Establishment and Characterization of a Novel Fibroblastic Cell Line (SCI13D) Derived from the Broncho-Alveolar Lavage of a Patient with Fibrotic Hypersensitivity Pneumonitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient, Bronchoalveolar Fluid Collection, and Histological Sample Assessment
2.2. Cell Line Establishment and Culture
2.3. mRNA Extraction and Quantitative Real-Time RT-PCR
2.4. Morphology, Cyto-Fluorographic, and Immunofluorescence Analyses
2.5. Immunocytochemistry of the SCI13D Cell Line
2.6. Proliferation and Growth Kinetic Determination
2.7. Wound Sratch Test
2.8. Determination of the Total Cellular F-Actin with/without Pirfenidone Treatment
2.9. β-Galactosidase Assessment
2.10. Cytogenetics
2.11. Short Tandem Repeat Analysis
2.12. Statistical Analysis
3. Results
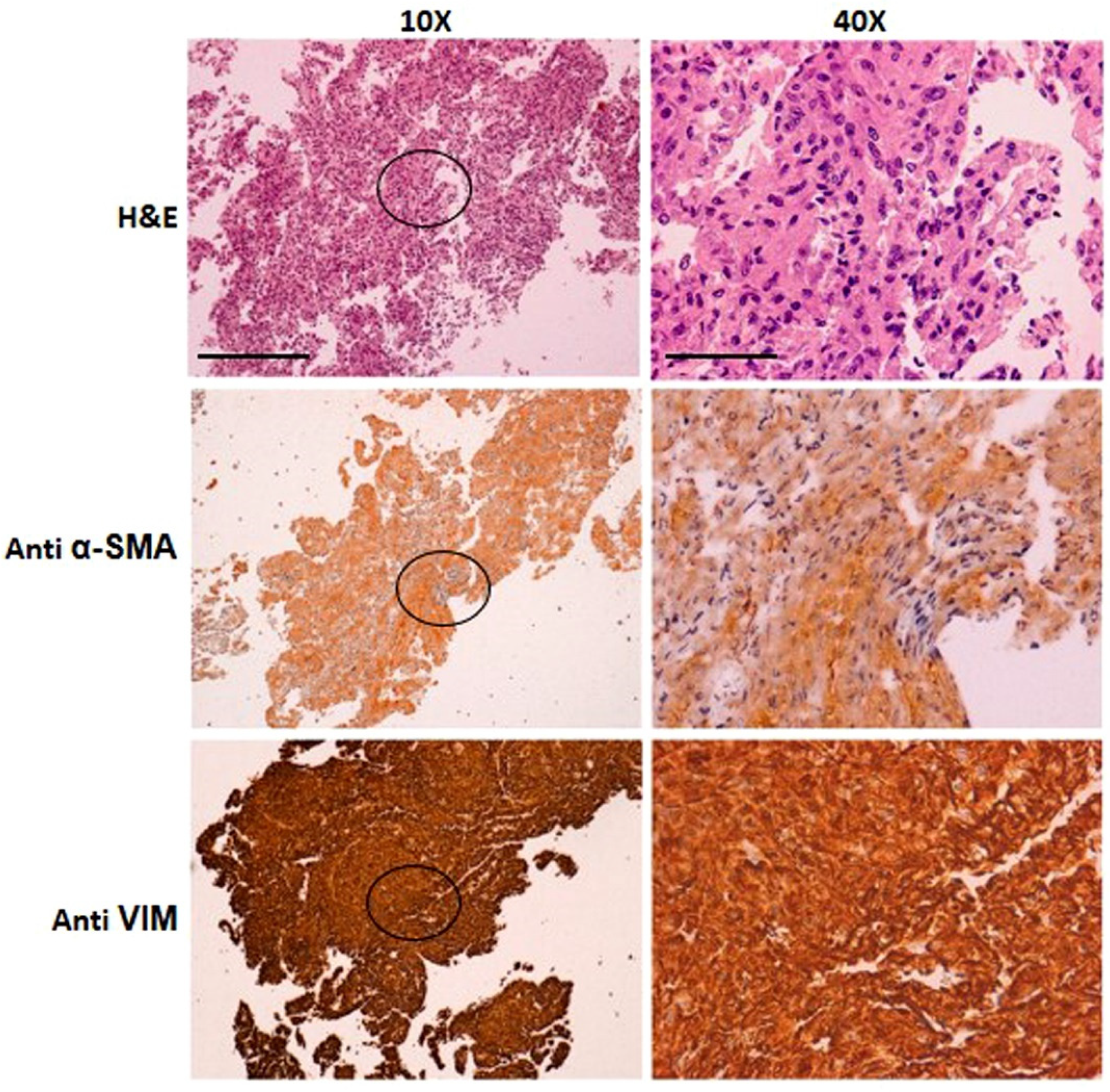
3.1. Patient’s Clinical and Diagnostic Features
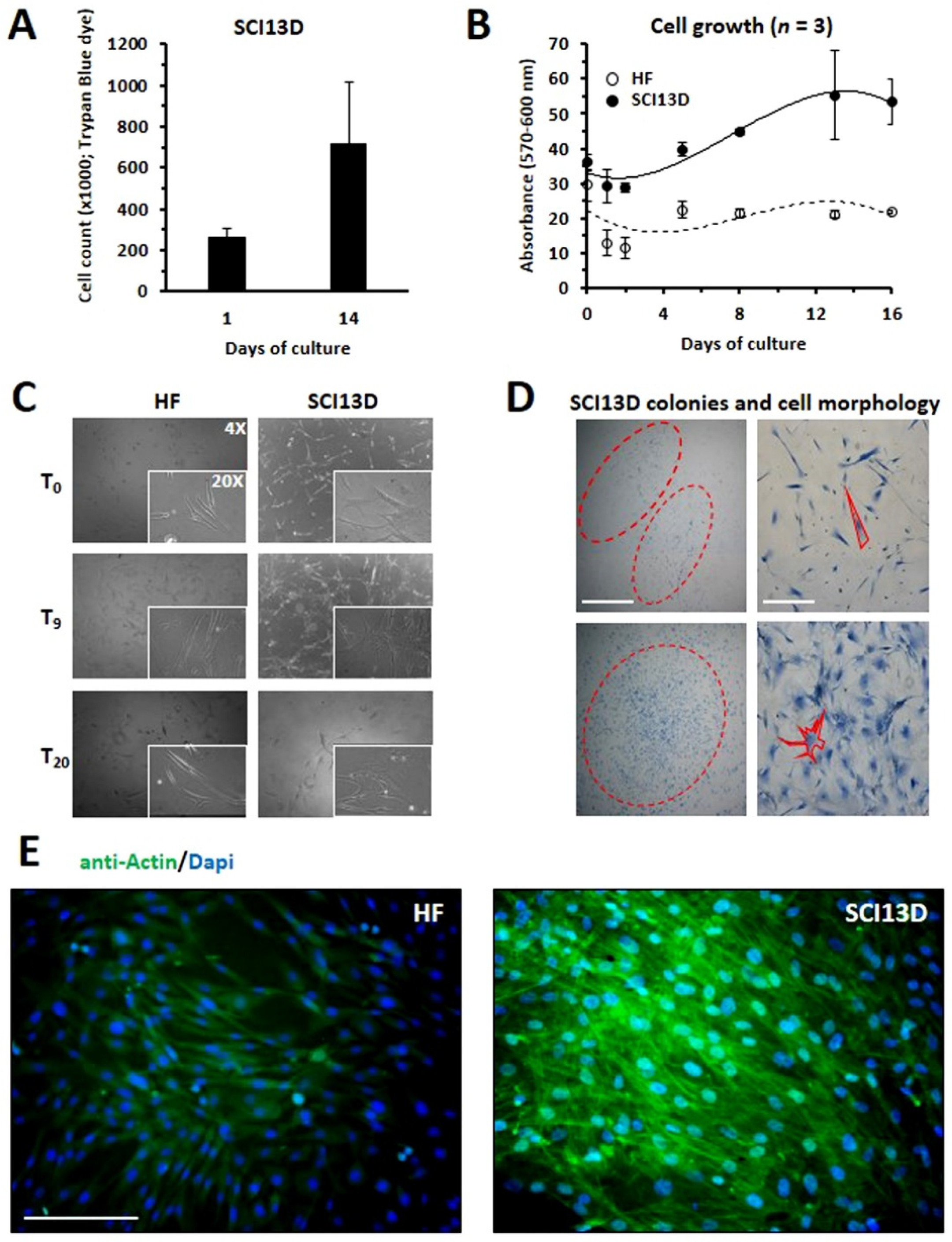
3.2. Establishment of a Unique Fibroblastic Cell Line (SCI13D) from fHP Patient
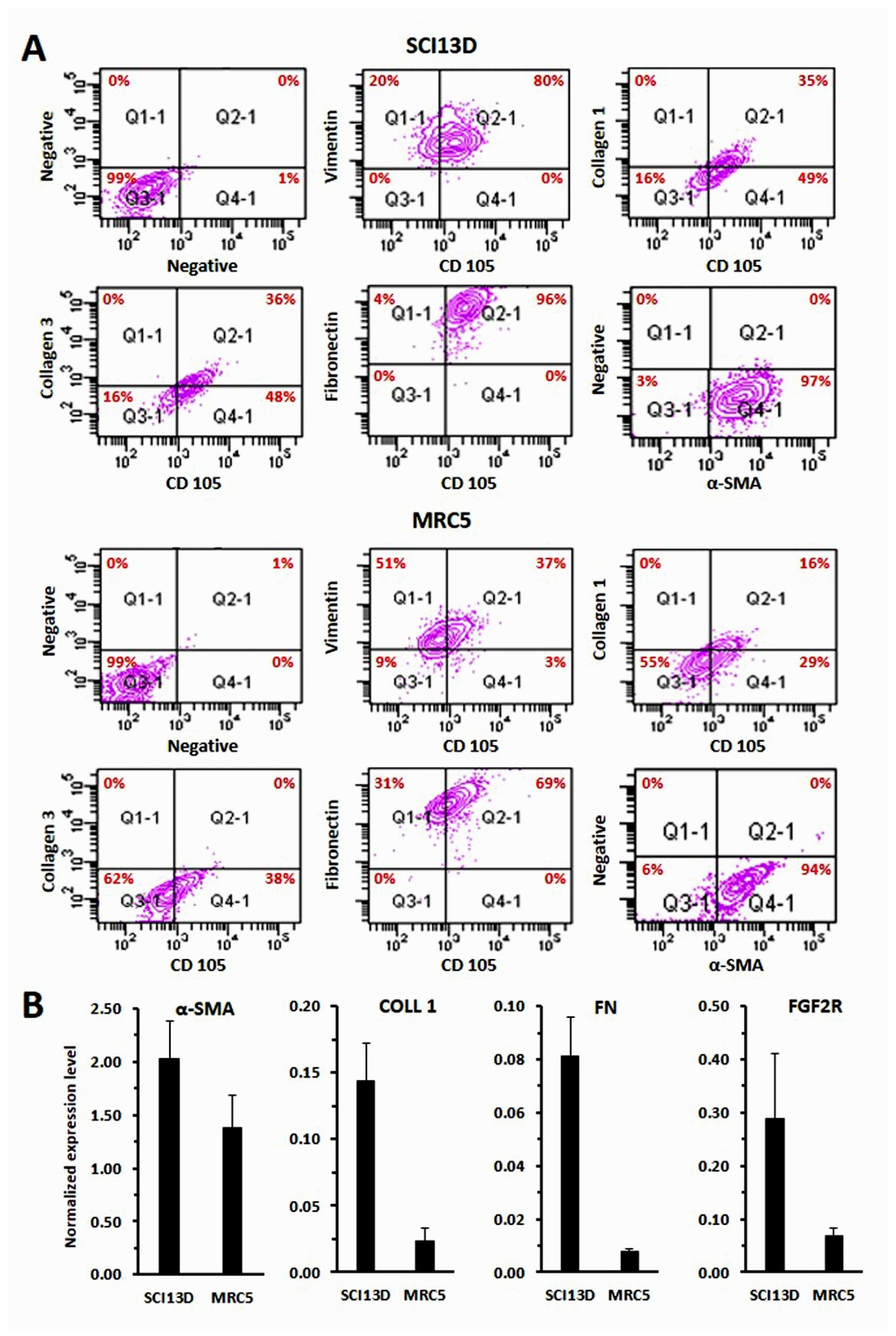
3.3. Immunocytochemical Characteristic of the SCI13D Cell Line
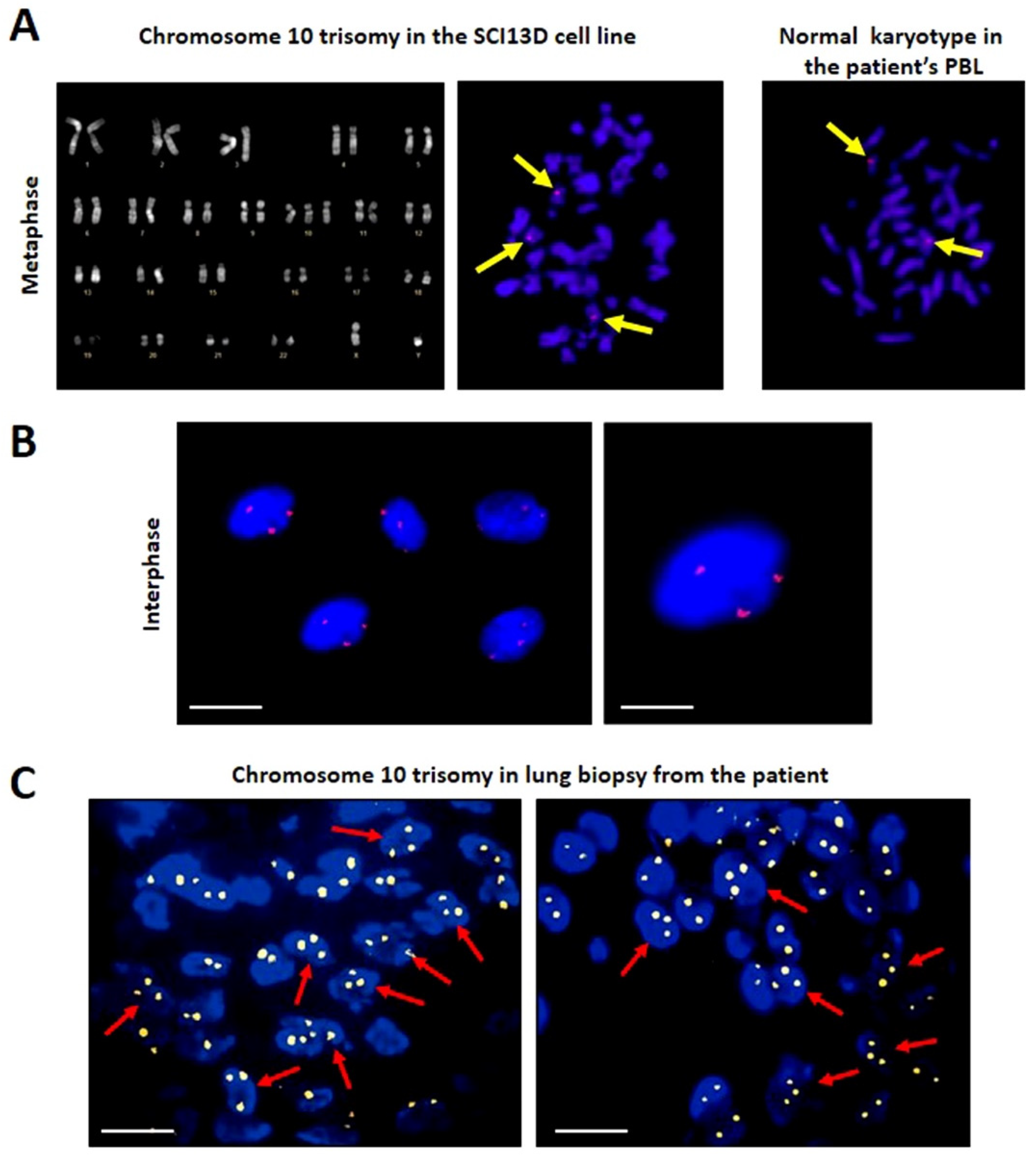
3.4. Karyotype and FISH
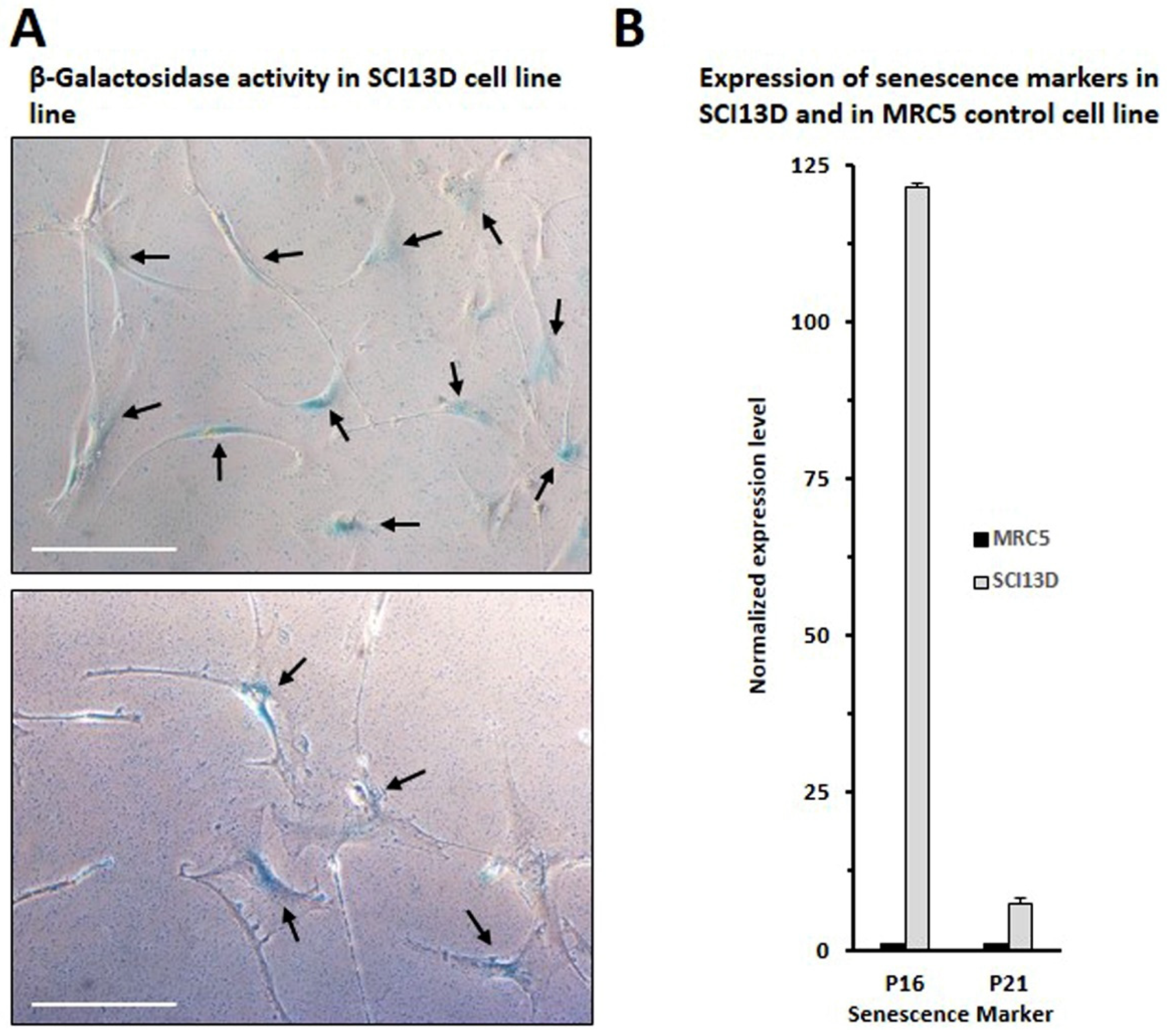
3.5. Authenticity of the SCI13D Cell Line
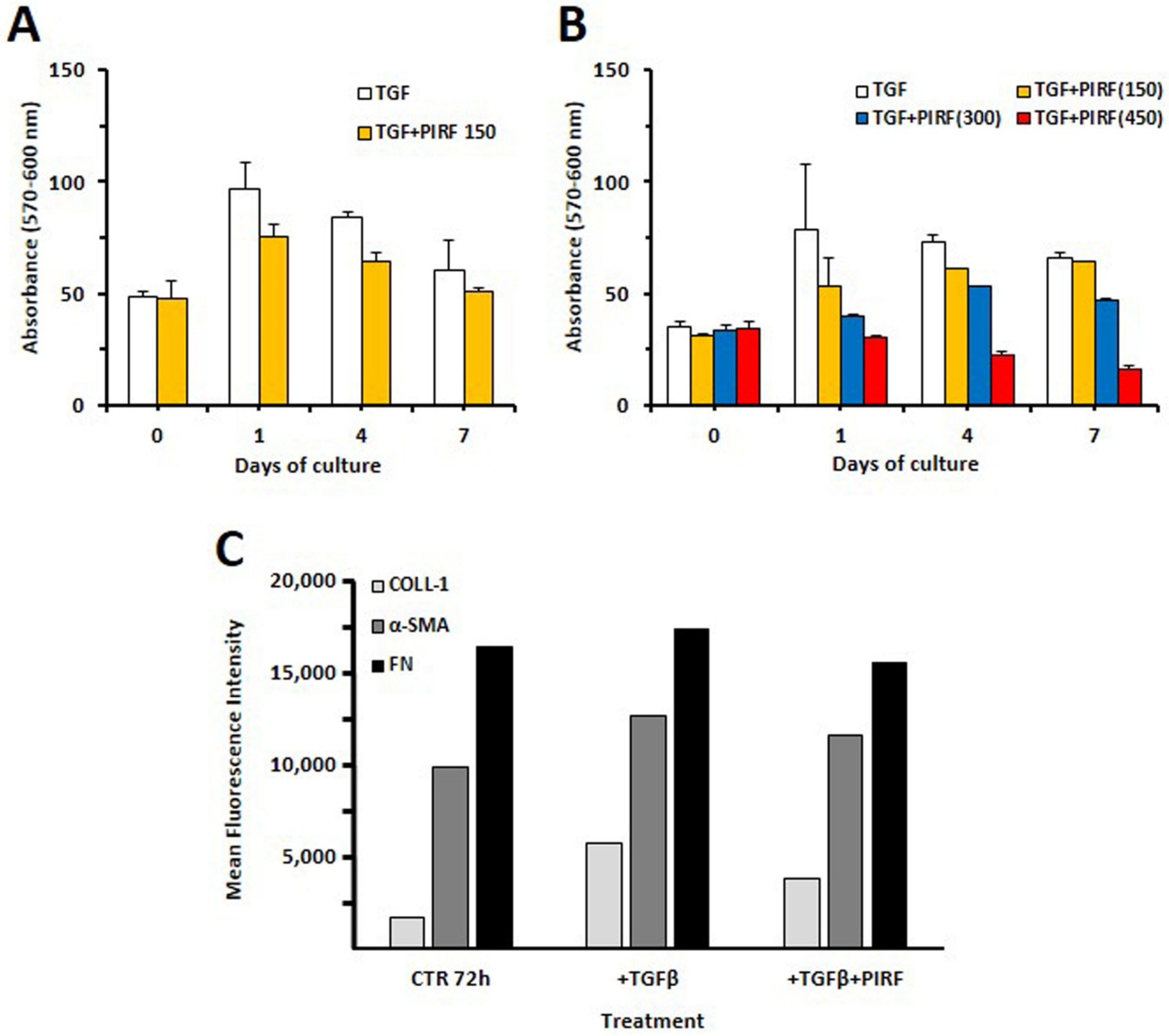
3.6. Inhibition of Proliferation by Antifibrotic Therapy
