Correlation of Pulse Wave Transit Time with Pulmonary Artery Pressure in a Porcine Model of Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Anaesthesia
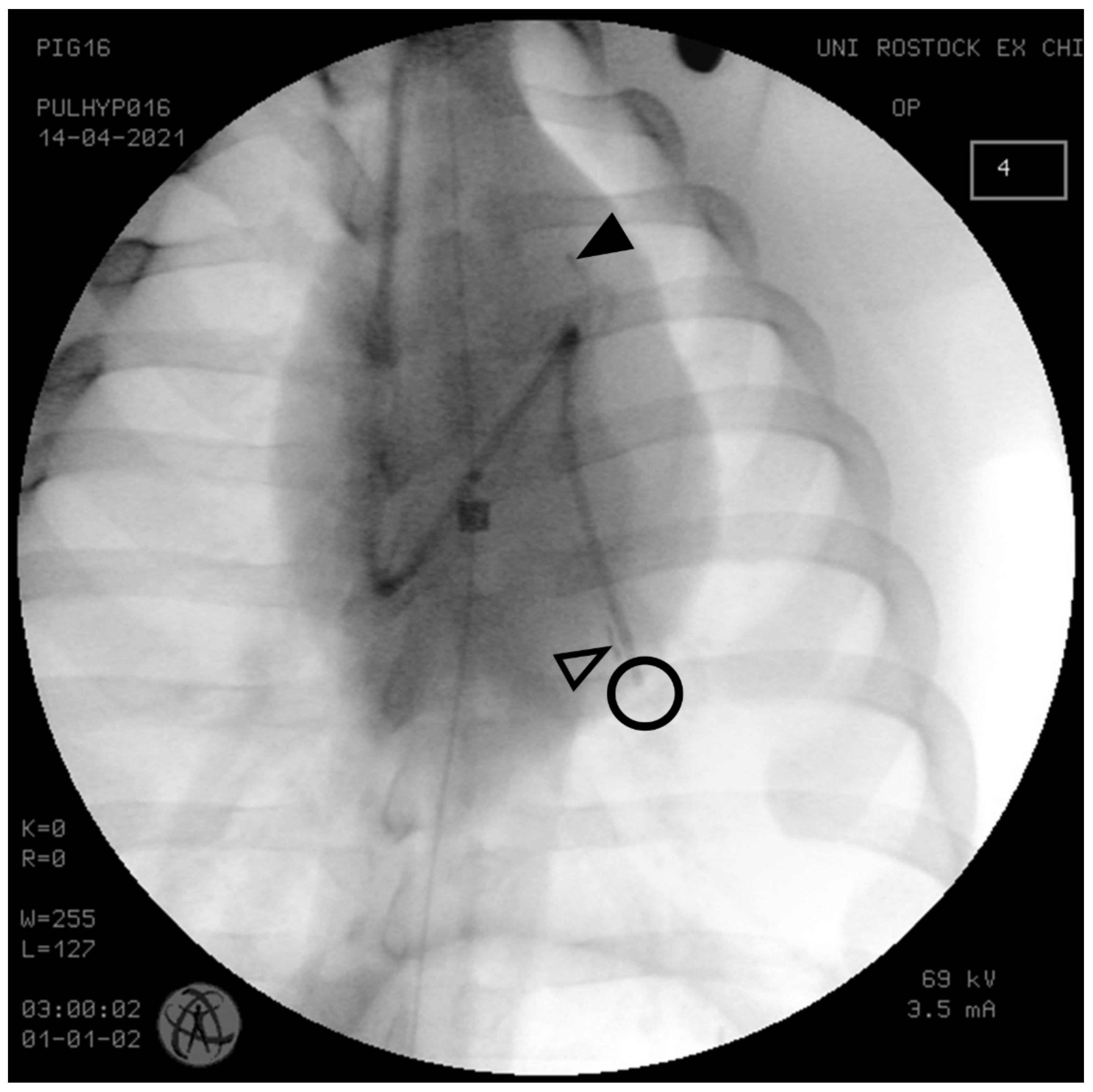
2.2. Instrumentation
2.3. Induction of PH
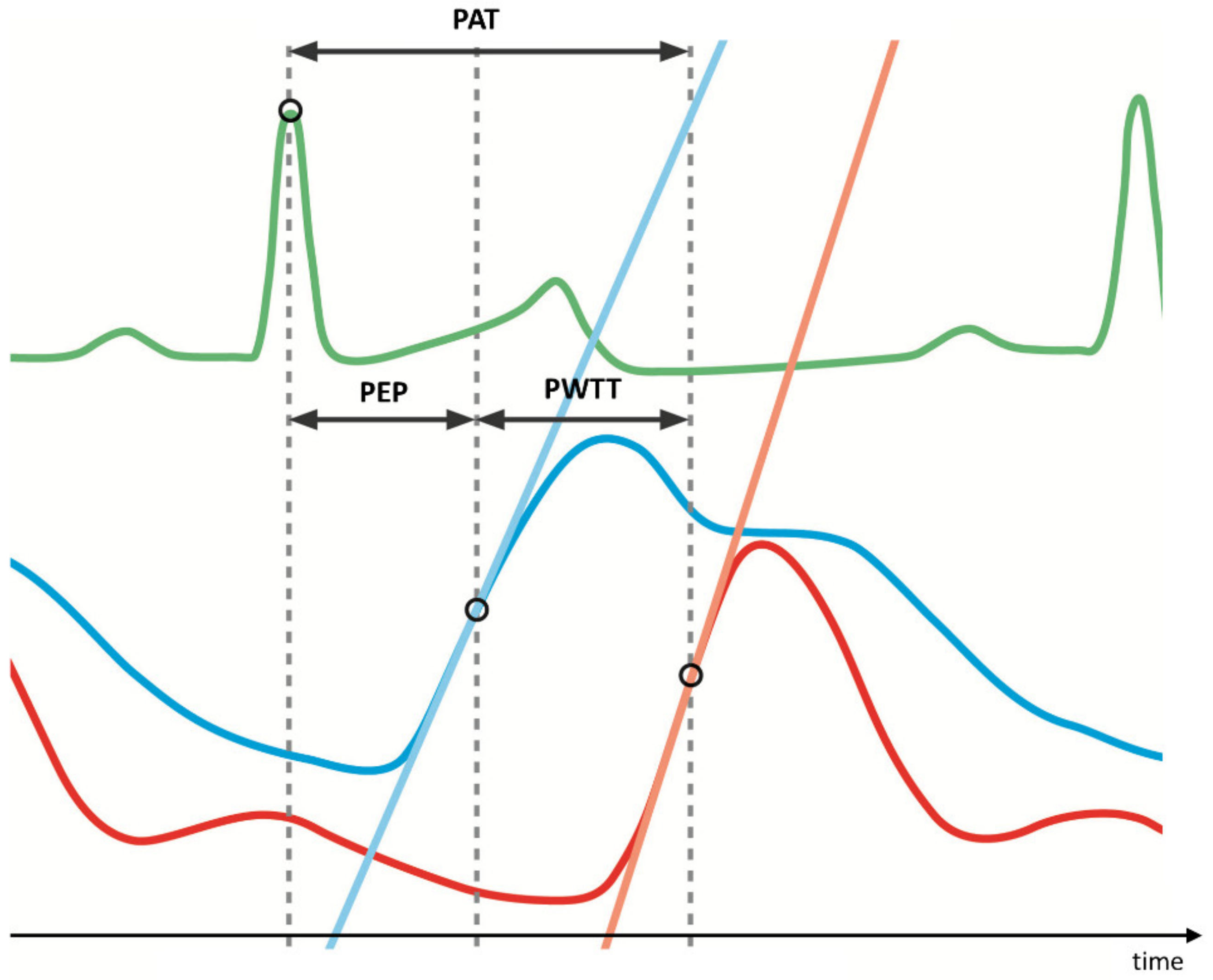
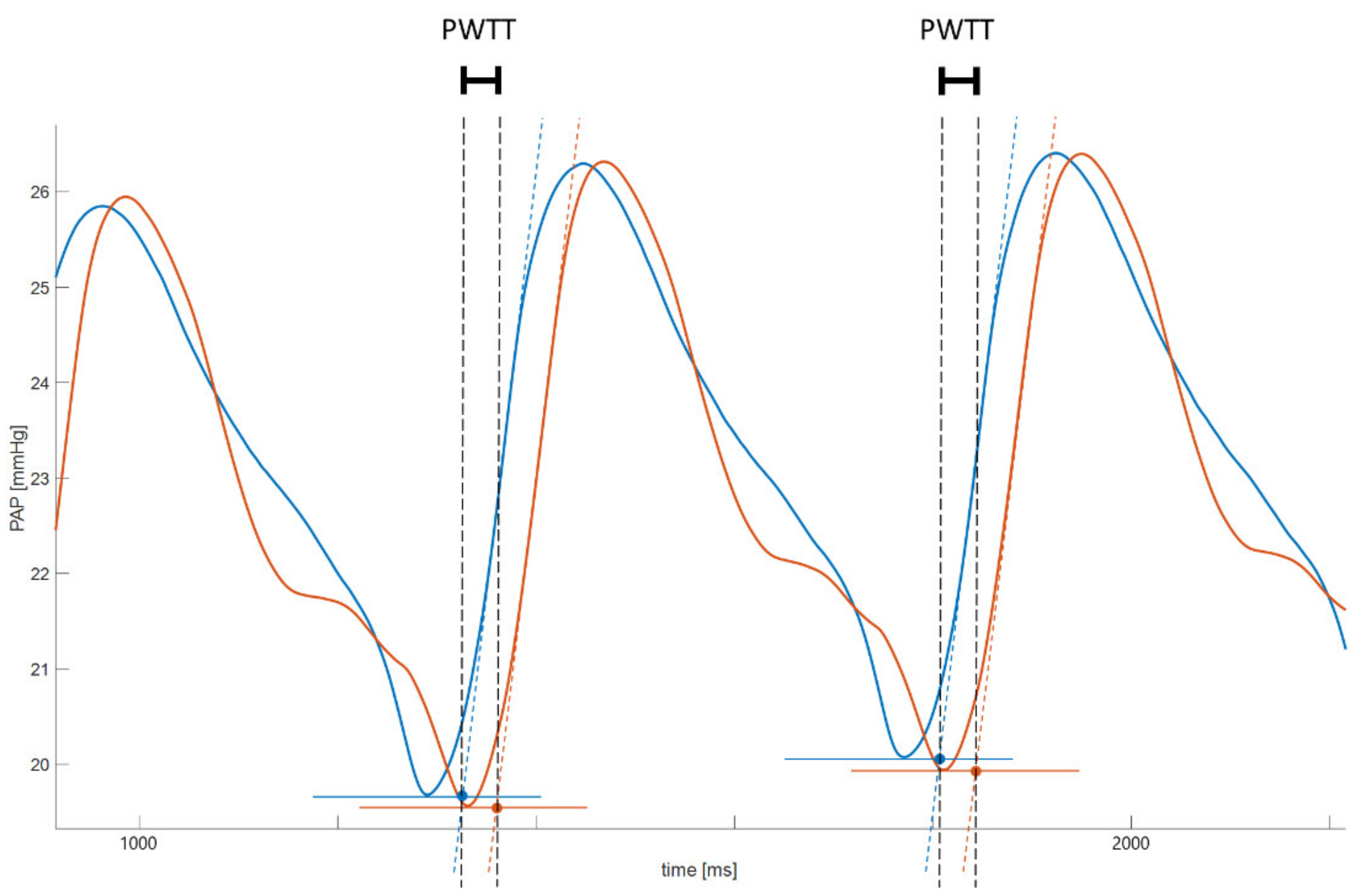
2.4. Data Acquisition and Processing
2.5. Statistics
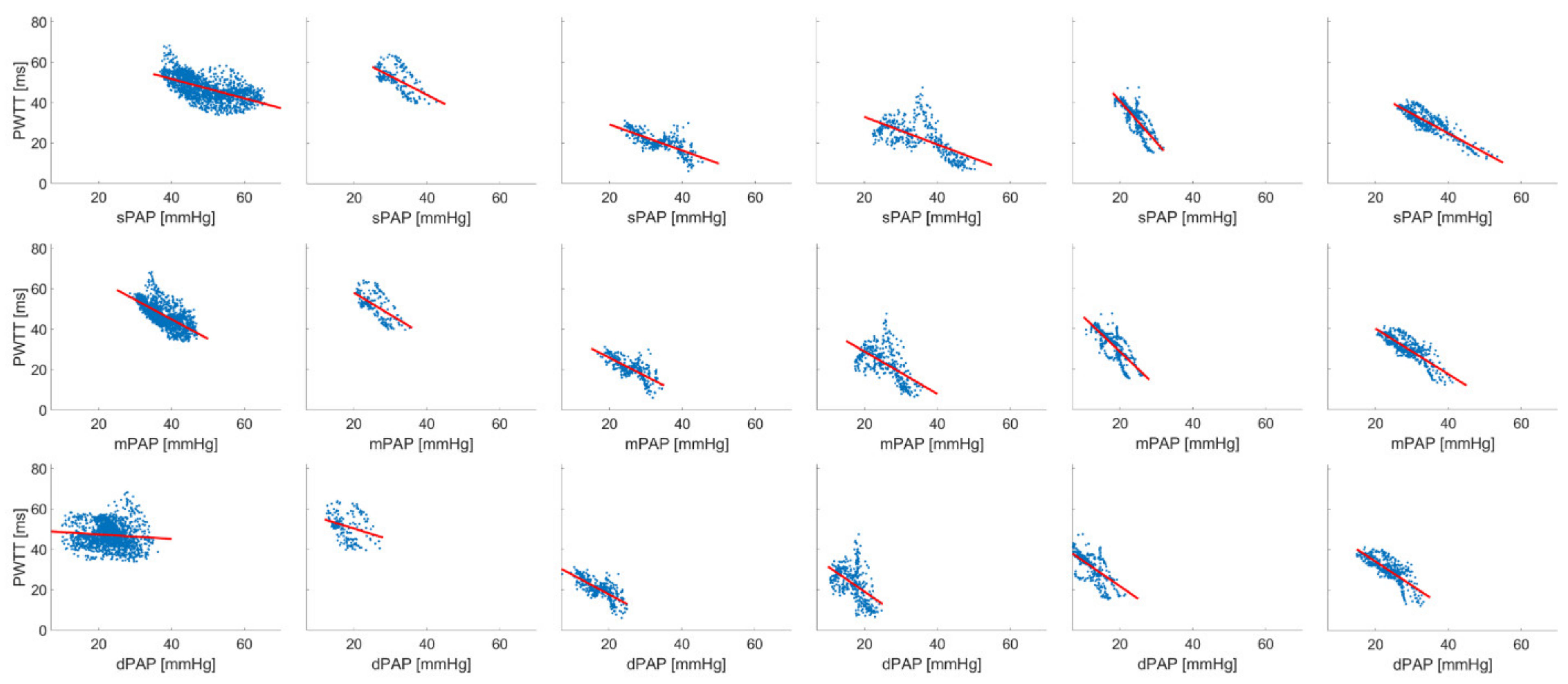
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Ibrahim, E.-S.H.; Bajwa, A.A.; White, R.D. Assessment of Pulmonary Arterial Hypertension by Magnetic Resonance Imaging. Tomography 2015, 1, 23–29. [Google Scholar] [CrossRef]
- Schäfer, M.; Wilson, N.; Ivy, D.D.; Ing, R.; Abman, S.; Browne, L.P.; Morgan, G.; Ross, M.; McLennan, D.; Barker, A.J.; et al. Noninvasive wave intensity analysis predicts functional worsening in children with pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H968–H977. [Google Scholar] [CrossRef] [PubMed]
- Jernstedt, G.C.; Newcomer, J.P. Blood pressure and pulse wave velocity measurement for operant conditioning of autonomic responding. Behav. Res. Methods Instrum. 1974, 6, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Peter, L.; Noury, N.; Cerny, M. A review of methods for non-invasive and continuous blood pressure monitoring: Pulse transit time method is promising? IRBM 2014, 35, 271–282. [Google Scholar] [CrossRef]
- Proença, M.; Braun, F.; Solà, J.; Adler, A.; Lemay, M.; Thiran, J.-P.; Rimoldi, S.F. Non-invasive monitoring of pulmonary artery pressure from timing information by EIT: Experimental evaluation during induced hypoxia. Physiol. Meas. 2016, 37, 713–726. [Google Scholar] [CrossRef]
- Proença, M.; Braun, F.; Lemay, M.; Solà, J.; Adler, A.; Riedel, T.; Messerli, F.H.; Thiran, J.-P.; Rimoldi, S.F.; Rexhaj, E. Non-invasive pulmonary artery pressure estimation by electrical impedance tomography in a controlled hypoxemia study in healthy subjects. Sci. Rep. 2020, 10, 21462. [Google Scholar] [CrossRef]
