Plasma Concentrations of Lysophosphatidic Acid and Autotaxin in Abstinent Patients with Alcohol Use Disorder and Comorbid Liver Disease
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants and Recruitment
2.2. Ethics Statements
2.3. Clinical Assessments
2.4. Collection of Plasma Samples
2.5. Determination of LPA and ATX
2.6. Biochemical Parameters Related to Liver Function
2.7. Statistical Analysis
3. Results
3.1. Biological Characteristics and Plasma Concentrations of LPA and ATX
3.2. Biological and Clinical Characteristics of the Alcohol Group Based on Liver Disease
3.3. Plasma Concentrations of LPA and ATX Based on Liver Disease
3.4. Correlation Analysis between AUD-Related Variables and Plasma Concentrations of LPA and ATX in the Alcohol Group Based on Liver Disease
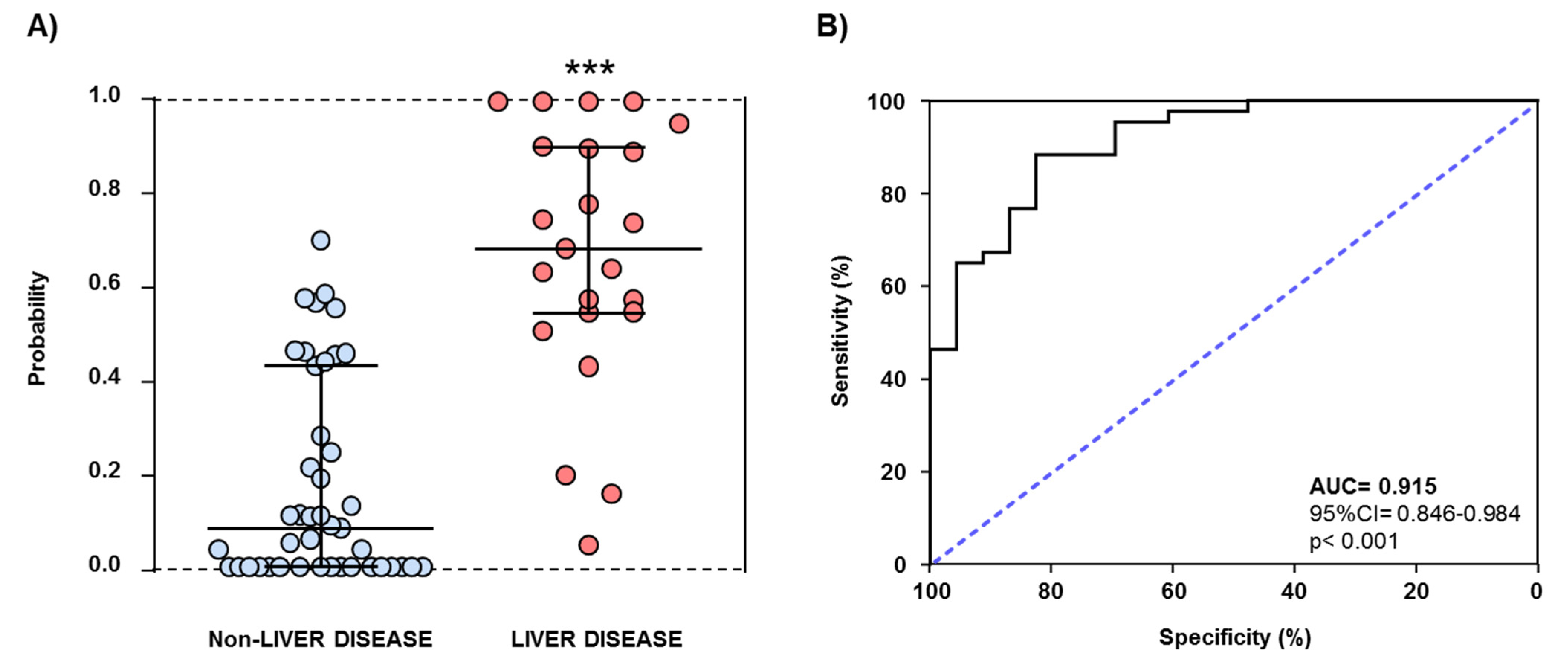
3.5. AUD-Related Variables and Plasma Concentrations of ATX and LPA as Predictors of Liver Disease
4. Discussion
Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Garcia Marchena, N.; Araos, P.; Pavon, F.J.; Ponce, G.; Pedraz, M.; Serrano, A.; Arias, F.; Romero-Sanchiz, P.; Suarez, J.; Pastor, A.; et al. Psychiatric comorbidity and plasma levels of 2-acyl-glycerols in outpatient treatment alcohol users. Analysis of gender differences. Adicciones 2016, 29, 83–96. [Google Scholar]
- Garcia-Marchena, N.; Silva-Pena, D.; Martin-Velasco, A.I.; Villanua, M.A.; Araos, P.; Pedraz, M.; Maza-Quiroga, R.; Romero-Sanchiz, P.; Rubio, G.; Castilla-Ortega, E.; et al. Decreased plasma concentrations of BDNF and IGF-1 in abstinent patients with alcohol use disorders. PLoS ONE 2017, 12, e0187634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic pancreatitis. Nat. Rev. Dis. Primers 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Golfieri, L.; Caputo, F.; Grandi, S.; Andreone, P. Multidisciplinary View of Alcohol Use Disorder: From a Psychiatric Illness to a Major Liver Disease. Biomolecules 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marchena, N.; Pizarro, N.; Pavon, F.J.; Martinez-Huelamo, M.; Flores-Lopez, M.; Requena-Ocana, N.; Araos, P.; Silva-Pena, D.; Suarez, J.; Santin, L.J.; et al. Potential association of plasma lysophosphatidic acid (LPA) species with cognitive impairment in abstinent alcohol use disorders outpatients. Sci. Rep. 2020, 10, 17163. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Pavon, F.J.; Sanchez-Marin, L.; Estivill-Torrus, G.; Pedraza, C.; Blanco, E.; Suarez, J.; Santin, L.; Rodriguez de Fonseca, F.; Serrano, A. Both genetic deletion and pharmacological blockade of lysophosphatidic acid LPA1 receptor results in increased alcohol consumption. Neuropharmacology 2016, 103, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Marin, L.; Ladron de Guevara-Miranda, D.; Manas-Padilla, M.C.; Alen, F.; Moreno-Fernandez, R.D.; Diaz-Navarro, C.; Perez-Del Palacio, J.; Garcia-Fernandez, M.; Pedraza, C.; Pavon, F.J.; et al. Systemic blockade of LPA1/3 lysophosphatidic acid receptors by ki16425 modulates the effects of ethanol on the brain and behavior. Neuropharmacology 2018, 133, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [Green Version]
- Riaz, A.; Huang, Y.; Johansson, S. G-Protein-Coupled Lysophosphatidic Acid Receptors and Their Regulation of AKT Signaling. Int. J. Mol. Sci. 2016, 17, 215. [Google Scholar] [CrossRef] [Green Version]
- Geraldo, L.H.M.; Spohr, T.; Amaral, R.F.D.; Fonseca, A.; Garcia, C.; Mendes, F.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef]
- Aoki, J.; Inoue, A.; Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 2008, 1781, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, P.K.; Orsi, S.A.; Moody, M.; Moore, A.N. A role for hippocampal Rho-ROCK pathway in long-term spatial memory. Biochem. Biophys. Res. Commun. 2004, 322, 893–898. [Google Scholar] [CrossRef]
- Rosell-Valle, C.; Pedraza, C.; Manuel, I.; Moreno-Rodriguez, M.; Rodriguez-Puertas, R.; Castilla-Ortega, E.; Carames, J.M.; Gomez Conde, A.I.; Zambrana-Infantes, E.; Ortega-Pinazo, J.; et al. Chronic central modulation of LPA/LPA receptors-signaling pathway in the mouse brain regulates cognition, emotion, and hippocampal neurogenesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110156. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yin, N.; Zhang, J. The Expression Regulation and Biological Function of Autotaxin. Cells 2021, 10, 939. [Google Scholar] [CrossRef]
- Benesch, M.G.; Ko, Y.M.; McMullen, T.P.; Brindley, D.N. Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. FEBS Lett. 2014, 588, 2712–2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleli, T.; Martin, D.; Kronenberger, B.; Brunner, F.; Koberle, V.; Grammatikos, G.; Farnik, H.; Martinez, Y.; Finkelmeier, F.; Labocha, S.; et al. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis--a prospective cohort study. PLoS ONE 2014, 9, e103532. [Google Scholar] [CrossRef]
