Stable Gastric Pentadecapeptide BPC 157 Heals Established Vesicovaginal Fistula and Counteracts Stone Formation in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Procedure
2.4. Experimental Protocol after Surgery
2.5. Vesicovaginal Fistula Assessment Protocol
2.5.1. Fistula Leakage
2.5.2. Urinary Leaking through Vagina
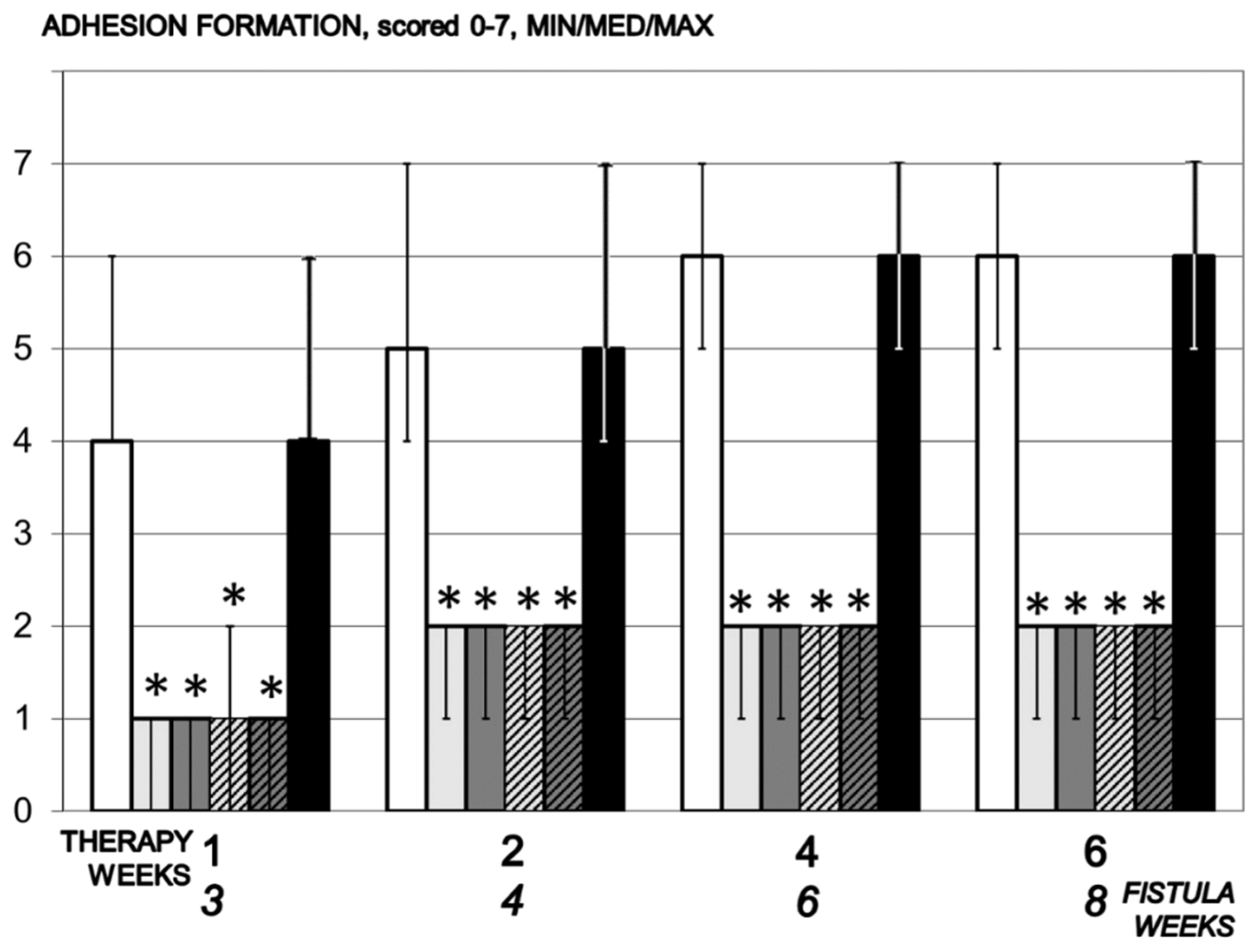
2.5.3. Adhesion
2.5.4. Microlithiasis or Stone Formation in Bladder
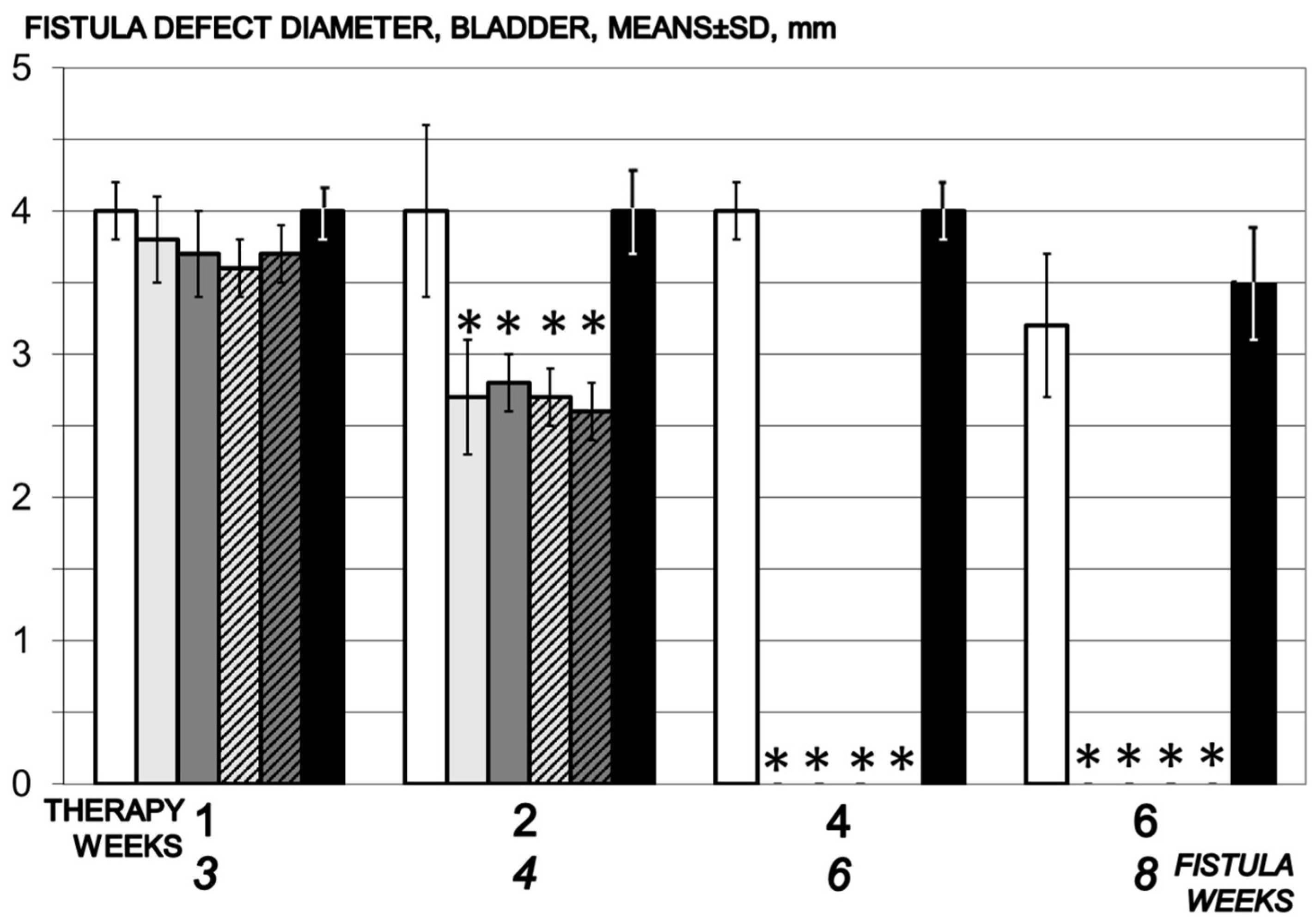
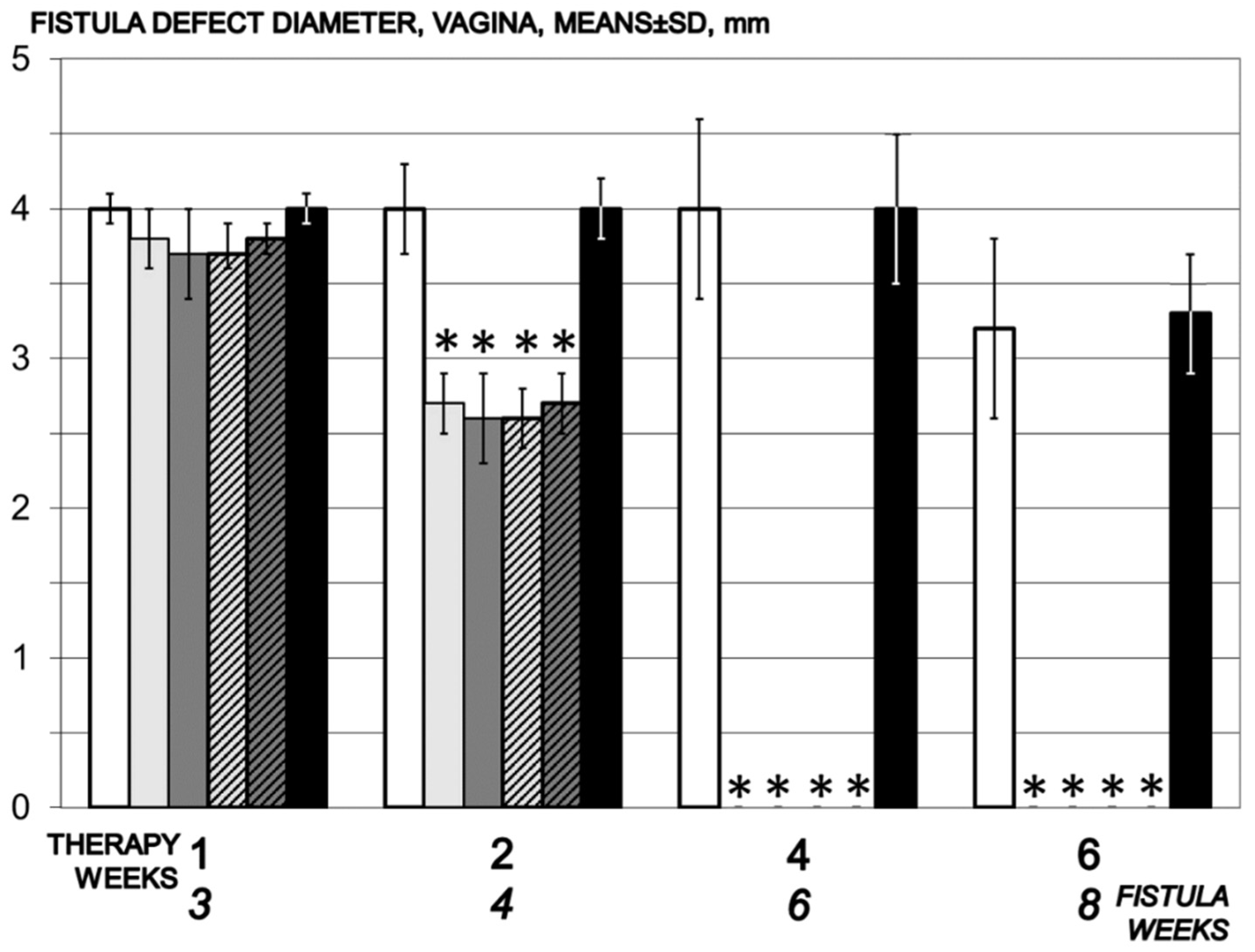
2.5.5. Vesical Defect, Vaginal Defect, Fistula Assessment
2.6. Statistical Analyses
3. Results
3.1. Fistula Leakage
3.2. Urinary Leaking through Vagina
3.3. Microlithiasis or Stone Formation in Bladder
3.4. Adhesions
3.5. Fistula
3.5.1. Vesical Defect, Vaginal Defect, Fistula Assessment
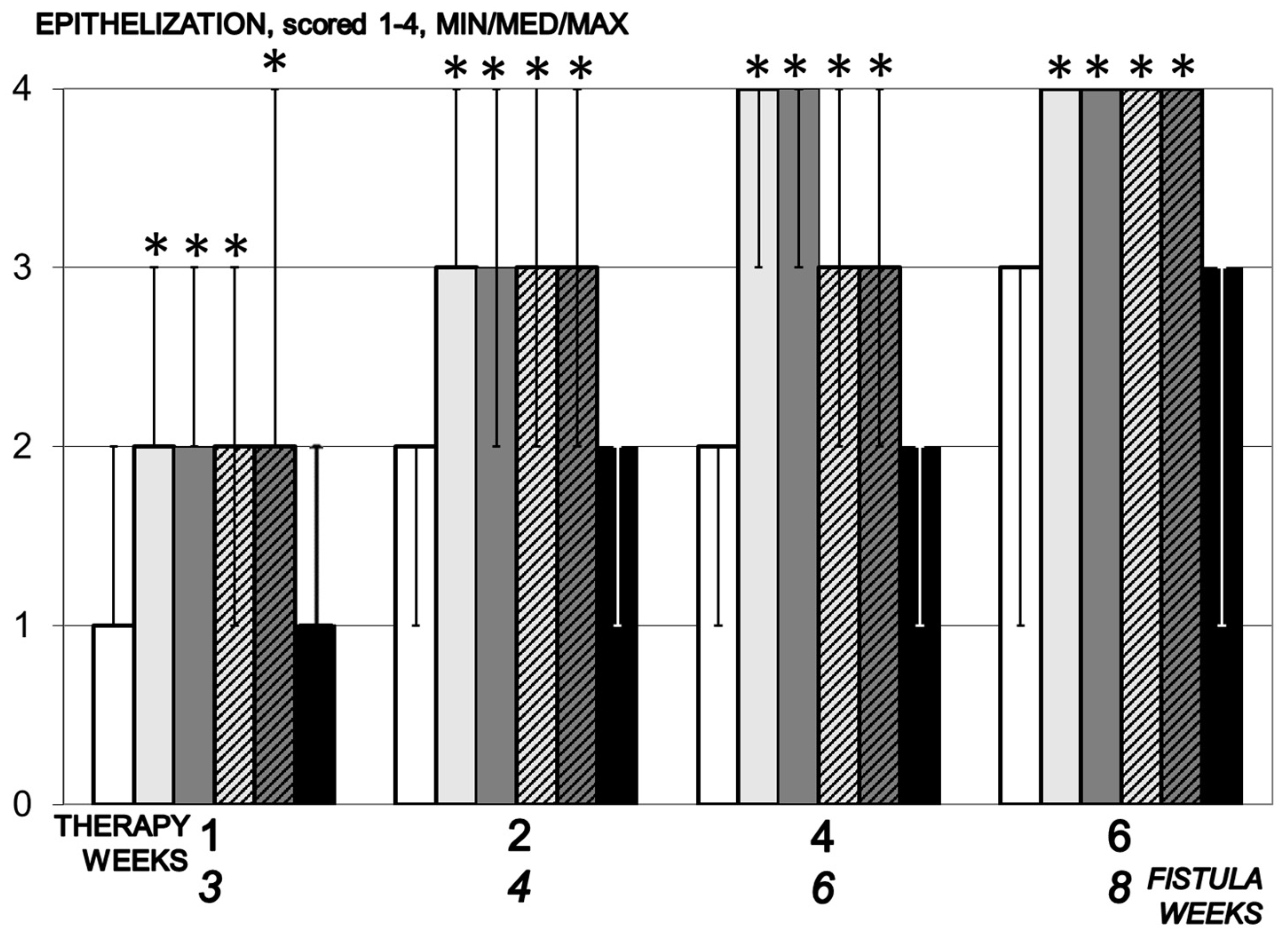
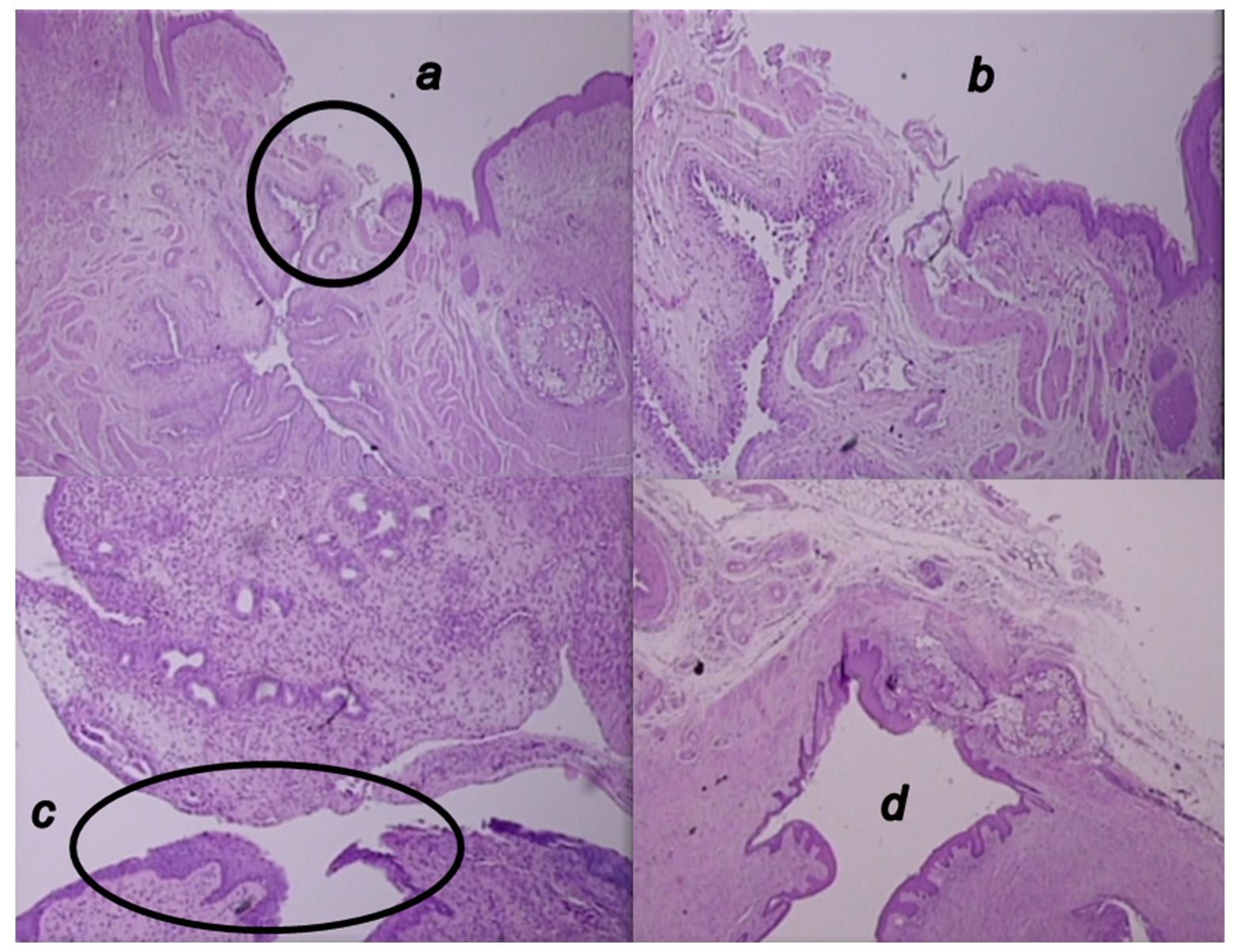
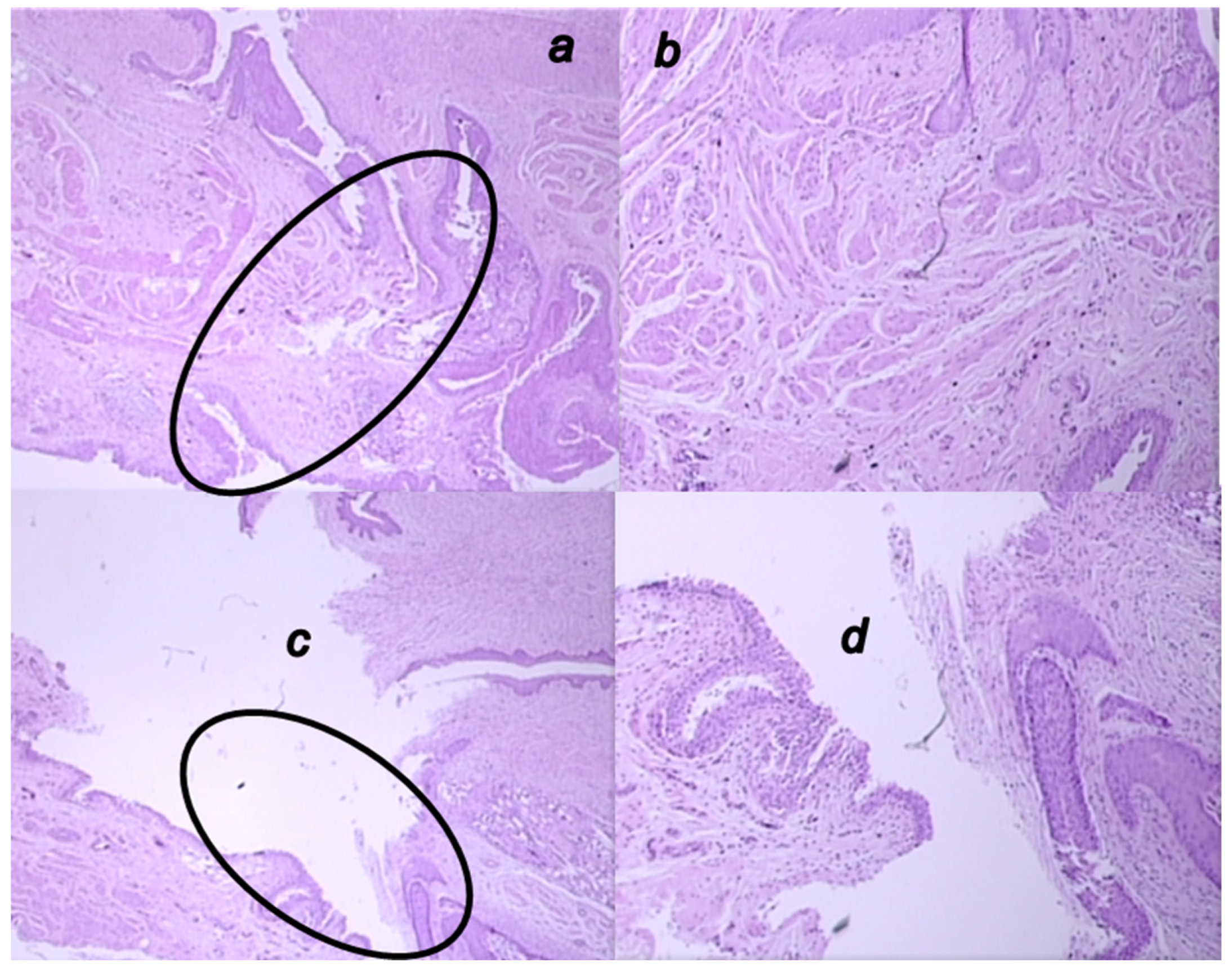
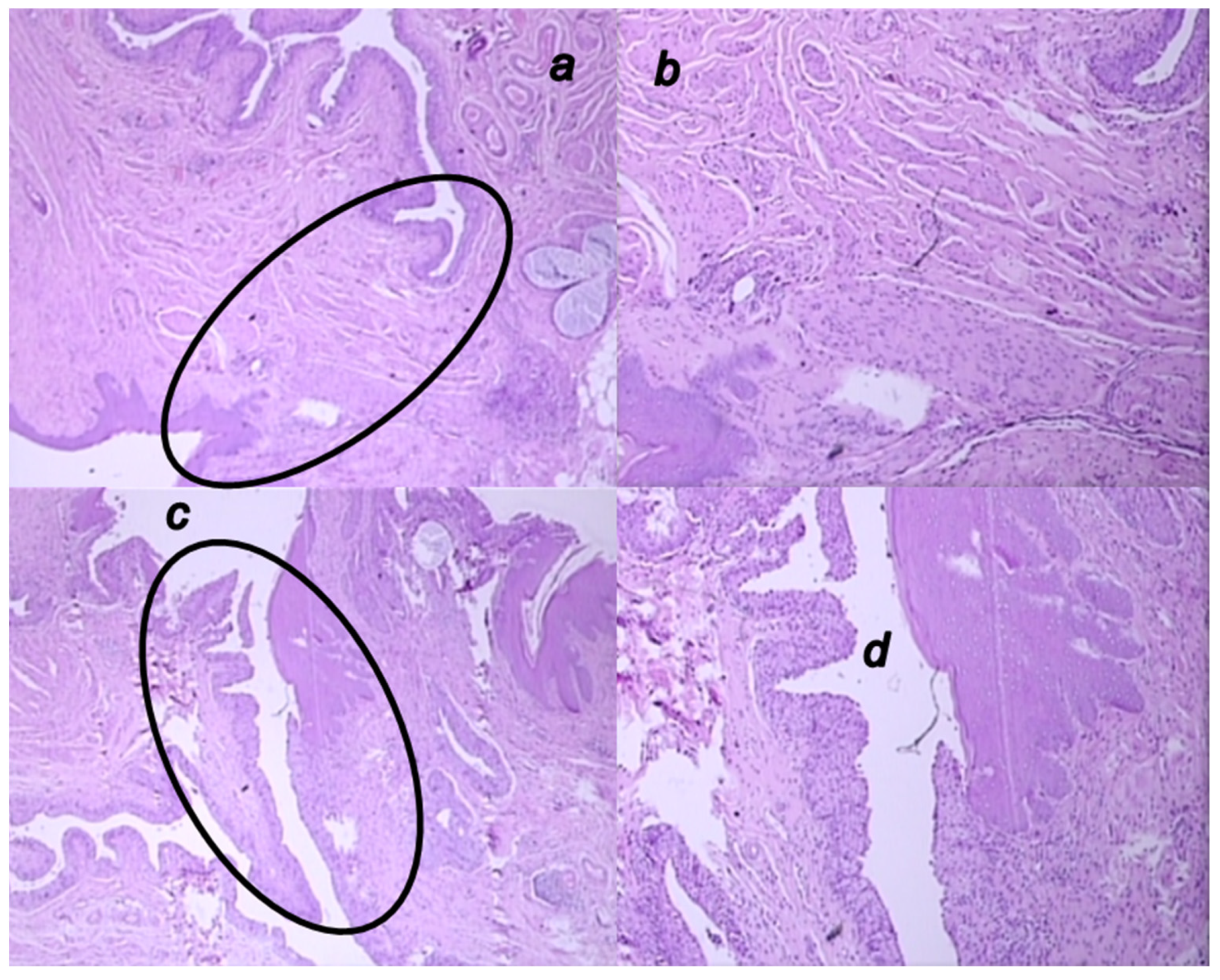
3.5.2. Epithelization
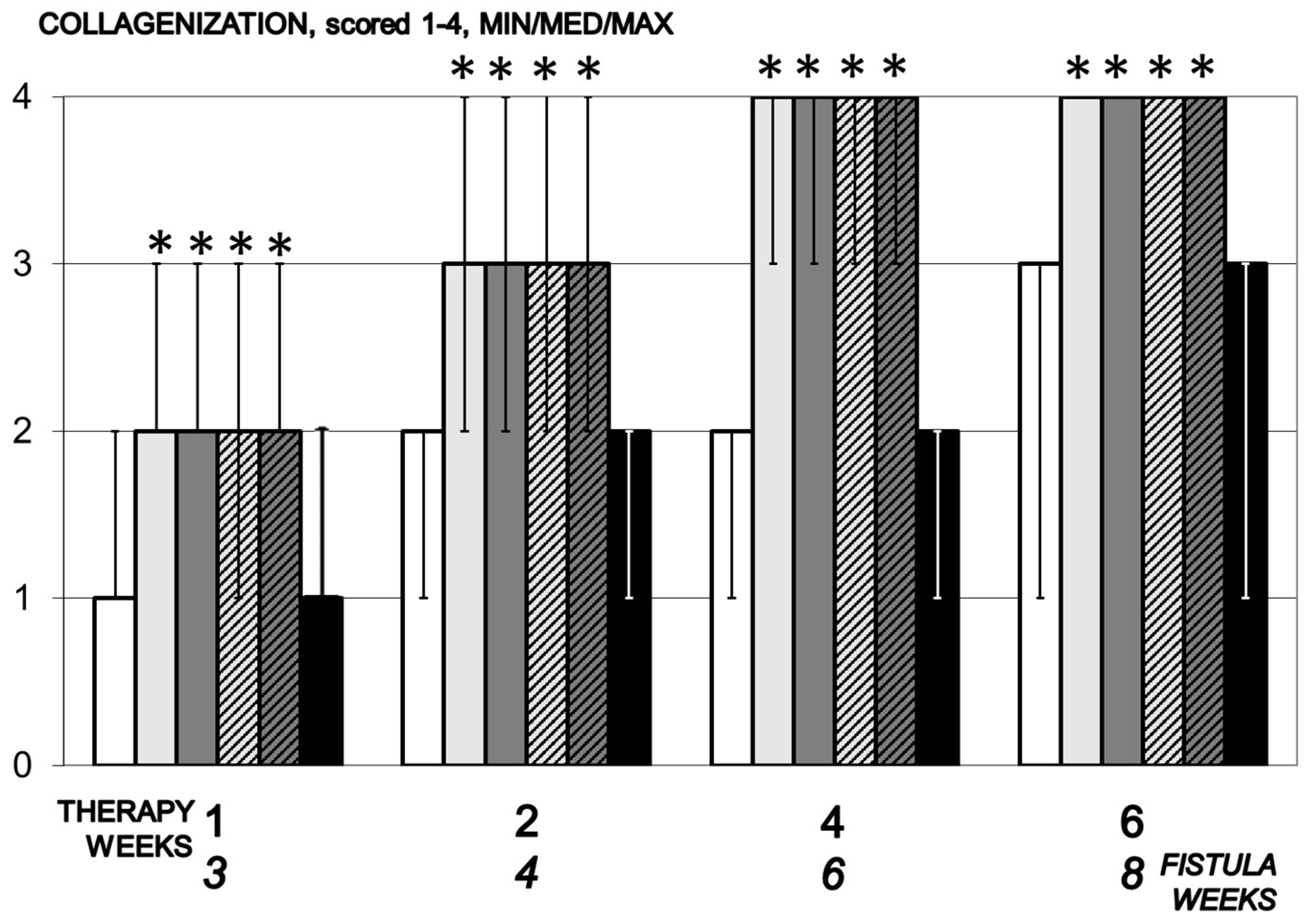
3.5.3. Collagenization
3.5.4. Inflammation
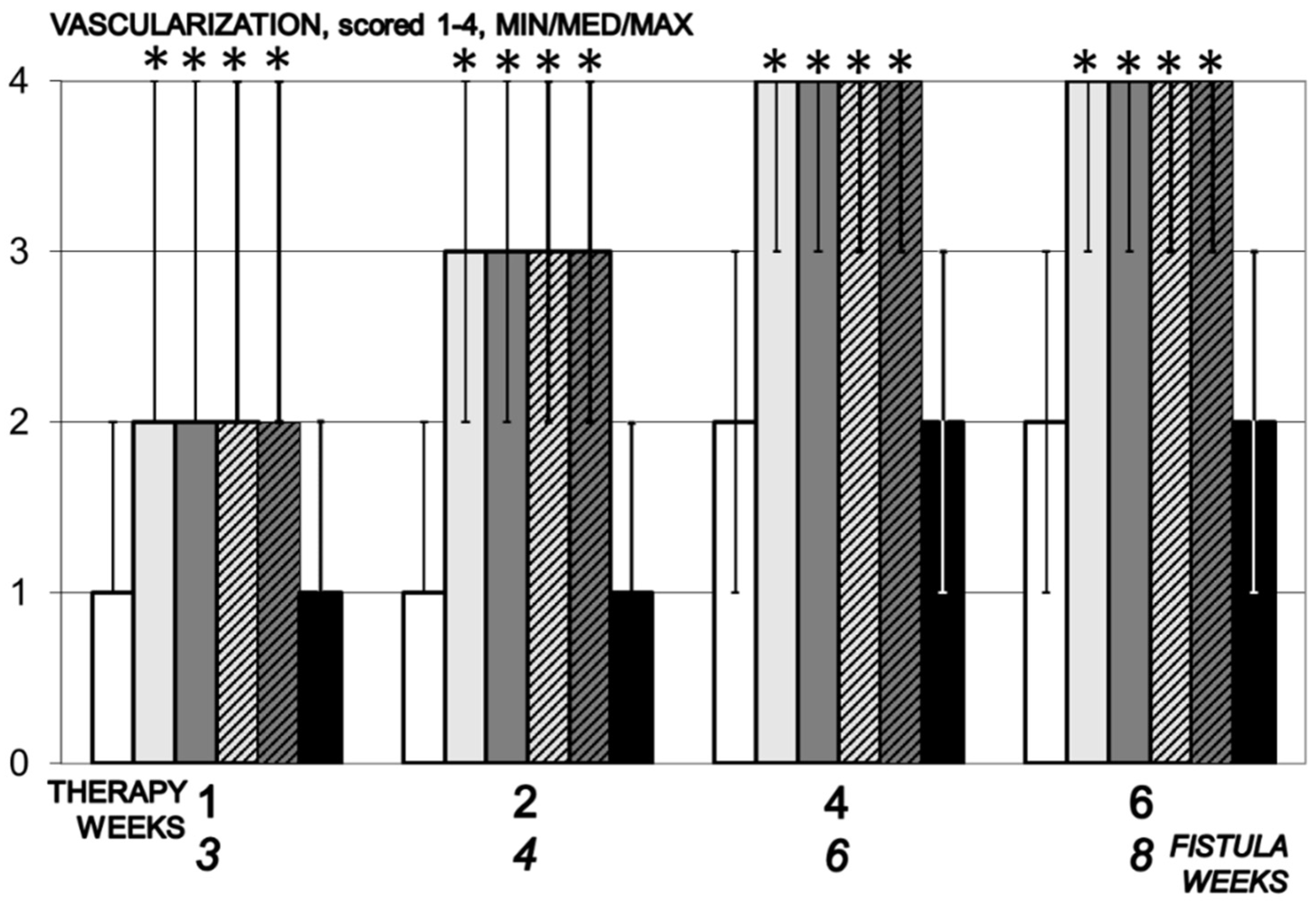
3.5.5. Neovascularization
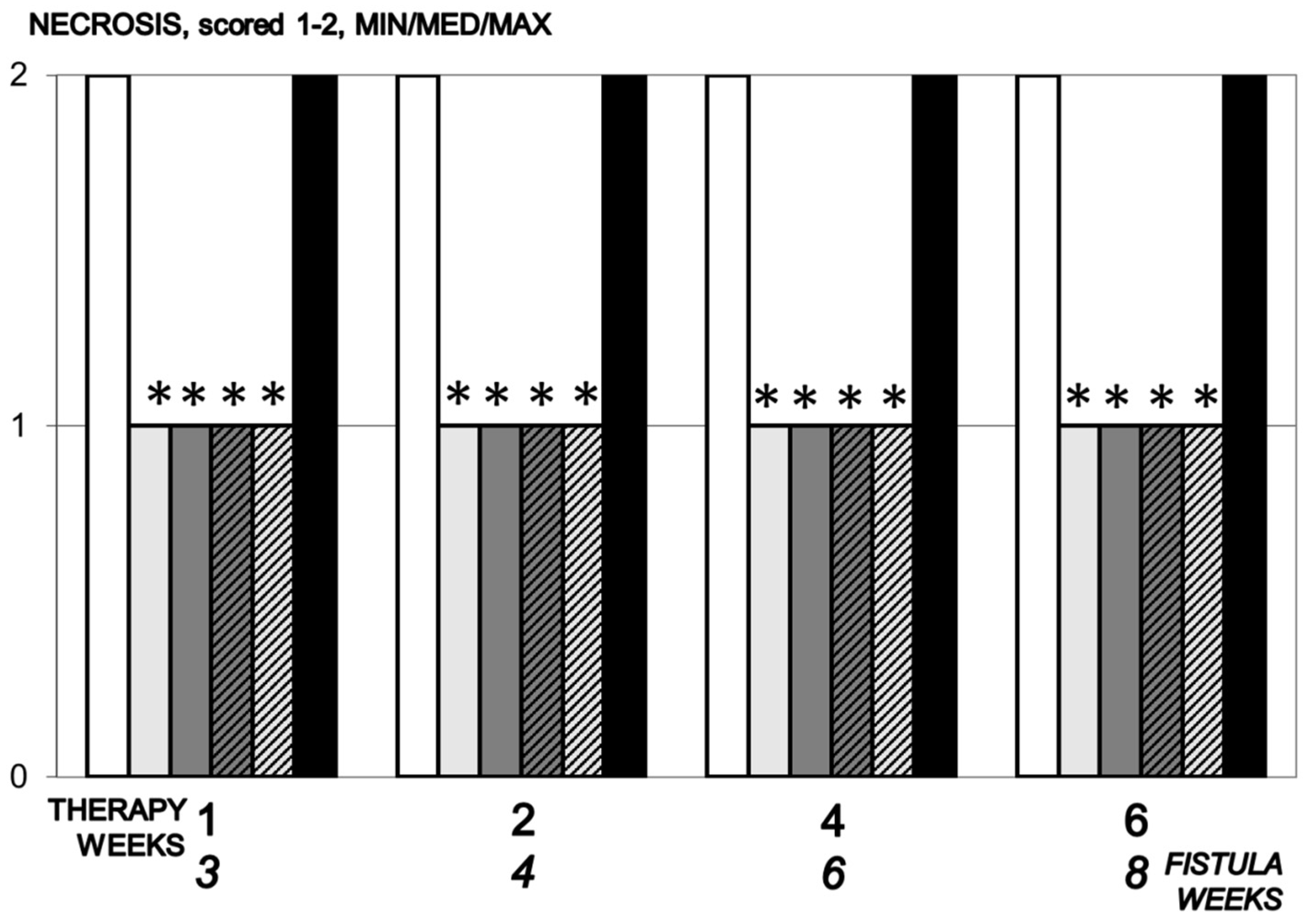
3.5.6. Necrosis
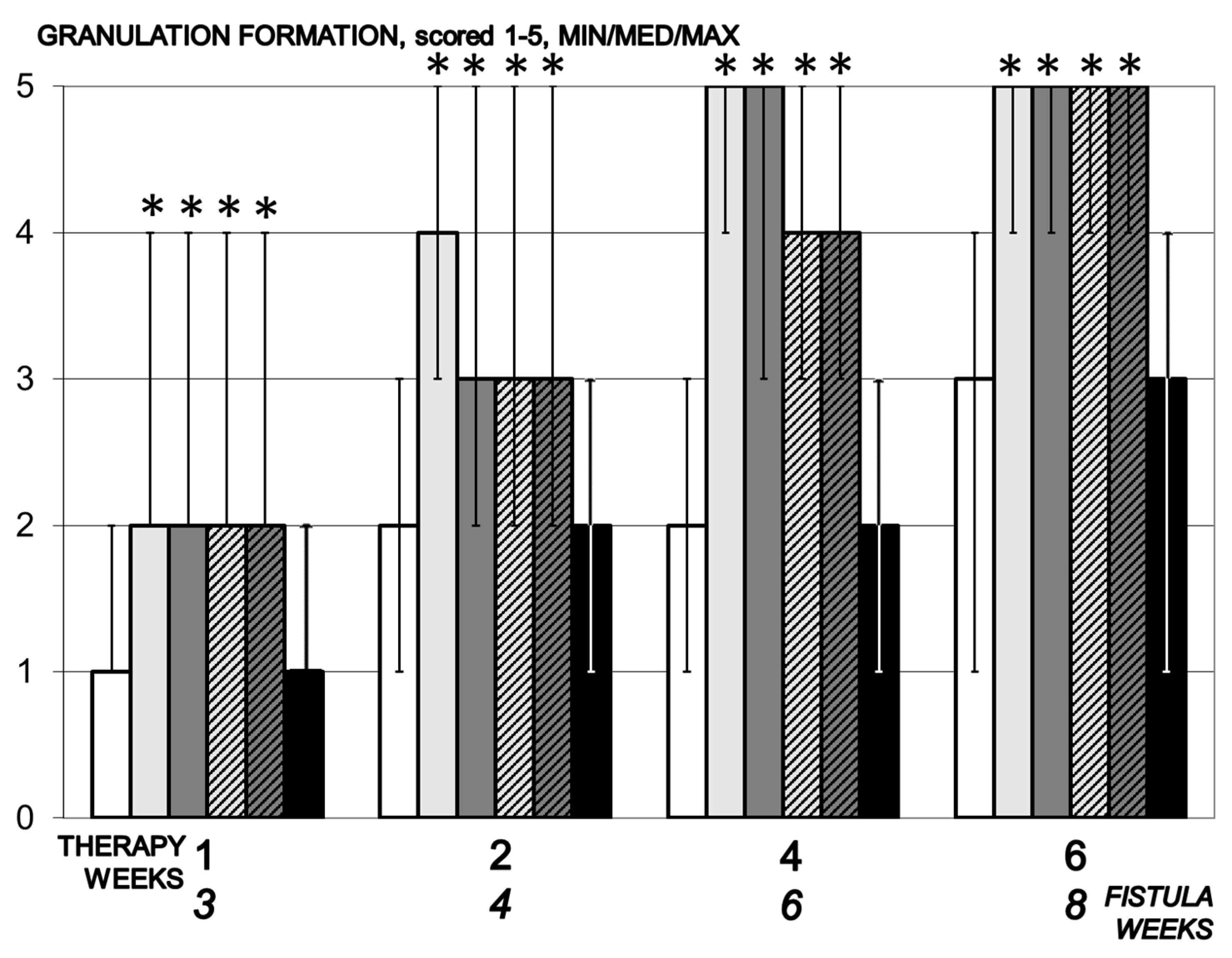
3.5.7. Granulation
3.6. Microscopy Presentation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikiric, P.; Hahm, K.B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157, Robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, and Selye’s stress coping response: Progress, achievements, and the future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Sever, M.; Klicek, R.; Blagaic, A.B.; Tvrdeic, A.; Kralj, T.; Kovac, K.K.; Vukojevic, J.; Siroglavic, M.; et al. Fistulas healing. Stable gastric pentadecapeptide BPC 157 therapy. Curr. Pharm. Des. 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Cesarec, V.; Becejac, T.; Misic, M.; Djakovic, Z.; Olujic, D.; Drmic, D.; Brcic, L.; Rokotov, D.S.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur. J. Pharmacol. 2013, 701, 203–212. [Google Scholar] [CrossRef]