- Proença, M.; Braun, F.; Solà, J.; Thiran, J.-P.; Lemay, M. Noninvasive pulmonary artery pressure monitoring by EIT: A model-based feasibility study. Med. Biol. Eng. Comput. 2017, 55, 949–963. [Google Scholar] [CrossRef]
- Ochiai, R.; Takeda, J.; Hosaka, H.; Sugo, Y.; Tanaka, R.; Soma, T. The relationship between modified pulse wave transit time and cardiovascular changes in isoflurane anesthetized dogs. J. Clin. Monit. Comput. 1999, 15, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Stompór, T.; Rajzer, M.; Sułowicz, W.; Dembińska-Kieć, A.; Janda, K.; Kawecka-Jaszcz, K.; Wójcik, K.; Tabor, B.; Zdzienicka, A.; Janusz-Grzybowska, E. An association between aortic pulse wave velocity, blood pressure and chronic inflammation in ESRD patients on peritoneal dialysis. Int. J. Artif. Organs 2003, 26, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solà, J.; Adler, A.; Santos, A.; Tusman, G.; Sipmann, F.S.; Bohm, S.H. Non-invasive monitoring of central blood pressure by electrical impedance tomography: First experimental evidence. Med. Biol. Eng. Comput. 2011, 49, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Geddes, L.A.; Voelz, M.H.; Babbs, C.F.; Bourland, J.D.; Tacker, W.A. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology 1981, 18, 71–74. [Google Scholar] [CrossRef]
- Milnor, W.R.; Conti, C.R.; Lewis, K.B.; O’Rourke, M.F. Pulmonary arterial pulse wave velocity and impedance in man. Circ. Res. 1969, 25, 637–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemla, D.; Weatherald, J.; Lau, E.M.T.; Savale, L.; Boucly, A.; Attal, P.; Jaïs, X.; Parent, F.; Girerd, B.; Simonneau, G.; et al. Clinical and Hemodynamic Correlates of Pulmonary Arterial Stiffness in Incident, Untreated Patients With Idiopathic Pulmonary Arterial Hypertension. Chest 2018, 154, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Moertl, D.; Jankowski, P.; Tyrka, A.; Sobień, B.; Podolec, P. Pulmonary artery pulse wave velocity in idiopathic pulmonary arterial hypertension. Can. J. Cardiol. 2013, 29, 683–690. [Google Scholar] [CrossRef]
