Analgesic and Anti-Inflammatory Effects of the Synthetic Neurosteroid Analogue BNN27 during CFA-Induced Hyperalgesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
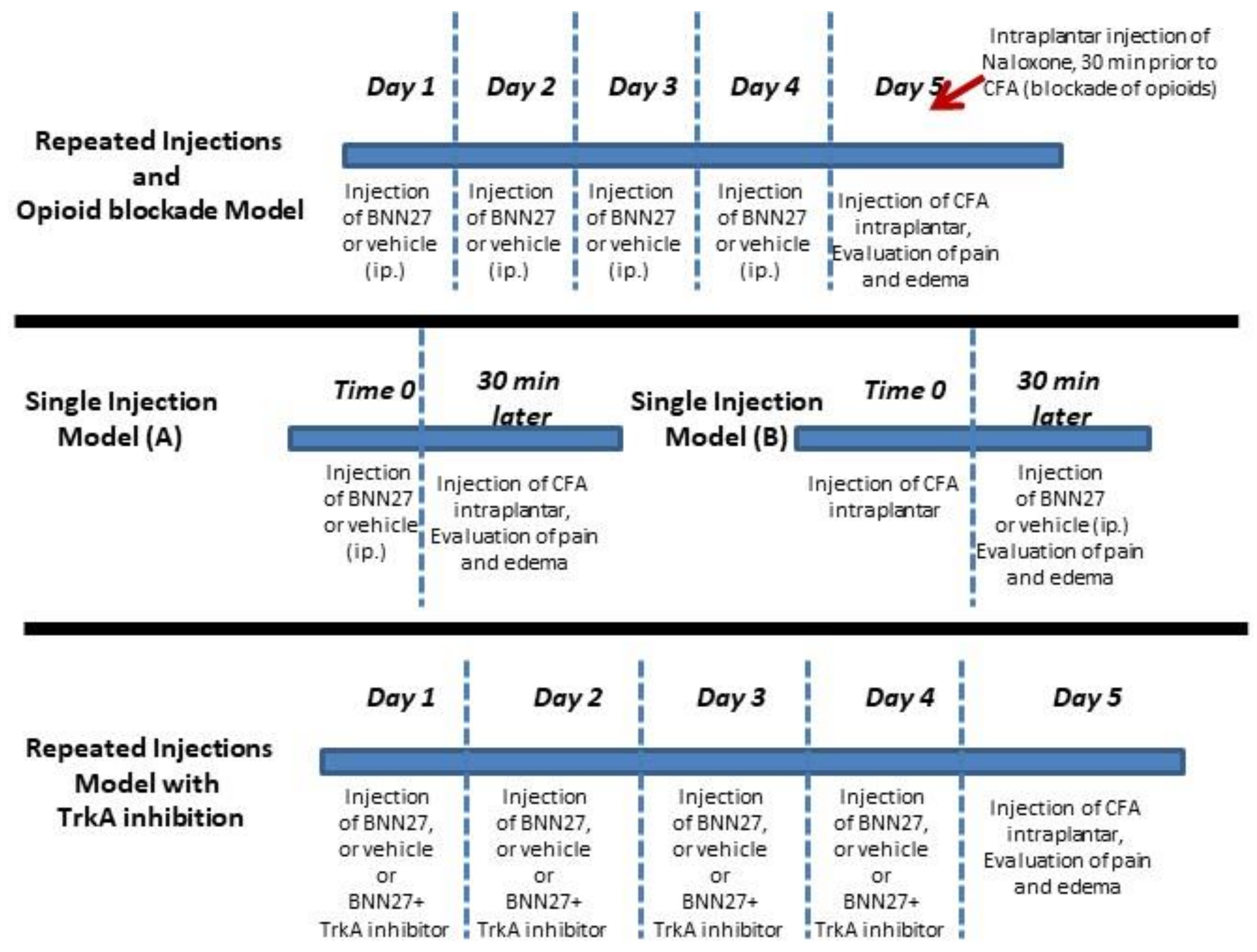
2.2. Animal Treatment