- Skorjanec, S.; Dolovski, Z.; Kocman, I.; Brcic, L.; Blagaic Boban, A.; Batelja, L.; Coric, M.; Sever, M.; Klicek, R.; Berkopic, L.; et al. Therapy for unhealed gastrocutaneous fistulas in rats as a model for analogous healing of persistent skin wounds and persistent gastric ulcers: Stable gastric pentadecapeptide BPC 157, atropine, ranitidine, and omeprazole. Dig. Dis. Sci. 2009, 54, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Skorjanec, S.; Kokot, A.; Drmic, D.; Radic, B.; Sever, M.; Klicek, R.; Kolenc, D.; Zenko, A.; Lovric Bencic, M.; Belosic Halle, Z.; et al. Duodenocutaneous fistula in rats as a model for “wound healing-therapy” in ulcer healing: The effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and L-arginine. J. Physiol. Pharmacol. 2015, 66, 581–590. [Google Scholar] [PubMed]
- Klicek, R.; Sever, M.; Radic, B.; Drmic, D.; Kocman, I.; Zoricic, I.; Vuksic, T.; Ivica, M.; Barisic, I.; Ilic, S.; et al. Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: Role of the nitric oxide-system. J. Pharmacol. Sci. 2008, 108, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Grgic, T.; Grgic, D.; Drmic, D.; Sever, A.Z.; Petrovic, I.; Sucic, M.; Kokot, A.; Klicek, R.; Sever, M.; Seiwerth, S.; et al. Stable gastric pentadecapeptide BPC 157 heals rat colovesical fistula. Eur. J. Pharmacol. 2016, 780, 1–7. [Google Scholar] [CrossRef]
- Baric, M.; Sever, A.Z.; Vuletic, L.B.; Rasic, Z.; Sever, M.; Drmic, D.; Pavelic-Turudic, T.; Sucic, M.; Vrcic, H.; Seiwerth, S.; et al. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci. 2016, 148, 63–70. [Google Scholar] [CrossRef]