- Shao, X.; Uojima, H.; Setsu, T.; Okubo, T.; Atsukawa, M.; Furuichi, Y.; Arase, Y.; Hidaka, H.; Tanaka, Y.; Nakazawa, T.; et al. Usefulness of autotaxin for the complications of liver cirrhosis. World J. Gastroenterol. 2020, 26, 97–108. [Google Scholar] [CrossRef]
- Nie, C.; Zhang, L.; Chen, X.; Li, Y.; Ha, F.; Liu, H.; Han, T. Autotaxin: An Early Warning Biomarker for Acute-on-chronic Liver Failure. J. Clin. Transl. Hepatol. 2020, 8, 240–245. [Google Scholar] [CrossRef]
- Honda, Y.; Imajo, K.; Kobayashi, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Yoneda, M.; Kobayashi, N.; Saito, S.; Nakajima, A. Autotaxin is a valuable biomarker for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2019, 49, 1136–1146. [Google Scholar] [CrossRef]
- Torrens, M.; Serrano, D.; Astals, M.; Perez-Dominguez, G.; Martin-Santos, R. Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Hasin, D.; Samet, S.; Nunes, E.; Meydan, J.; Matseoane, K.; Waxman, R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. Am. J. Psychiatry 2006, 163, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Robins, L.N.; Wing, J.; Wittchen, H.U.; Helzer, J.E.; Babor, T.F.; Burke, J.; Farmer, A.; Jablenski, A.; Pickens, R.; Regier, D.A.; et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch. Gen. Psychiatry 1988, 45, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Li, B.; Huang, J.; Zhong, M.; Li, L.; Duan, C.; Zeng, S.; Huang, J.; Liu, W.; Lu, J.; et al. Lysophosphatidic Acid is a Biomarker for Peritoneal Carcinomatosis of Gastric Cancer and Correlates with Poor Prognosis. Genet. Test. Mol. Biomark. 2017, 21, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, A.; Budkowska, M.; Dolegowska, B.; Chlubek, D.; Safranow, K. Lysophosphatidic acid plasma concentrations in healthy subjects: Circadian rhythm and associations with demographic, anthropometric and biochemical parameters. Lipids Health Dis. 2017, 16, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaffe, E.; Magkrioti, C.; Aidinis, V. Deregulated Lysophosphatidic Acid Metabolism and Signaling in Liver Cancer. Cancers 2019, 11, 1626. [Google Scholar] [CrossRef] [Green Version]
- Benesch, M.G.; Zhao, Y.Y.; Curtis, J.M.; McMullen, T.P.; Brindley, D.N. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J. Lipid Res. 2015, 56, 1134–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- le Balle, F.; Simon, M.F.; Meijer, S.; Fourcade, O.; Chap, H. Membrane sidedness of biosynthetic pathways involved in the production of lysophosphatidic acid. Adv. Enzym. Regul. 1999, 39, 275–284. [Google Scholar] [CrossRef]
