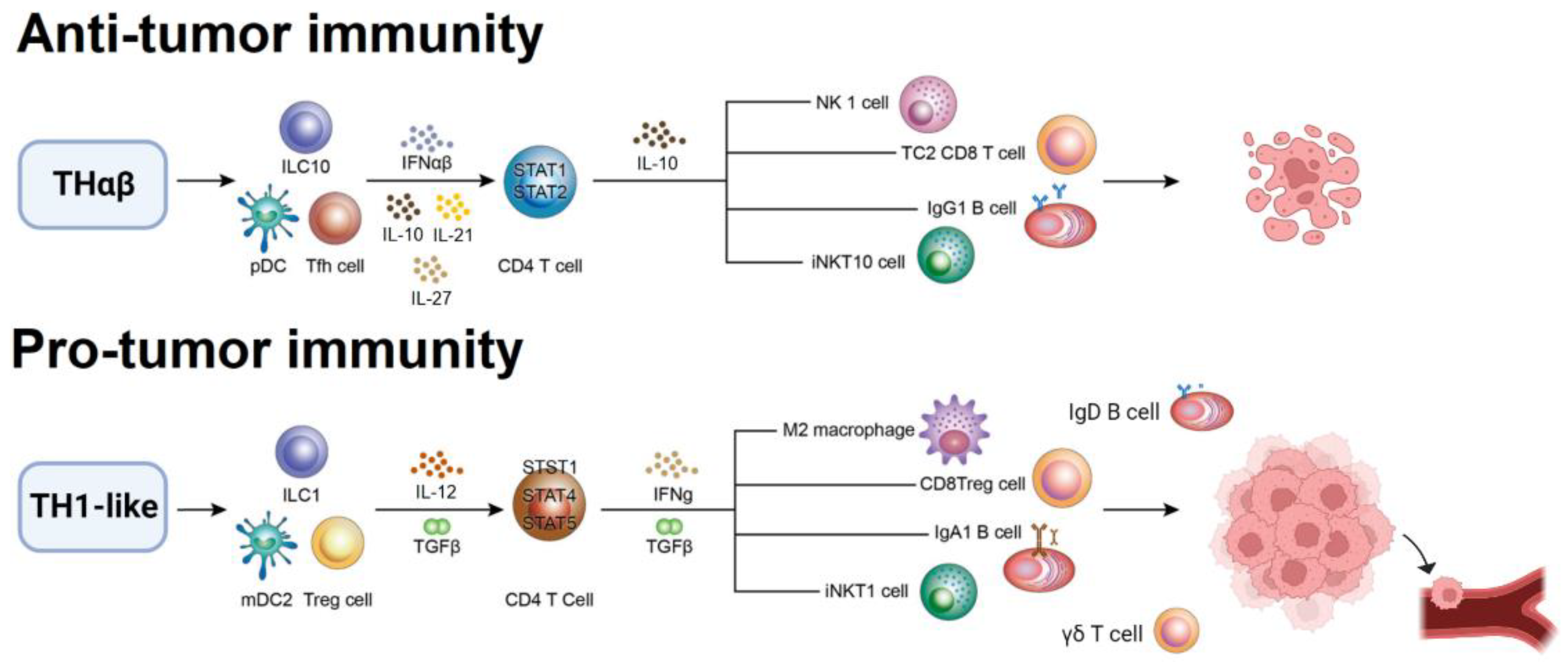
2.5. Pro-Tumor Immunological Pathway
2.6. Anti-Tumor Immunological Pathway
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.C. A Framework of All Discovered Immunological Pathways and Their Roles for Four Specific Types of Pathogens and Hypersensitivities. Front. Immunol. 2020, 11, 1992. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two Types of Murine Helper T Cell Clone. I. Definition According to Profiles of Lymphokine Activities and Secreted Proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Hu, W.C. The Central THαβ Immunity Associated Cytokine: Il-10 Has a Strong Anti-tumor Ability Toward Established Cancer Models In Vivo and Toward Cancer Cells In Vitro. Front. Oncol. 2021, 11, 655554. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C. Human Immune Responses to Plasmodium falciparum Infection: Molecular Evidence for a Suboptimal THαβ and TH17 Bias Over Ideal and Effective Traditional TH1 Immune Response. Malar. J. 2013, 12, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zan, H.; Cerutti, A.; Dramitinos, P.; Schaffer, A.; Casali, P. CD40 Engagement Triggers Switching to IgA1 and IgA2 in Human B Cells Through Induction of Endogenous TGF-Beta: Evidence for TGF-Beta but Not IL-10-Dependent Direct S Mu-->S Alpha and Sequential S Mu–>S Alpha and Sequential S Mu–>S Gamma, S Gamma-->S Alpha DNA Recombination. J. Immunol. 1998, 161, 5217–5225. [Google Scholar]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B Helper T Cells Express CXC Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Kondo, T.; Takata, H.; Yokota, S.; Takiguchi, M. Functional and Phenotypic Analysis of Human Memory CD8+ T Cells Expressing CXCR3. J. Leukoc. Biol. 2006, 80, 320–329. [Google Scholar] [CrossRef]
- Tomiyama, H.; Takata, H.; Matsuda, T.; Takiguchi, M. Phenotypic Classification of Human CD8+ T Cells Reflecting Their Function: Inverse Correlation Between Quantitative Expression of CD27 and Cytotoxic Effector Function. Eur. J. Immunol. 2004, 34, 999–1010. [Google Scholar] [CrossRef]
- Wen, T.H.; Tsai, K.W.; Wu, Y.J.; Liao, M.T.; Lu, K.C.; Hu, W.C. The Framework for Human Host Immune Responses to Four Types of Parasitic Infections and Relevant Key JAK/STAT Signaling. Int. J. Mol. Sci. 2021, 22, 13310. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 Induces IL-4, IL-5, and IL-13 and Th2-Associated Pathologies In Vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef] [Green Version]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 Therapies for Asthma. Cochrane Database Syst. Rev. 2017, 9, CD010834. [Google Scholar] [CrossRef] [PubMed]