- Shephard, S.N.; Lengmang, S.J.; Kirschner, C.V. Bladder stones in vesicovaginal fistula: Is concurrent repair an option? Experience with 87 patients. Int. Urogynecol. J. 2017, 28, 569–574. [Google Scholar] [CrossRef]
- Francisca Kholis, K.; Palinrungi, M.A.; Syarif, S.S.; Faruk, M. Bladder stones associated with vesicovaginal fistula: A case report. Int. J. Surg. Case Rep. 2020, 75, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Barry, D.; Khatry, S.K.; Klasen, E.M.; Singh, M.; LeClerq, S.C.; Katz, J.; Tielsch, J.M.; Mullany, L.C. Validation of an obstetric fistula screening questionnaire in rural Nepal: A community-based cross-sectional and nested case-control study with clinical examination. BJOG 2017, 124, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.A.; Gormley, A. State of the art for treatment of vesicovaginal fistula. Curr. Urol. Rep. 2017, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Holtz, S.A. Social and economic consequences of obstetric fistula: Life changed forever? Int. J. Gynaecol. Obstet. 2007, 99 (Suppl. 1), S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Angioli, R.; Penalver, M.; Muzii, L.; Mendez, L.; Mirhashemi, R.; Bellati, F.; Crocè, C.; Benedetti Panici, P. Guidelines of how to manage vesicovaginal fistula. Crit. Rev. Oncol. Hematol. 2003, 48, 295–304. [Google Scholar] [CrossRef]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Bateljam, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Devel. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef]
- Djakovic, Z.; Djakovic, I.; Cesarec, V.; Madzarac, G.; Becejac, T.; Zukanovic, G.; Drmic, D.; Batelja, L.; Zenko Sever, A.; Kolenc, D.; et al. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J. Gastroenterol. 2016, 22, 9127–9140. [Google Scholar] [CrossRef]