- Gupta, A.; Sharifov, O.F.; Lloyd, S.G.; Tallaj, J.A.; Aban, I.; Dell’italia, L.J.; Denney, T.S.; Gupta, H. Novel Noninvasive Assessment of Pulmonary Arterial Stiffness Using Velocity Transfer Function. J. Am. Heart Assoc. 2018, 7, e009459. [Google Scholar] [CrossRef] [Green Version]
- Du Percie Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Kischkel, S.; Bergt, S.; Brock, B.; von Grönheim, J.; Herbst, A.; Epping, M.-J.; Matheis, G.; Novosel, E.; Schneider, J.; Warnke, P.; et al. In Vivo Testing of Extracorporeal Membrane Ventilators: iLA-Activve Versus Prototype I-Lung. ASAIO J. 2017, 63, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mosing, M.; Kutter, A.P.N.; Iff, S.; Raszplewicz, J.; Mauch, J.; Bohm, S.H.; Tusman, G. The effects of cardiac output and pulmonary arterial hypertension on volumetric capnography derived-variables during normoxia and hypoxia. J. Clin. Monit. Comput. 2015, 29, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hlastala, M.P.; Lamm, W.J.E.; Karp, A.; Polissar, N.L.; Starr, I.R.; Glenny, R.W. Spatial distribution of hypoxic pulmonary vasoconstriction in the supine pig. J. Appl. Physiol. 2004, 96, 1589–1599. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Arand, P.W.; Shroff, S.G.; Feldman, T.; Carroll, J.D. Determination of pulse wave velocities with computerized algorithms. Am. Heart J. 1991, 121, 1460–1470. [Google Scholar] [CrossRef]
- Solà, J.; Vetter, R.; Renevey, P.; Chételat, O.; Sartori, C.; Rimoldi, S.F. Parametric estimation of pulse arrival time: A robust approach to pulse wave velocity. Physiol. Meas. 2009, 30, 603–615. [Google Scholar] [CrossRef]
- Field, A.P. Is the meta-analysis of correlation coefficients accurate when population correlations vary? Psychol. Methods 2005, 10, 444–467. [Google Scholar] [CrossRef] [Green Version]