3.7. Pirfenidone Slightly Inhibited Type 1 Collagen Expression in TGF-β-Treated SCI13D Cells
3.8. Inhibition of SCI13D Migration by Pirfenidone
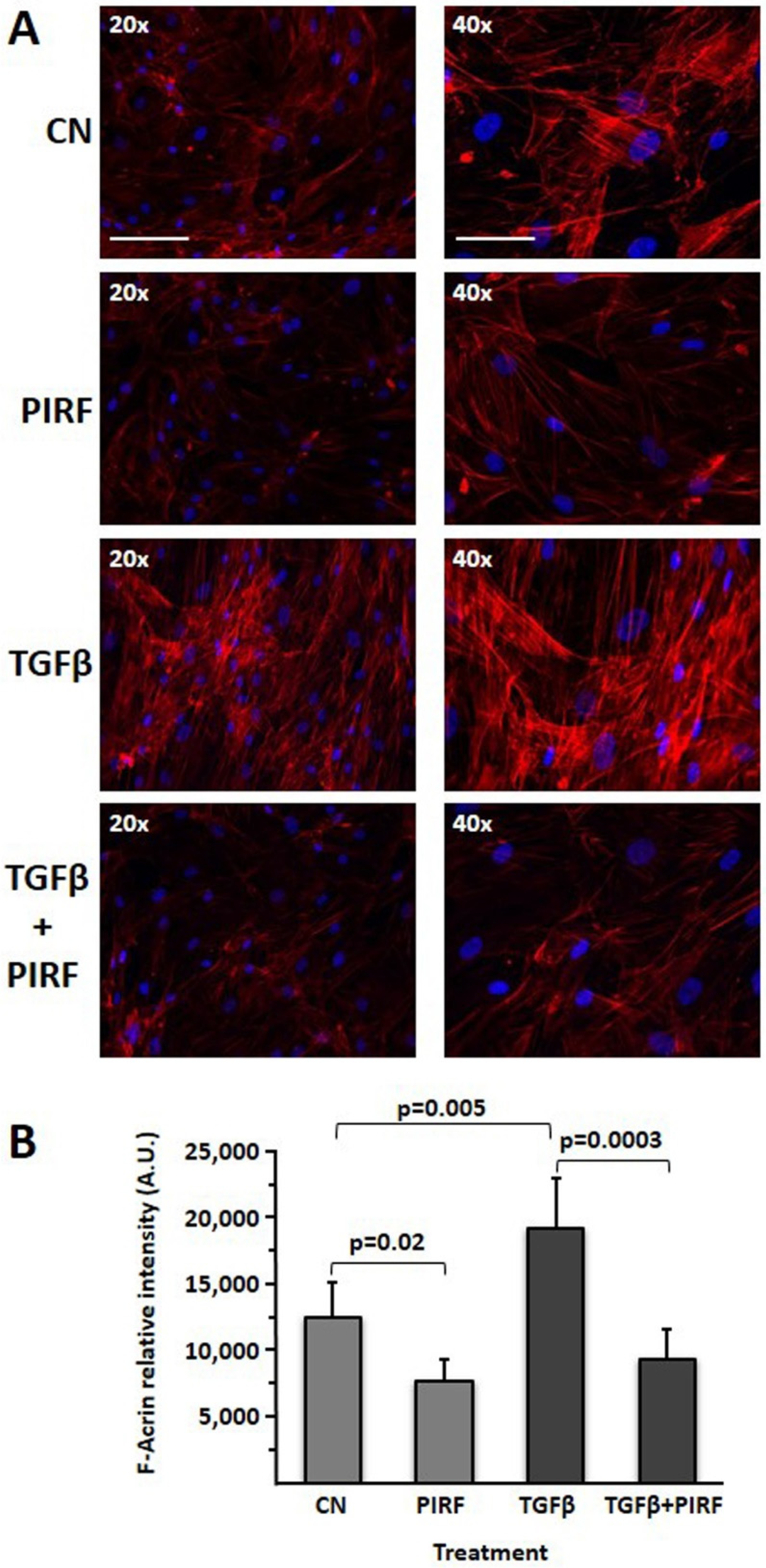
3.9. Reduction of the TGFβ-Induced Profibrotic Phenotype by Pirfenidone in SCI13D Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Morisset, J.; Johannson, K.A.; Jones, K.D.; Wolters, P.J.; Collard, H.R.; Walsh, S.L.F.; Ley, B.; Collaborators, H.P.D. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: An international modified Delphi survey. Am. J. Respir. Crit. Care Med. 2018, 197, 1036–1044. [Google Scholar] [CrossRef]
- Varone, F.; Iovene, B.; Sgalla, G.; Calvello, M.; Calabrese, A.; Larici, A.R.; Richeldi, L. Fibrotic hypersensitivity pneumonitis: Diagnosis and management. Lung 2020, 198, 429–440. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of hypersensitivity pneumonitis in adults. An Official ATS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Costabel, U.; Miyazaki, Y.; Pardo, A.; Koschel, D.; Bonella, F.; Spagnolo, P.; Guzman, J.; Ryerson, C.J.; Selman, M. Hypersensitivity pneumonitis. Nat. Rev. Dis. Primers 2020, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, P.; Rask-Andersen, A.; Hoglund, S.; Kolmodin-Hedman, B.; Read Guernsey, J. Incidence of organic dust toxic syndrome and allergic alveolitis in Swedish farmers. Int. Arch. Allergy Appl. Immunol. 1988, 87, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.E.; Baldwin, C.I.; Allen, A.; Todd, A.; Bourke, S.J. Pigeon fanciers’ lung: A complex disease? Clin. Exp. Allergy 1999, 29, 166–175. [Google Scholar] [CrossRef] [PubMed]