- Sever, M.; Klicek, R.; Radic, B.; Brcic, L.; Zoricic, I.; Drmic, D.; Ivica, M.; Barisic, I.; Ilic, S.; Berkopic, L.; et al. Gastric pentadecapeptide BPC 157 and short bowel syndrome in rats. Dig. Dis. Sci. 2009, 54, 2070–2083. [Google Scholar] [CrossRef]
- Lojo, N.; Rasic, Z.; Sever, A.Z.; Kolenc, D.; Vukusic, D.; Drmic, D.; Zoricic, I.; Sever, M.; Seiwerth, S.; Sikiric, P. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 h-short-bowel rats. PLoS ONE 2016, 11, e0162590. [Google Scholar] [CrossRef]
- Klicek, R.; Kolenc, D.; Suran, J.; Drmic, D.; Brcic, L.; Aralica, G.; Sever, M.; Holjevac, J.; Radic, B.; Turudic, T.; et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizo ne brain injuries and motor disability. J. Physiol. Pharmacol. 2013, 64, 597–612. [Google Scholar] [PubMed]
- Vuksic, T.; Zoricic, I.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Cesarec, V.; Berkopic, L.; Keller, N.; Blagaic, A.B.; et al. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL14736, Pliva, Croatia) heals ileoileal anastomosis in the rat. Surg. Today 2007, 37, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Jadrijevic, S.; Seiwerth, S.; Sosa, T.; Deskovic, S.; Perovic, D.; Aralica, G.; Grabarevic, Z.; Rucman, R.; Petek, M.; et al. Long-lasting cytoprotection after pentadecapeptide BPC 157, ranitidine, sucralfate or cholestyramine application in reflux oesophagitis in rats. J. Physiol. Paris 1999, 93, 467–477. [Google Scholar] [CrossRef]
- Sikirić, P.; Mikus, D.; Seiwerth, S.; Grabarević, Z.; Rucman, R.; Petek, M.; Jagić, V.; Turković, B.; Rotkvić, I.; Mise, S.; et al. Pentadecapeptide BPC 157, cimetidine, ranitidine, bromocriptine, and atropine effect in cysteamine lesions in totally gastrectromized rats: A model for cytoprotective studies. Dig. Dis. Sci. 1999, 42, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Sikirić, P.; Seiwerth, S.; Desković, S.; Grabarević, Z.; Marović, A.; Rucman, R.; Petek, M.; Konjevoda, P.; Jadrijević, S.; Sosa, T.; et al. New model of cytoprotection/adaptive cytoprotection in rats: Endogenous small irritants, antiulcer agents and indomethacin. Eur. J. Pharmacol. 1999, 364, 23–31. [Google Scholar] [CrossRef]