- Gesche, H.; Grosskurth, D.; Küchler, G.; Patzak, A. Continuous blood pressure measurement by using the pulse transit time: Comparison to a cuff-based method. Eur. J. Appl. Physiol. 2012, 112, 309–315. [Google Scholar] [CrossRef]
- Ngim, C.A.; Abdul Rahman, A.R.; Ibrahim, A. Pulse wave velocity as an index of arterial stiffness: A comparison between newly diagnosed (untreated) hypertensive and normotensive middle-aged Malay men and its relationship with fasting insulin. Acta Cardiol. 1999, 54, 277–282. [Google Scholar] [PubMed]
- Nürnberger, J.; Dammer, S.; Opazo Saez, A.; Philipp, T.; Schäfers, R.F. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J. Hum. Hypertens. 2003, 17, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Park, C.G.; Park, J.S.; Suh, S.Y.; Choi, C.U.; Kim, J.W.; Kim, S.H.; Lim, H.E.; Rha, S.W.; Seo, H.S.; et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: Invasive study. J. Hum. Hypertens. 2007, 21, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Steptoe, A.; Smulyan, H.; Gribbin, B. Pulse wave velocity and blood pressure change: Calibration and applications. Psychophysiology 1976, 13, 488–493. [Google Scholar] [CrossRef]
- Doig, J.R.; Adam, A.H.; Fleming, J.E.; Whitfield, C.R. Fetal cardiac electromechanical intervals during labour and effects of hypoxia and cord compression. Br. J. Obstet. Gynaecol. 1982, 89, 145–148. [Google Scholar] [CrossRef]
- Rasmussen, J.P.; Bech-Jansen, P. Influence of transient hypoxia and reoxygenation on the pre-ejection period of the heart. Acta Anaesthesiol. Scand. 1974, 18, 5–11. [Google Scholar] [CrossRef]
- Organ, L.W.; Bernstein, A.; Rowe, I.H.; Smith, K.C. The pre-ejection period of the fetal heart: Detection during labor with Doppler ultrasound. Am. J. Obstet. Gynecol. 1973, 115, 369–376. [Google Scholar] [CrossRef]