- May, J.J.; Stallones, L.; Darrow, D.; Pratt, D.S. Organic dust toxicity (pulmonary mycotoxicosis) associated with silo unloading. Thorax 1986, 41, 919–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, H.; Kaproth-Joslin, K.; Hobbs, S.K. Hypersensitivity pneumonitis. Semin. Roentgenol. 2019, 54, 37–43. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Donnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuramochi, J.; Inase, N.; Miyazaki, Y.; Kawachi, H.; Takemura, T.; Yoshizawa, Y. Lung cancer in chronic hypersensitivity pneumonitis. Respiration 2011, 82, 263–267. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nagai, K.; Yokose, T.; Yoshida, J.; Nishimura, M.; Takahashi, K.; Suzuki, K.; Kakinuma, R.; Nishiwaki, Y. Clinicopathological characteristics of surgically resected lung cancer associated with idiopathic pulmonary fibrosis. J. Surg. Oncol. 2001, 76, 53–57. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.S.; Shim, T.S.; Lim, C.M.; Koh, Y.; Lee, S.D.; Kim, W.S.; Kim, W.D.; Lee, J.S.; Song, K.S. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2001, 17, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Barisione, E.; Salio, M.; Romagnoli, M.; Pratico, A.; Bargagli, E.; Corbetta, L. Competence in transbronchial cryobiopsy. Panminerva Med. 2019, 61, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Shyti, G.; Rosalbino, F.; Maccio, D.; Scarabelli, L.; Quarto, R.; Giannoni, P. A comparative evaluation between new ternary zirconium alloys as alternative metals for orthopedic and dental prosthetic devices. Int. J. Artif. Organs 2014, 37, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Quercioli, A.; Mach, F.; Bertolotto, M.; Lenglet, S.; Vuilleumier, N.; Galan, K.; Pagano, S.; Braunersreuther, V.; Pelli, G.; Pistoia, V.; et al. Receptor activator of NF- kappaB ligand (RANKL) increases the release of neutrophil products associated with coronary vulnerability. Thromb. Haemost. 2012, 107, 124–139. [Google Scholar] [PubMed] [Green Version]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef]
- Jacobson, K.; Thompson, A.; Browne, G.; Shasserre, C.; Seelig, S.A.; King, W. Automation of fluorescence in situ hybridization pretreatment: A comparative study of different sample types. Mol. Diagn. 2000, 5, 209–220. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.Y.; El-Baz, L.M.; House, S.; Cilvik, S.N.; Dorry, S.J.; Shoukry, N.M.; Salem, M.L.; Hafez, H.S.; Dulin, N.O.; Ornitz, D.M.; et al. Fibroblast growth factor 2 decreases bleomycin-induced pulmonary fibrosis and inhibits fibroblast collagen production and myofibroblast differentiation. J. Pathol. 