- Masure, D.; Vlaminck, J.; Wang, T.; Chiers, K.; Van den Broeck, W.; Vercruysse, J.; Geldhof, P. A Role for Eosinophils in the Intestinal Immunity Against Infective Ascaris suum Larvae. PLoS Negl. Trop. Dis. 2013, 7, e2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vliagoftis, H.; Lacy, P.; Luy, B.; Adamko, D.; Hollenberg, M.; Befus, D.; Moqbel, R. Mast Cell Tryptase Activates Peripheral Blood Eosinophils to Release Granule-Associated Enzymes. Int. Arch. Allergy Immunol. 2004, 135, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Brombacher, F.; Pushparaj, P.N.; Arendse, B.; McSharry, C.; Alexander, J.; Chaudhuri, R.; Thomson, N.C.; McKenzie, A.N.; McInnes, I.; et al. Interleukin-33 Amplifies IgE Synthesis and Triggers Mast Cell Degranulation via Interleukin-4 in Naive Mice. Allergy 2012, 67, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Mukai, K.; Tsujimura, Y.; Ishiwata, K.; Kawano, Y.; Minegishi, Y.; Watanabe, N.; Karasuyama, H. Basophils Are Essential Initiators of a Novel Type of Chronic Allergic Inflammation. Blood 2007, 110, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Romagnani, P.; De Paulis, A.; Beltrame, C.; Annunziato, F.; Dente, V.; Maggi, E.; Romagnani, S.; Marone, G. Tryptase-Chymase Double-Positive Human Mast Cells Express the Eotaxin Receptor CCR3 and Are Attracted by CCR3-Binding Chemokines. Am. J. Pathol. 1999, 155, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Basu, R.; O’Quinn, D.B.; Silberger, D.J.; Schoeb, T.R.; Fouser, L.; Ouyang, W.; Hatton, R.D.; Weaver, C.T. Th22 Cells Are an Important Source of IL-22 for Host Protection Against Enteropathogenic Bacteria. Immunity 2012, 37, 1061–1075. [Google Scholar] [CrossRef] [Green Version]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 Cells Represent a Distinct Human T Cell Subset Involved in Epidermal Immunity and Remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [Green Version]
- Tsou, A.; Chen, P.J.; Tsai, K.W.; Hu, W.C.; Lu, K.C. THαβ Immunological Pathway as Protective Immune Response Against Prion Diseases: An Insight for Prion Infection Therapy. Viruses 2022, 14, 408. [Google Scholar] [CrossRef]
- Krovi, S.H.; Gapin, L. Invariant Natural Killer T Cell Subsets-More Than Just Developmental Intermediates. Front. Immunol. 2018, 9, 1393. [Google Scholar] [CrossRef] [Green Version]
- Prochazkova, J.; Pokorna, K.; Holan, V. IL-12 Inhibits the TGF-Beta-Dependent T Cell Developmental Programs and Skews the TGF-Beta-Induced Differentiation into a Th1-Like Direction. Immunobiology 2012, 217, 74–82. [Google Scholar] [CrossRef]