- Aoki, J.; Taira, A.; Takanezawa, Y.; Kishi, Y.; Hama, K.; Kishimoto, T.; Mizuno, K.; Saku, K.; Taguchi, R.; Arai, H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002, 277, 48737–48744. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.W.; Choi, K.Y.; Min, D.S. Functional regulation of phospholipase D expression in cancer and inflammation. J. Biol. Chem. 2014, 289, 22575–22582. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.G.; Di Paolo, G. Phospholipase D in brain function and Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Krzystanek, M.; Krzystanek, E.; Skalacka, K.; Palasz, A. Enhancement in Phospholipase D Activity as a New Proposed Molecular Mechanism of Haloperidol-Induced Neurotoxicity. Int. J. Mol. Sci. 2020, 21, 9265. [Google Scholar] [CrossRef]
- Isaksson, A.; Walther, L.; Hansson, T.; Andersson, A.; Alling, C. Phosphatidylethanol in blood (B-PEth): A marker for alcohol use and abuse. Drug Test. Anal. 2011, 3, 195–200. [Google Scholar] [CrossRef]
- Garcia-Marchena, N.; Araos, P.F.; Barrios, V.; Sanchez-Marin, L.; Chowen, J.A.; Pedraz, M.; Castilla-Ortega, E.; Romero-Sanchiz, P.; Ponce, G.; Gavito, A.L.; et al. Plasma Chemokines in Patients with Alcohol Use Disorders: Association of CCL11 (Eotaxin-1) with Psychiatric Comorbidity. Front. Psychiatry 2016, 7, 214. [Google Scholar] [CrossRef]
- Garcia-Marchena, N.; Maza-Quiroga, R.; Serrano, A.; Barrios, V.; Requena-Ocana, N.; Suarez, J.; Chowen, J.A.; Argente, J.; Rubio, G.; Torrens, M.; et al. Abstinent patients with alcohol use disorders show an altered plasma cytokine profile: Identification of both interleukin 6 and interleukin 17A as potential biomarkers of consumption and comorbid liver and pancreatic diseases. J. Psychopharmacol. 2020, 34, 1250–1260. [Google Scholar] [CrossRef]
- Ikeda, H.; Kobayashi, M.; Kumada, H.; Enooku, K.; Koike, K.; Kurano, M.; Sato, M.; Nojiri, T.; Kobayashi, T.; Ohkawa, R.; et al. Performance of autotaxin as a serum marker for liver fibrosis. Ann. Clin. Biochem. 2018, 55, 469–477. [Google Scholar] [CrossRef]