2018, 246, 54–66. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Meng, X.; Zhang, K.; Li, X.; Wang, C.; Xiang, Z.; Hu, K.; Han, X. Inhibition of Wnt/beta-catenin signaling suppresses bleomycin-induced pulmonary fibrosis by attenuating the expression of TGF-beta1 and FGF-2. Exp. Mol. Pathol. 2016, 101, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Morizumi, S.; Sato, S.; Koyama, K.; Okazaki, H.; Chen, Y.; Goto, H.; Kagawa, K.; Ogawa, H.; Nishimura, H.; Kawano, H.; et al. Blockade of pan-fibroblast growth factor receptors mediates bidirectional effects in lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 63, 317–326. [Google Scholar] [CrossRef]
- Alvarez, D.; Cardenes, N.; Sellares, J.; Bueno, M.; Corey, C.; Hanumanthu, V.S.; Peng, Y.; D’Cunha, H.; Sembrat, J.; Nouraie, M.; et al. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L1164–L1173. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, Z. Fibroblast senescence in idiopathic pulmonary fibrosis. Front. Cell Dev. Biol. 2020, 8, 593283. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.J.; Tarmey, T.; Ferreira, A.; Mangaonkar, A.A.; Ferrer, A.; Patnaik, M.M.; Wylam, M.E.; Jenkins, S.M.; Spears, G.M.; Yi, E.S.; et al. Pathology, radiology, and genetics of interstitial lung disease in patients with shortened telomeres. Am. J. Surg. Pathol. 2021, 45, 871–884. [Google Scholar]