- Goncharova, N.S.; Andreeva, E.M.; Vakhrushev, A.D.; Leandro, H.I.C.; Murashova, L.A.; Voronin, S.E.; Korobchenko, L.E.; Mitrofanova, L.B.; Skorik, Y.A.; Galagudza, M.M.; et al. Modeling of Acute Pulmonary Arterial Hypertension in Pigs Using a Stable Thromboxane A2 Analogue (U46619): Dose Adjustment and Assessment of Hemodynamic Reactions. Bull. Exp. Biol. Med. 2021, 170, 729–733. [Google Scholar] [CrossRef]
- Tan, W.; Madhavan, K.; Hunter, K.S.; Park, D.; Stenmark, K.R. Vascular stiffening in pulmonary hypertension: Cause or consequence? (2013 Grover Conference series). Pulm. Circ. 2014, 4, 560–580. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.-H.; Chung, H.-W.; Yu, H.-Y.; Tseng, W.-Y.I. Estimation of pulse wave velocity in main pulmonary artery with phase contrast MRI: Preliminary investigation. J. Magn. Reson. Imaging 2006, 24, 1303–1310. [Google Scholar] [CrossRef]
- Swift, A.J.; Rajaram, S.; Hurdman, J.; Hill, C.; Davies, C.; Sproson, T.W.; Morton, A.C.; Capener, D.; Elliot, C.; Condliffe, R.; et al. Noninvasive Estimation of PA Pressure, Flow, and Resistance With CMR Imaging. JACC Cardiovasc. Imaging 2013, 6, 1036–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, G.; Reiter, U.; Kovacs, G.; Olschewski, H.; Fuchsjäger, M. Blood Flow Vortices along the Main Pulmonary Artery Measured with MR Imaging for Diagnosis of Pulmonary Hypertension. Radiology 2015, 275, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Laffon, E.; Laurent, F.; Bernard, V.; De Boucaud, L.; Ducassou, D.; Marthan, R. Noninvasive assessment of pulmonary arterial hypertension by MR phase-mapping method. J. Appl. Physiol. 2001, 90, 2197–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeleveld, R.J.; Marcus, J.T.; Boonstra, A.; Postmus, P.E.; Marques, K.; Bronzwaer, J.G.; Vonk-Noordegraaf, A. A comparison of noninvasive MRI-based methods of estimating pulmonary artery pressure in pulmonary hypertension. J. Magn. Reson. Imaging 2005, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