- Hrelec, M.; Klicek, R.; Brcic, L.; Brcic, I.; Cvjetko, I.; Seiwerth, S.; Sikiric, P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 161–165. [Google Scholar]
- Gjurasin, M.; Miklic, P.; Zupancic, B.; Perovic, D.; Zarkovic, K.; Brcic, L.; Kolenc, D.; Radic, B.; Seiwerth, S.; Sikiric, P. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul. Pept. 2010, 160, 33–41. [Google Scholar] [CrossRef]
- Jandric, I.; Vrcic, H.; Jandric Balen, M.; Kolenc, D.; Brcic, L.; Radic, B.; Drmic, D.; Seiwerth, S.; Sikiric, P. Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med. Sci. Monit. Basic Res. 2013, 19, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef]
- National Toxicology Program. Carcinogenesis bioassay of melamine in F344/N rats and B6C3F l Mice. NTP Rep. Melamine 1983, 245, 55–57. [Google Scholar]
- Weil, C.S.; Carpenter, C.P.; Smyth, H.F., Jr. Urinary bladder calculus and tumor response following either repeated feeding of diethylene glycol or calcium oxalate stone implantation. Ind. Med. Surg. 1967, 36, 55–57. [Google Scholar]
- Vasudevan, S.; Laconi, E.; Abanobi, S.E.; Rao, P.M.; Rajalakshmi, S.; Sarma, D.S. Effect of glycine on the induction of orotic aciduria and urinary bladder tumorigenesis in the rat. Toxicol. Pathol. 1987, 15, 194–197. [Google Scholar] [CrossRef]
- Jaffe, V.; Alexander, B.; Price, A.B.; Zanelli, G.D. The induction of bladder cancer in portally diverted rats. Br. J. Cancer 1992, 66, 470–473. [Google Scholar] [CrossRef][Green Version]
- Saclarides, T.J. Rectovaginal fistula. Surg. Clin. N. Am. 2002, 82, 1261–1272. [Google Scholar] [CrossRef]