- Newton, C.A.; Batra, K.; Torrealba, J.; Kozlitina, J.; Glazer, C.S.; Aravena, C.; Meyer, K.; Raghu, G.; Collard, H.R.; Garcia, C.K. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur. Respir. J. 2016, 48, 1710–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golec, M.; Wielscher, M.; Lemieszek, M.K.; Vierlinger, K.; Skorska, C.; Huetter, S.; Sitkowska, J.; Mackiewicz, B.; Gora-Florek, A.; Ziesche, R.; et al. Middle age enhances expression of innate immunity genes in a female mouse model of pulmonary fibrosis. Biogerontology 2017, 18, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Lopez-Otin, C.; Pardo, A. Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis. Eur. Respir. J. 2016, 48, 538–552. [Google Scholar] [CrossRef]
- Vasakova, M.; Selman, M.; Morell, F.; Sterclova, M.; Molina-Molina, M.; Raghu, G. Hypersensitivity pneumonitis: Current concepts of pathogenesis and potential targets for treatment. Am. J. Respir. Crit. Care Med. 2019, 200, 301–308. [Google Scholar] [CrossRef]
- Hwang, S.; Cavaliere, P.; Li, R.; Zhu, L.J.; Dephoure, N.; Torres, E.M. Consequences of aneuploidy in human fibroblasts with trisomy 21. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Xiao, L.; Du, Y.; Shen, Y.; He, Y.; Zhao, H.; Li, Z. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front. Biosci. 