- Anuradha, R.; George, P.J.; Hanna, L.E.; Chandrasekaran, V.; Kumaran, P.; Nutman, T.B.; Babu, S. IL-4-, TGF-Beta-, and IL-1-Dependent Expansion of Parasite Antigen-Specific Th9 Cells Is Associated with Clinical Pathology in Human Lymphatic Filariasis. J. Immunol. 2013, 191, 2466–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, K.; Hwang, Y.; Nikolaev, A.; Atreya, R.; Dornhoff, H.; Steiner, S.; Lehr, H.A.; Wirtz, S.; Vieth, M.; Waisman, A.; et al. TH9 Cells That Express the Transcription Factor PU.1 Drive T Cell-Mediated Colitis via IL-9 Receptor Signaling in Intestinal Epithelial Cells. Nat. Immunol. 2014, 15, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backert, I.; Koralov, S.B.; Wirtz, S.; Kitowski, V.; Billmeier, U.; Martini, E.; Hofmann, K.; Hildner, K.; Wittkopf, N.; Brecht, K.; et al. STAT3 Activation in Th17 and Th22 Cells Controls IL-22-Mediated Epithelial Host Defense During Infectious Colitis. J. Immunol. 2014, 193, 3779–3791. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Naqvi, R.A.; Khanna, N.; Pathak, P.; Rao, D.N. Th3 Immune Responses in the Progression of Leprosy via Molecular Cross-Talks of TGF-Beta, CTLA-4 and Cbl-b. Clin. Immunol. 2011, 141, 133–142. [Google Scholar] [CrossRef]
- Jiang, R.; Feng, X.; Guo, Y.; Lu, Q.; Hou, J.; Luo, K.; Fu, N.; Venuprasad, K.; Banchereau, J. T Helper Cells in Patients With Chronic Hepatitis B Virus Infection. Chin. Med. J. 2002, 115, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Chabab, G.; Barjon, C.; Bonnefoy, N.; Lafont, V. Pro-tumor γδ T Cells in Human Cancer: Polarization, Mechanisms of Action, and Implications for Therapy. Front. Immunol. 2020, 11, 2186. [Google Scholar] [CrossRef]
- Guan, H.; Zu, G.; Slater, M.; Elmets, C.; Xu, H. GammadeltaT Cells Regulate the Development of Hapten-Specific CD8+ Effector T Cells in Contact Autoimmunity Responses. J. Investig. Dermatol. 2002, 119, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Heiser, R.A.; Detanico, T.O.; Getahun, A.; Kirchenbaum, G.A.; Casper, T.L.; Aydintug, M.K.; Carding, S.R.; Ikuta, K.; Huang, H.; et al. γδ T Cells Affect IL-4 Production and B-Cell Tolerance. Proc. Natl. Acad. Sci. USA 2015, 112, E39–E48. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumor-Infiltrating Gammadelta T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-Like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.G.; Little, C.B.; Yenson, V.M.; Jackson, C.J.; McCracken, S.A.; Warning, J.; Stevens, V.; Gallery, E.G.; Morris, J.M. Anti-IgD Antibody Attenuates Collagen-Induced Arthritis by Selectively Depleting Mature B-cells and Promoting Immune Tolerance. J. Autoimmun. 2010, 35, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.G. Immune-Modulation via IgD B-Cell Receptor Suppresses Allergic Skin Inflammation in Experimental Contact Autoimmunity Models Despite of a Th2-Favoured Humoral Response. Immunol. Lett. 2018, 203, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Wu, X.; Qiu, D.; Gao, L.; Liu, H.; Fan, Z.; Huang, F.; Jin, Z.; Sun, J.; Li, Y.; et al. Regulatory γδ T Cells Induced by G-CSF Participate in Acute Graft-versus-host Disease Regulation in G-CSF-Mobilized Allogeneic Peripheral Blood Stem Cell Transplantation. J. Transl. Med. 2018, 16, 144. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Appleton, S.E.; Stadnyk, A.; Lee, T.D.; Nashan, B.A. CD8+ γδ T Regulatory Cells Mediate Kidney Allograft Prolongation After Oral Exposure to Alloantigen. Transpl. Int. 2008, 21, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Okada, T.; Ansel, K.M. Cutting Edge: Distinct Waves of BCL6 Expression During T Follicular Helper Cell Development. J. Immunol. 2011, 187, 2089–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, V.L.; Ma, C.S.; Avery, D.T.; Li, Y.; Good, K.L.; Corcoran, L.M.; de Waal Malefyt, R.; Tangye, S.G. Cytokine-Mediated Regulation of Human B Cell Differentiation into Ig-Secreting Cells: Predominant Role of IL-21 Produced by CXCR5+ T Follicular Helper Cells. J. Immunol. 2007, 179, 8180–8190. [Google Scholar] [CrossRef] [Green Version]
- Barsheshet, Y.; Wildbaum, G.; Levy, E.; Vitenshtein, A.; Akinseye, C.; Griggs, J.; Lira, S.A.; Karin, N. CCR8(+)FOXp3(+) Treg Cells as Master Drivers of Immune Regulation. Proc. Natl. Acad. Sci. USA 2017, 114, 6086–6091. [Google Scholar] [CrossRef] [Green Version]
- Leppkes, M.; Becker, C.; Ivanov, I.I.; Hirth, S.; Wirtz, S.; Neufert, C.; Pouly, S.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; et al. RORgamma-Expressing Th17 Cells Induce Murine Chronic Intestinal Inflammation via Redundant Effects of IL-17A and IL-17F. Gastroenterology 2009, 136, 257–267. [Google Scholar] [CrossRef]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef] [Green Version]
- Pawelek, J.M.; Chakraborty, A.K. Fusion of Tumour Cells with Bone Marrow-Derived Cells: A Unifying Explanation for Metastasis. Nat. Rev. Cancer 2008, 8, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Gschmeissner, S.; East, N.; Balkwill, F.R. Enhancement of Metastatic Potential by Gamma-Interferon. Cancer Res. 1991, 51, 4020–4027. [Google Scholar] [PubMed]
- Ding, J.; Jin, W.; Chen, C.; Shao, Z.; Wu, J. Tumor Associated Macrophage x Cancer Cell Hybrids May Acquire Cancer Stem Cell Properties in Breast Cancer. PLoS ONE 2012, 7, e41942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The Chemotaxis of M1 and M2 Macrophages Is Regulated by Different Chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Dirkx, A.E.; Oude Egbrink, M.G.; Wagstaff, J.; Griffioen, A.W. Monocyte/Macrophage Infiltration in Tumors: Modulators of Angiogenesis. J. Leukoc. Biol. 2006, 80, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, T.; Ankathil, R.; Remani, P.; Sasidharan, V.K.; Vijayan, K.K.; Vasudevan, D.M. Serum Immunoglobulins in Patients with Carcinoma of the Oral Cavity, Uterine Cervix and Breast. Cancer Immunol. Immunother. 1986, 22, 76–79. [Google Scholar] [CrossRef]