- Lindberg, J.; Rickardsson, E.; Andersen, M.; Lund, L. Formation of a vesicovaginal fistula in a pig model. Res. Rep. Urol. 2015, 7, 113–116. [Google Scholar] [CrossRef]
- Cogan, S.L.; Paraiso, M.F.; Bedaiwy, M.A. Formation of vesicovaginal fistulas in laparoscopic hysterectomy with electrosurgically induced cystotomy in female mongrel dogs. Am. J. Obstet. Gynecol. 2002, 187, 1510–1513. [Google Scholar] [CrossRef]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded Superior Mesenteric Artery and Vein. Therapy with the Stable Gastric Pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and the permanent occlusion of the superior sagittal sinus in rat. Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell. Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar] [PubMed]
- Luetic, K.; Sucic, M.; Vlainic, J.; Halle, Z.B.; Strinic, D.; Vidovic, T.; Luetic, F.; Marusic, M.; Gulic, S.; Pavelic, T.T.; et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef] [PubMed]
- Amic, F.; Drmic, D.; Bilic, Z.; Krezic, I.; Zizek, H.; Peklic, M.; Klicek, R.; Pajtak, A.; Amic, E.; Vidovic, T.; et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5366–5378. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Milkovic Perisa, M.; Horvat Pavlov, K.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef]
- Masnec, S.; Kokot, A.; Zlatar, M.; Kalauz, M.; Kunjko, K.; Radic, B.; Klicek, R.; Drmic, D.; Lazic, R.; Brcic, L.; et al. Perforating corneal injury in rat and pentadecapeptide BPC 157. Exp. Eye Res. 2015, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Veljaca, M.; Pavić-Sladoljev, D.; Mildner, B.; Brajsa, K.; Krnic, Z.; Bubenik, M.; Stipanicic, S.; Tabak-Slosic, M.; Brnic, L.; Khan, Z.; et al. Safety, tolerability and pharmacokinetics of PL 14736, a novel agent for treatment of ulcerative colitis, in healthy male volunteers. Gut 2003, 51 (Suppl. III), A309. [Google Scholar]