2012, 17, 2667–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, S.M.; Penke, L.R.; King, D.; Pham, T.X.; Ligresti, G.; Peters-Golden, M. Myofibroblast dedifferentiation proceeds via distinct transcriptomic and phenotypic transitions. JCI Insight 2021, 6, e144799. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Muraglia, A.; Campanile, G.; Cancedda, R.; Quarto, R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 1997, 138, 4456–4462. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Maher, T.M. Chronic hypersensitivity pneumonitis; an enigmatic and frequently fatal disease. Eur. Respir. Rev. 2020, 29. [Google Scholar] [CrossRef]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef] [PubMed]


| Cells Assessed | ||
|---|---|---|
| STR Locus | PBL | SCI13D |
| D5S818 | 11,12 | 11,12 |
| D13S17 | 9,11 | 9,11 |
| D7S820 | 11 | 11 |
| D16S539 | 9,14 | 9,14 |
| VWA | 16 | 16 |
| TH01 | 6 | 6 |
| AM | x, y | x, y |
| TPOX | 9,10 | 9,10 |
| CSF1PO | 11,12 | 11,12 |
| D21S11 | 27,30 | 27,30 |
| D3S1358 | 16 | 16 |
| D18S51 | 13,16 | 13,16 |
| Penta E | 7,17 | 7,17 |
| Penta D | 9,11 | 9,11 |
| D8S1179 | 14,15 | 14,15 |
| FGA | 19,22 | 19,22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoni, P.; Grosso, M.; Fugazza, G.; Nizzari, M.; Capra, M.C.; Bianchi, R.; Fiocca, R.; Salvi, S.; Montecucco, F.; Bertolotto, M.; et al. Establishment and Characterization of a Novel Fibroblastic Cell Line (SCI13D) Derived from the Broncho-Alveolar Lavage of a Patient with Fibrotic Hypersensitivity Pneumonitis. Biomedicines 2021, 9, 1193. https://doi.org/10.3390/biomedicines9091193