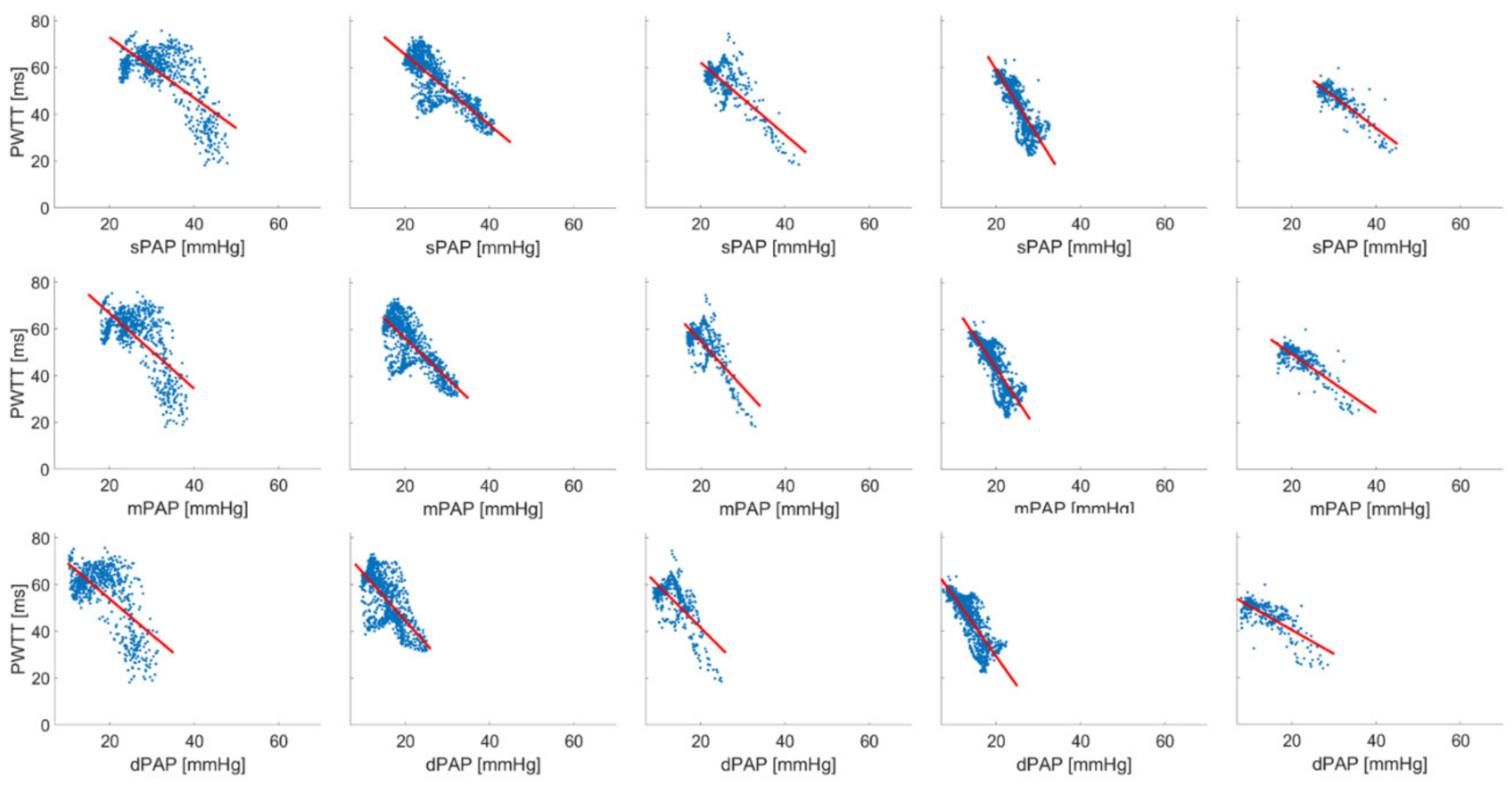

| A | sPAP [mmHg] | mPAP [mmHg] | dPAP [mmHg] | PWTT [ms] | PWV [m/s] |
| 1 | 42.5 ± 2.9 | 33.0 ± 1.9 | 22.5 ± 2.8 | 54.9 ± 3.4 | n/a |
| 2 | 28.7 ± 2.3 | 23.2 ± 2.0 | 15.9 ± 2.5 | 55.1 ± 4.1 | 1.9 ± 0.1 |
| 3 | 25.6 ± 1.3 | 20.1 ± 1.5 | 11.9 ± 1.4 | 27.2 ± 2.2 | 2.3 ± 0.2 |
| 4 | 25.0 ± 2.2 | 19.5 ± 1.8 | 12.6 ± 1.5 | 24.2 ± 2.4 | 2.7 ± 0.3 |
| 5 | 21.5 ± 1.6 | 15.0 ± 2.1 | 9.3 ± 2.7 | 38.6 ± 2.7 | 2.7 ± 0.2 |
| 6 | 29.1 ± 1.8 | 23.7 ± 2.0 | 18.6 ± 2.5 | 35.5 ± 2.9 | 2.0 ± 0.2 |
| B | sPAP [mmHg] | mPAP [mmHg] | dPAP [mmHg] | PWTT [ms] | PWV [m/s] |
| 1 | n/a | n/a | n/a | n/a | n/a |
| 2 | 25.0 ± 2.0 | 20.5 ± 1.9 | 13.5 ± 2.5 | 62.6 ± 4.8 | 1.6 ± 0.1 |
| 3 | 21.8 ± 1.8 | 16.4 ± 1.6 | 12.0 ± 2.0 | 57.8 ± 7.2 | 1.6 ± 0.2 |
| 4 | 22.9 ± 1.6 | 18.4 ± 1.7 | 11.6 ± 2.5 | 54.2 ± 5.3 | 1.2 ± 0.1 |
| 5 | 21.1 ± 1.2 | 15.4 ± 1.6 | 10.0 ± b1.8 | 55.6 ± 2.3 | 2.0 ± 0.1 |
| 6 | 27.9 ± 1.7 | 19.5 ± 2.2 | 11.2 ± 3.1 | 47.9 ± 3.4 | 1.7 ± 0.1 |
| A | sPAP [mmHg] | mPAP [mmHg] | dPAP [mmHg] | PWTT [ms] | PWV [m/s] |
| 1 | 60.4 ± 3.1 | 43.9 ± 1.8 | 20.0 ± 5.6 | 42.9 ± 3.3 | n/a |
| 2 | 37.5 ± 2.2 | 30.8 ± 2.1 | 21.6 ± 2.9 | 42.6 ± 0.7 | 2.4 ± 0.2 |
| 3 | 42.1 ± 1.9 | 31.4 ± 1.6 | 22.3 ± 1.5 | 13.4 ± 4.1 | 5.0 ± 1.5 |
| 4 | 47.3 ± 1.4 | 32.4 ± 1.6 | 21.3 ± 1.7 | 10.2 ± 2.0 | 6.5 ± 1.2 |
| 5 | 28.4 ± 1.8 | 22.3 ± 2.1 | 16.3 ± 2.8 | 21.0 ± 5.2 | 5.3 ± 1.2 |
| 6 | 46.8 ± 4.4 | 36.6 ± 2.7 | 29.5 ± 2.3 | 16.0 ± 3.5 | 4.5 ± 0.8 |
| B | sPAP [mmHg] | mPAP [mmHg] | dPAP [mmHg] | PWTT [ms] | PW [m/s] |
| 1 | n/a | n/a | n/a | n/a | n/a |
| 2 | 44.8 ± 1.5 | 35.1 ± 1.7 | 27.1 ± 2.0 | 30.8 ± 6.0 | 3.4 ± 0.7 |
| 3 | 38.4 ± 1.4 | 29.6 ± 1.5 | 20.6 ± 2.1 | 38.0 ± 3.8 | 2.5 ± 0.2 |
| 4 | 39.7 ± 2.1 | 29.5 ± 2.4 | 21.0 ± 3.2 | 26.5 ± 6.7 | 2.6 ± 0.6 |
| 5 | 29.2 ± 1.8 | 23.5 ± 1.8 | 17.9 ± 1.9 | 33.0 ± 4.2 | 3.5 ± 0.5 |
| 6 | 40.5 ± 3.4 | 31.8 ± 2.9 | 23.7 ± 2.9 | 30.2 ± 6.3 | 2.8 ± 0.5 |