- Ruenzi, M.; Stolte, M.; Veljaca, M.; Oreskovic, K.; Peterson, J. Ulcerative Colitis Study Group. A multicenter, randomized, double blind, placebo controlled phase II study of PL 14736 enema in the treatment of mild-to-moderate ulcerative colitis. Gastroenterology 2005, 128, 584. [Google Scholar]
- Veljaca, M.; Lesch, C.A.; Pllana, R.; Sanchez, B.; Chan, K.; Guglietta, A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J. Pharmacol. Exp. Ther. 1994, 272, 417–422. [Google Scholar]
- Sikiric, P.; Seiwerth, S.; Aralica, G.; Perovic, D.; Staresinic, M.; Anic, T.; Gjurasin, M.; Prkacin, I.; Separovic, J.; Stancic-Rokotov, D.; et al. Therapy effect of antiulcer agents on new chronic cysteamine colon lesion in rat. J. Physiol. Paris 2001, 95, 283–288. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Balen, I.; Aralica, G.; Gjurasin, M.; Komericki, L.; Perovic, D.; Ziger, T.; Anic, T.; et al. Cysteamine-colon and cysteamine-duodenum lesions in rats. Attenuation by gastric pentadecapeptide BPC 157, cimetidine, ranitidine, atropine, omeprazole, sulphasalazine and methylprednisolone. J. Physiol. Paris 2001, 95, 261–270. [Google Scholar] [CrossRef]
- Drmic, D.; Kolenc, D.; Ilic, S.; Bauk, L.; Sever, M.; Zenko Sever, A.; Luetic, K.; Suran, J.; Seiwerth, S.; Sikiric, P. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J. Gastroenterol. 2017, 23, 5304–5312. [Google Scholar] [CrossRef]
- Ilic, S.; Brcic, I.; Mester, M.; Filipovic, M.; Sever, M.; Klicek, R.; Barisic, I.; Radic, B.; Zoricic, Z.; Bilic, V.; et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J. Physiol. Pharmacol. 2009, 60, 107–114. [Google Scholar]