- Bellentani, S.; Saccoccio, G.; Costa, G.; Tiribelli, C.; Manenti, F.; Sodde, M.; Saveria Croce, L.; Sasso, F.; Pozzato, G.; Cristianini, G.; et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997, 41, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Rehm, J.; Taylor, B.; Mohapatra, S.; Irving, H.; Baliunas, D.; Patra, J.; Roerecke, M. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol Rev. 2010, 29, 437–445. [Google Scholar] [CrossRef]
- Brodersen, C.; Koen, E.; Ponte, A.; Sanchez, S.; Segal, E.; Chiapella, A.; Fernandez, M.; Torres, M.; Tripodi, V.; Lemberg, A. Cognitive function in patients with alcoholic and nonalcoholic chronic liver disease. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Edwin, D.; Flynn, L.; Klein, A.; Thuluvath, P.J. Cognitive impairment in alcoholic and nonalcoholic cirrhotic patients. Hepatology 1999, 30, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, M.; Crabb, D.; Xu, Y.; Liangpunsakul, S. Ethanol-induced alterations in fatty acid-related lipids in serum and tissues in mice. Alcohol. Clin. Exp. Res. 2011, 35, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Group | p-Value | ||
|---|---|---|---|---|
| Control (N = 70) | Alcohol (N = 66) | |||
| Age (years) | Mean ± SD | 47.8 ± 6.5 | 48.1 ± 5.7 | 0.760 a |
| BMI (kg/m2) | Mean ± SD | 24.2 ± 2.1 | 25.1 ± 3.4 | 0.060 a |
| Sex (men) | N (%) | 60 (85.7) | 56 (84.8) | 0.887 b |
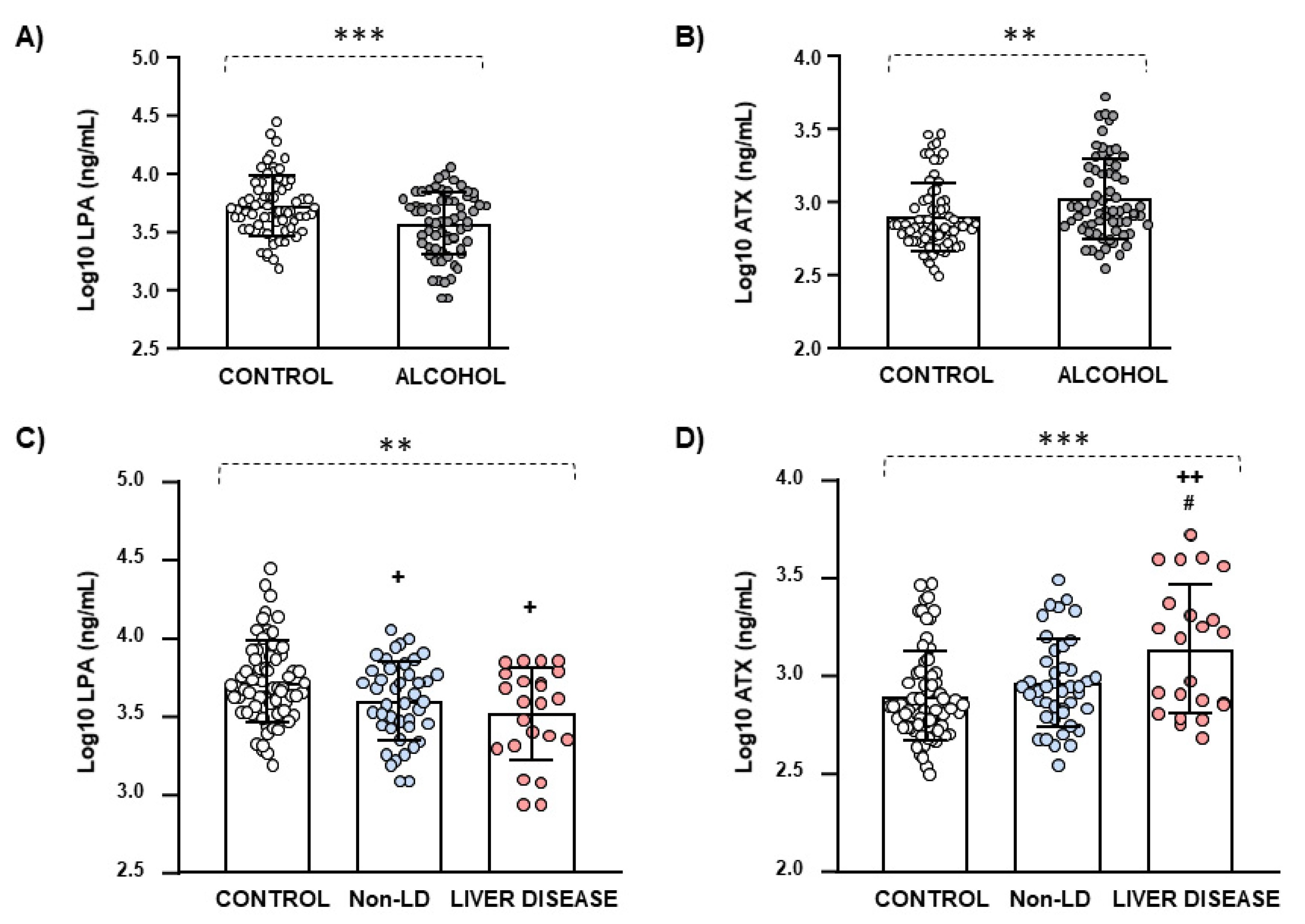
| LPA (ng/mL) | Median (IQR) | 4917.1 (4393.0) | 3972.7 (3726.4) | 0.009 c |
| ATX (ng/mL) | Median (IQR) | 696.5 (472.6) | 885.2 (1049.8) | 0.004 c |
| Variable | Subgroup | p-Value | ||
|---|---|---|---|---|
| Non-Liver Disease (N = 43) | Liver Disease (N = 23) | |||
| Age (years) | Mean ± SD | 48.1 ± 6.4 | 48.2 ± 4.2 | 0.911 a |
| BMI (kg/m2) | Mean ± SD | 24.6 ± 3.3 | 25.9 ± 3.5 | 0.155 a |
| Sex (men) | N (%) | 34 (79.1) | 22 (95.7) | 0.073 b |
| Problematic alcohol use (years) | Mean ± SD | 17.4 ± 11.2 | 19.3 ± 9.9 | 0.510 a |
| Last period of alcohol abstinence (days) | Median (IQR) | 120.0 (120.0) | 120.0 (120.0) | 0.760 c |
| DSM criteria for AUD (0–11) | Median (IQR) | 8.0 (2.0) | 7.0 (3.0) | 0.313 c |
| Mental disorders | N (%) | 28 (65.1) | 13 (56.5) | 0.493 b |
| Mood disorders | N (%) | 20 (46.5) | 8 (34.8) | 0.713 b |
| Anxiety disorders | N (%) | 11 (25.6) | 4 (17.4) | 0.728 b |
| Psychotic disorders | N (%) | 6 (14.0) | 1 (4.3) | 0.425 b |
| Personality disorders | N (%) | 8 (18.6) | 5 (21.7) | 0.760 b |
| Substance use disorders | N (%) | 17 (39.5) | 10 (43.5) | 0.756 b |
| Cocaine | N (%) | 15 (34.9) | 8 (34.8) | 0.993 b |