| A | sPAP | mPAP | dPAP | ||||||||||
| Pig | n | Linear Regression | r2 | F | p | Linear Regression | r2 | F | p | Linear Regression | r2 | F | p |
| 1 | 1611 | PWTT = 70.945 − (0.481 * sPAP) | 0.356 | 888.151 | <0.001 | PWTT = 83.645 − (0.969 * mPAP) | 0.499 | 1601.15 | <0.001 | PWTT = 49.662 − (0.112 * dPAP) | 0.00885 | 14.364 | <0.001 |
| 2 | 686 | PWTT = 98.938 − (1.298 * sPAP) | 0.502 | 689.041 | <0.001 | PWTT = 99.054 − (1.616 * mPAP) | 0.466 | 596.726 | <0.001 | PWTT = 84.597 − (1.537 * dPAP) | 0.430 | 517.007 | <0.001 |
| 3 | 333 | PWTT = 42.133 − (0.646 * sPAP) | 0.547 | 400.226 | <0.001 | PWTT = 44.194 − (0.917 * mPAP) | 0.534 | 379.604 | <0.001 | PWTT = 37.053 − (0.967 * dPAP) | 0.542 | 391.071 | <0.001 |
| 4 | 377 | PWTT = 46.632 − (0.683 * sPAP) | 0.399 | 248.757 | <0.001 | PWTT = 44.595 − (0.815 * mPAP) | 0.210 | 90.716 | <0.001 | PWTT = 43.850 − (1.239 * dPAP) | 0.245 | 121.935 | <0.001 |
| 5 | 426 | PWTT = 81.659 − (2.047 * sPAP) | 0.649 | 783.579 | <0.001 | PWTT = 62.936 − (1.719 * mPAP) | 0.597 | 628.24 | <0.001 | PWTT = 46.610 − (1.243 * dPAP) | 0.447 | 342.462 | <0.001 |
| 6 | 440 | PWTT = 63.723 − (0.972 * sPAP) | 0.765 | 1422.27 | <0.001 | PWTT = 62.645 − (1.129 * mPAP) | 0.686 | 955.157 | <0.001 | PWTT = 58.002 − (1.188 * dPAP) | 0.628 | 738.206 | <0.001 |
| B | sPAP * | mPAP | dPAP | ||||||||||
| Pig | n | Linear Regression | r2 | F | p | Linear Regression | r2 | F | p | Linear Regression | r2 | F | p |
| 1 | 686 | PWTT = 98.938 − (1.298 * sPAP) | 0.502 | 689.041 | <0.001 | PWTT = 99.054 − (1.616 * mPAP) | 0.466 | 596.726 | <0.001 | PWTT = 84.597 − (1.537 * dPAP) | 0.430 | 517.007 | <0.001 |
| 2 | 1022 | PWTT = 95.676 − (1.501 * sPAP) | 0.671 | 2083.98 | <0.001 | PWTT = 90.743 − (1.719 * mPAP) | 0.598 | 1518.03 | <0.001 | PWTT = 85.160 − (2.027 * dPAP) | 0.503 | 1030.82 | <0.001 |