- Ilic, S.; Drmic, D.; Franjic, S.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: Diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011, 88, 535–542. [Google Scholar] [CrossRef]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Brcic, L.; Radic, B.; Djuzel, V.; Blagaic, A.B.; Romic, Z.; Dzidic, S.; et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur. J. Pharmacol. 2011, 667, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Ribarić, J.; Smodiš Škerl, M.; Vlainić, J.; Sikirić, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasic, D.; Zenko Sever, A.; Rasic, F.; Strbe, S.; Rasic, Z.; Djuzel, A.; Duplancic, B.; Boban Blagaic, A.; Skrtic, A.; Seiwerth, S.; et al. Stable Gastric Pentadecapeptide BPC 157 Heals Established Vesicovaginal Fistula and Counteracts Stone Formation in Rats. Biomedicines 2021, 9, 1206. https://doi.org/10.3390/biomedicines9091206
Rasic D, Zenko Sever A, Rasic F, Strbe S, Rasic Z, Djuzel A, Duplancic B, Boban Blagaic A, Skrtic A, Seiwerth S, et al. Stable Gastric Pentadecapeptide BPC 157 Heals Established Vesicovaginal Fistula and Counteracts Stone Formation in Rats. Biomedicines. 2021; 9(9):1206. https://doi.org/10.3390/biomedicines9091206
Chicago/Turabian StyleRasic, Domagoj, Anita Zenko Sever, Fran Rasic, Sanja Strbe, Zarko Rasic, Antonija Djuzel, Bozidar Duplancic, Alenka Boban Blagaic, Anita Skrtic, Sven Seiwerth, and et al. 2021. "Stable Gastric Pentadecapeptide BPC 157 Heals Established Vesicovaginal Fistula and Counteracts Stone Formation in Rats" Biomedicines 9, no. 9: 1206. https://doi.org/10.3390/biomedicines9091206
APA StyleRasic, D., Zenko Sever, A., Rasic, F., Strbe, S., Rasic, Z., Djuzel, A., Duplancic, B., Boban Blagaic, A., Skrtic, A., Seiwerth, S., Sikiric, P., & Sever, M. (2021). Stable Gastric Pentadecapeptide BPC 157 Heals Established Vesicovaginal Fistula and Counteracts Stone Formation in Rats. Biomedicines, 9(9), 1206. https://doi.org/10.3390/biomedicines9091206