- Sabouri, Z.; Perotti, S.; Spierings, E.; Humburg, P.; Yabas, M.; Bergmann, H.; Horikawa, K.; Roots, C.; Lambe, S.; Young, C.; et al. IgD Attenuates the IgM-Induced Anergy Response in Transitional and Mature B Cells. Nat. Commun. 2016, 7, 13381. [Google Scholar] [CrossRef] [Green Version]
- Berman, R.M.; Suzuki, T.; Tahara, H.; Robbins, P.D.; Narula, S.K.; Lotze, M.T. Systemic Administration of Cellular IL-10 Induces an Effective, Specific, and Long-Lived Immune Response Against Established Tumors in Mice. J. Immunol. 1996, 157, 231–238. [Google Scholar]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J.; et al. IL-10 Elicits IFNgamma-Dependent Tumor Immune Surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef] [Green Version]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Stewart, M.D.; Choi, Y.; Johnson, G.A.; Yu-Lee, L.Y.; Bazer, F.W.; Spencer, T.E. Roles of Stat1, Stat2, and Interferon Regulatory Factor-9 (IRF-9) in Interferon Tau Regulation of IRF-1. Biol. Reprod. 2002, 66, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, G.D.; He, X.B.; Sun, Q.; Chen, S.; Wan, K.; Xu, X.; Feng, X.; Li, P.P.; Chen, B.; Xiong, M.M. The oncolytic virus in cancer diagnosis and treatment. Front. Oncol. 2020, 10, 1786. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Wong, D.J.; Infante, J.R.; Korn, W.M.; Aljumaily, R.; Papadopoulos, K.P.; Autio, K.A.; Pant, S.; Bauer, T.M.; Drakaki, A.; et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): A multicentre, muticohort, open-label, phase 1b trial. Lancet Oncol. 2019, 20, 1544–1555. [Google Scholar] [CrossRef]

| Immune Pathways | Driven Cytokines, ILCs, DC | Transcription Factors | Effector Cells | CD4 T Cells | B Cells | NKT Cells | Pathogen/ Pathogenesis | Autoimmune |
|---|---|---|---|---|---|---|---|---|
| Initiatory | ||||||||
| Tfh | IL-21, FDC, LTi | STAT1, STAT3, STAT5B | IL-21 CD4 T cells | IgG B cells | iNKTfh | Subacute infection | ||
| Eradicable immunities | ||||||||
| TH1 | IL-12, ILC1, mDC2 | STAT4 | Macrophages (M1), CTL (Tc1, EM4) | IFN-γ CD4 T cells | IgG3 B cells | iNKT1 | Intracellular micro-organisms (bacteria, fungi and protozoa) | Type 4 DTH |
| TH2 (TH2a) | IL-4, iILC2, LC | STAT6, STAT1 | Eosinophils (iEOS), mast cells (MCt) | IL-4, IL-5 CD4 T cells | IgG4 B cells | iNKT2 | Endoparasites (Helminths) | Type 1 allergy (IgG4) |
| TH2 (TH2b) | IL-4, nILC2, LC | STAT6, STAT3 | Basophils, mast cells (MCct) | IL-4, IL-13 CD4 T cells | IgE B cells | iNKT2 | Ectoparasites (Insects) | Type 1 allergy (IgE) |