| 3 | 444 | PWTT = 93.021 − (1.542 * sPAP) | 0.597 | 653.743 | <0.001 | PWTT = 93.907 − (1.967 * mPAP) | 0.547 | 534.506 | <0.001 | PWTT = 77.779 − (1.806 * dPAP) | 0.467 | 386.649 | <0.001 |
| 4 | 1040 | PWTT = 117.075 − (2.902 * sPAP) | 0.750 | 3114.64 | <0.001 | PWTT = 97.682 − (2.717 * mPAP) | 0.730 | 2804.00 | <0.001 | PWTT = 80.169 − (2.548 * dPAP) | 0.709 | 2531.34 | <0.001 |
| 5 | 290 | PWTT = 88.233 − (1.354 * sPAP) | 0.716 | 725.429 | <0.001 | PWTT = 74.310 − (1.249 * mPAP) | 0.652 | 539.333 | <0.001 | PWTT = 60.839 − (1.024 * dPAP) | 0.551 | 354.094 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller-Graf, F.; Merz, J.; Bandorf, T.; Albus, C.F.; Henkel, M.; Krukewitt, L.; Kühn, V.; Reuter, S.; Vollmar, B.; Pulletz, S.; et al. Correlation of Pulse Wave Transit Time with Pulmonary Artery Pressure in a Porcine Model of Pulmonary Hypertension. Biomedicines 2021, 9, 1212. https://doi.org/10.3390/biomedicines9091212
Mueller-Graf F, Merz J, Bandorf T, Albus CF, Henkel M, Krukewitt L, Kühn V, Reuter S, Vollmar B, Pulletz S, et al. Correlation of Pulse Wave Transit Time with Pulmonary Artery Pressure in a Porcine Model of Pulmonary Hypertension. Biomedicines. 2021; 9(9):1212. https://doi.org/10.3390/biomedicines9091212
Chicago/Turabian StyleMueller-Graf, Fabian, Jonas Merz, Tim Bandorf, Chiara Felicitas Albus, Maike Henkel, Lisa Krukewitt, Volker Kühn, Susanne Reuter, Brigitte Vollmar, Sven Pulletz, and et al. 2021. "Correlation of Pulse Wave Transit Time with Pulmonary Artery Pressure in a Porcine Model of Pulmonary Hypertension" Biomedicines 9, no. 9: 1212. https://doi.org/10.3390/biomedicines9091212
APA StyleMueller-Graf, F., Merz, J., Bandorf, T., Albus, C. F., Henkel, M., Krukewitt, L., Kühn, V., Reuter, S., Vollmar, B., Pulletz, S., Böhm, S. H., Reuter, D. A., & Zitzmann, A. (2021). Correlation of Pulse Wave Transit Time with Pulmonary Artery Pressure in a Porcine Model of Pulmonary Hypertension. Biomedicines, 9(9), 1212. https://doi.org/10.3390/biomedicines9091212