Giannoni P, Grosso M, Fugazza G, Nizzari M, Capra MC, Bianchi R, Fiocca R, Salvi S, Montecucco F, Bertolotto M, et al. Establishment and Characterization of a Novel Fibroblastic Cell Line (SCI13D) Derived from the Broncho-Alveolar Lavage of a Patient with Fibrotic Hypersensitivity Pneumonitis. Biomedicines. 2021; 9(9):1193. https://doi.org/10.3390/biomedicines9091193
Chicago/Turabian StyleGiannoni, Paolo, Marco Grosso, Giuseppina Fugazza, Mario Nizzari, Maria Cristina Capra, Rita Bianchi, Roberto Fiocca, Sandra Salvi, Fabrizio Montecucco, Maria Bertolotto, and et al. 2021. "Establishment and Characterization of a Novel Fibroblastic Cell Line (SCI13D) Derived from the Broncho-Alveolar Lavage of a Patient with Fibrotic Hypersensitivity Pneumonitis" Biomedicines 9, no. 9: 1193. https://doi.org/10.3390/biomedicines9091193
APA StyleGiannoni, P., Grosso, M., Fugazza, G., Nizzari, M., Capra, M. C., Bianchi, R., Fiocca, R., Salvi, S., Montecucco, F., Bertolotto, M., Fais, F., Salio, M., Barisione, E., & de Totero, D. (2021). Establishment and Characterization of a Novel Fibroblastic Cell Line (SCI13D) Derived from the Broncho-Alveolar Lavage of a Patient with Fibrotic Hypersensitivity Pneumonitis. Biomedicines, 9(9), 1193. https://doi.org/10.3390/biomedicines9091193