| TH22 | IL-1, mDC1, ILC3 NCR+ | STAT3 | Neutrophils (N1) | IL-1, TNFα, IL-22 CD4 T cells | IgG2 B cells | iNKT17 | Extracellular micro-organisms (bacteria, fungi and protozoa) | Type 3 Immune complex |
| THαβ | IL-10, pDC, IFNα, ILC10 | STAT1, STAT2 | NK cells (NK1), CTL (Tc2, EM1) | IL-10 CD4 T cells | IgG1 B cells | iNKT10 | Infectious particles (Viruses and Prions) | Type 2 ADCC |
| Regulatory | ||||||||
| Treg | TGF-β, DCreg, ILCreg | STAT5A, STAT5B | TGF-β CD4 T cells | IgA B cells | iNKTreg | Chronic infection | ||
| Tolerable immunities | ||||||||
| TH1like | IL-12, TGF-β, ILC1 | STAT4, STAT5 | Macrophages (M2), CD8 T cells (EM3) | IFN-γ/TGF-β CD4 T cells | IgA1 B cells | iNKT1 | Intracellular micro-organisms (bacteria, fungi and protozoa) | Type 4 DTH |
| TH9 | IL-4, TGF-β, TSLP ILC2 | STAT6, STAT5 | Eosinophils(rEOS), basophils, mast cells (MMC9) | IL-9 CD4 T cells | IgA2 B cells | iNKT2 | Parasites (Helminths and Insects) | Type 1 allergy |
| TH17 | IL-6, TGF-β, ILC3 NCR- | STAT3, STAT5 | Neutrophils (N2) | IL-17 CD4 T cells | IgA2 B cells | iNKT17 | Extracellular micro-organisms (bacteria, fungi and protozoa) | Type 3 immune complex |
| TH3 | IL-10, TGF-β, ILC10 | STAT1, STAT2, STAT5 | NK cells (NK2), CD8 T cells (EM2) | IL-10/TGF-β CD4 T cells | IgA1 B cells | iNKT10 | Infectious particles (Viruses and Prions) | Type 2 ADCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Tsai, K.-W.; Lu, K.-C.; Shih, L.-J.; Hu, W.-C. Cancer as a Dysfunctional Immune Disorder: Pro-Tumor TH1-like Immune Response and Anti-Tumor THαβ Immune Response Based on the Complete Updated Framework of Host Immunological Pathways. Biomedicines 2022, 10, 2497. https://doi.org/10.3390/biomedicines10102497
Lee Y-H, Tsai K-W, Lu K-C, Shih L-J, Hu W-C. Cancer as a Dysfunctional Immune Disorder: Pro-Tumor TH1-like Immune Response and Anti-Tumor THαβ Immune Response Based on the Complete Updated Framework of Host Immunological Pathways. Biomedicines. 2022; 10(10):2497. https://doi.org/10.3390/biomedicines10102497
Chicago/Turabian StyleLee, Yi-Hsin, Kuo-Wang Tsai, Kuo-Cheng Lu, Li-Jane Shih, and Wan-Chung Hu. 2022. "Cancer as a Dysfunctional Immune Disorder: Pro-Tumor TH1-like Immune Response and Anti-Tumor THαβ Immune Response Based on the Complete Updated Framework of Host Immunological Pathways" Biomedicines 10, no. 10: 2497. https://doi.org/10.3390/biomedicines10102497
APA StyleLee, Y.-H., Tsai, K.-W., Lu, K.-C., Shih, L.-J., & Hu, W.-C. (2022). Cancer as a Dysfunctional Immune Disorder: Pro-Tumor TH1-like Immune Response and Anti-Tumor THαβ Immune Response Based on the Complete Updated Framework of Host Immunological Pathways. Biomedicines, 10(10), 2497. https://doi.org/10.3390/biomedicines10102497