| Cannabis | N (%) | 6 (14.0) | 5 (21.7) | 0.419 b |
| AST (0.0–40.0 U/L) | Mean ± SD | 28.4 ± 2.6 | 44.1 ± 5.8 | <0.001 a |
| GGT (0.0–45.0 U/L) | Mean ± SD | 36.2 ± 5.1 | 49.1 ± 6.5 | <0.001 a |
| ALT (0.0–40.0 U/L) | Mean ± SD | 34.9 ± 3.0 | 38.4 ± 6.2 | 0.017 a |
| LPA (ng/mL) | Median (IQR) | 3941.1 (3554.1) | 4004.4 (3994.0) | 0.364 c |
| ATX (ng/mL) | Median (IQR) | 883.2 (534.0) | 1574.5 (1982.2) | 0.079 c |
| Variable | LPA (ng/mL) a | ATX (ng/mL) a | ||||
|---|---|---|---|---|---|---|
| Alcohol Group | Alcohol Subgroup | Alcohol Group | Alcohol Subgroup | |||
| Non-Liver Disease | Liver Disease | Non-Liver Disease | Liver Disease | |||
| Problematic alcohol use (years) | r = +0.082 p = 0.514 | r = +0.158 p = 0.312 | r = −0.017 p = 0.938 | r = −0.049 p = 0.696 | r = −0.185 p = 0.235 | r = +0.072 p = 0.745 |
| Last period of alcohol abstinence (days) a | r = +0.157 p = 0.207 | r = +0.325 p = 0.033 | r = −0.183 p = 0.403 | r = +0.140 p = 0.263 | r = +0.326 p = 0.033 | r = −0.158 p = 0.470 |
| DSM criteria for AUD (0–11) | rho = +0.067 p = 0.590 | rho = +0.097 p = 0.538 | rho = −0.089 p = 0.686 | rho = −0.264 p = 0.032 | rho = −0.327 p = 0.033 | rho = −0.212 p = 0.332 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-López, M.; García-Marchena, N.; Pavon, F.J.; Lara, E.; Porras-Perales, O.; Araos, P.; Requena-Ocaña, N.; Torres-Galván, S.; Mañas-Padilla, M.C.; Rubio, G.; et al. Plasma Concentrations of Lysophosphatidic Acid and Autotaxin in Abstinent Patients with Alcohol Use Disorder and Comorbid Liver Disease. Biomedicines 2021, 9, 1207. https://doi.org/10.3390/biomedicines9091207
Flores-López M, García-Marchena N, Pavon FJ, Lara E, Porras-Perales O, Araos P, Requena-Ocaña N, Torres-Galván S, Mañas-Padilla MC, Rubio G, et al. Plasma Concentrations of Lysophosphatidic Acid and Autotaxin in Abstinent Patients with Alcohol Use Disorder and Comorbid Liver Disease. Biomedicines. 2021; 9(9):1207. https://doi.org/10.3390/biomedicines9091207
Chicago/Turabian StyleFlores-López, María, Nuria García-Marchena, Francisco Javier Pavon, Estrella Lara, Oscar Porras-Perales, Pedro Araos, Nerea Requena-Ocaña, Sandra Torres-Galván, M. Carmen Mañas-Padilla, Gabriel Rubio, and et al. 2021. "Plasma Concentrations of Lysophosphatidic Acid and Autotaxin in Abstinent Patients with Alcohol Use Disorder and Comorbid Liver Disease" Biomedicines 9, no. 9: 1207. https://doi.org/10.3390/biomedicines9091207