2.3. Behavioral Testing
2.4. Measurement of Corticosterone
2.5. Histological Studies
2.6. Myeloperoxidase (MPO) Assay
2.7. Measurement of Cytokines
2.8. Measurement of β-Endorphin
2.9. Quantitative Real-Time RT-PCR
2.10. Western Blot
2.11. Statistical Analysis
3. Results
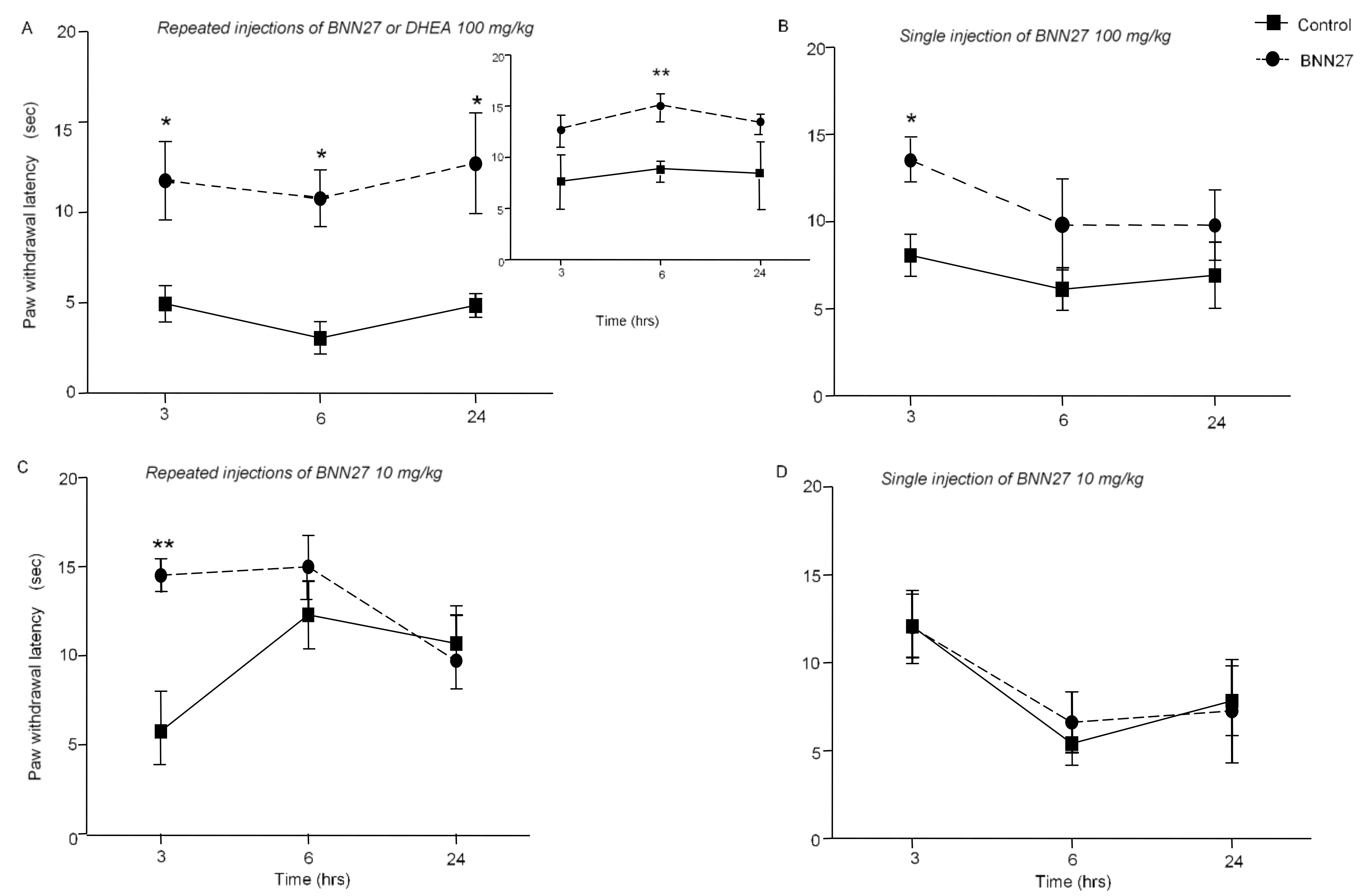
3.1. BNN27 Induced Analgesia in Mice under Inflammatory Conditions
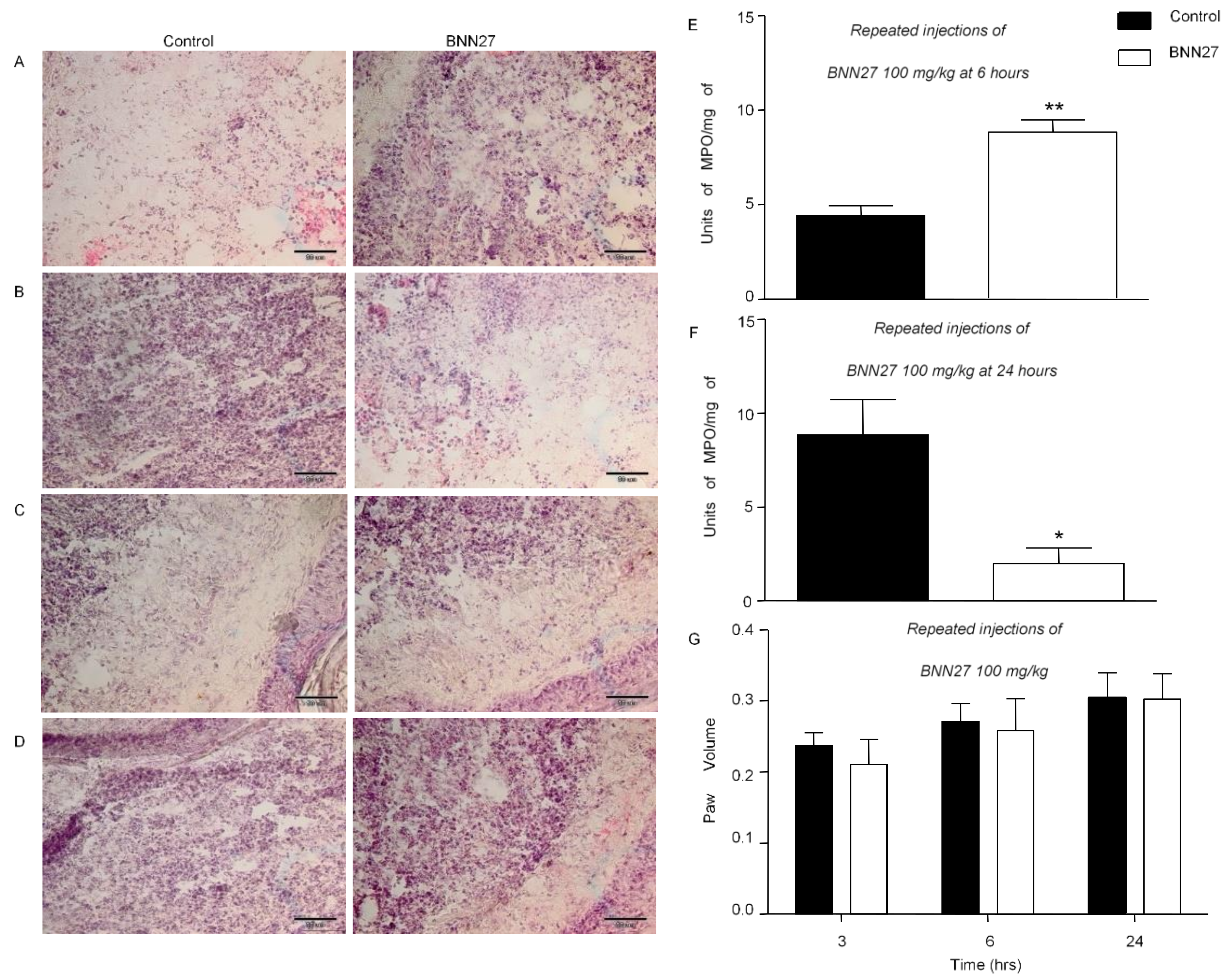
3.2. BNN27 Affected Paw Inflammatory Response
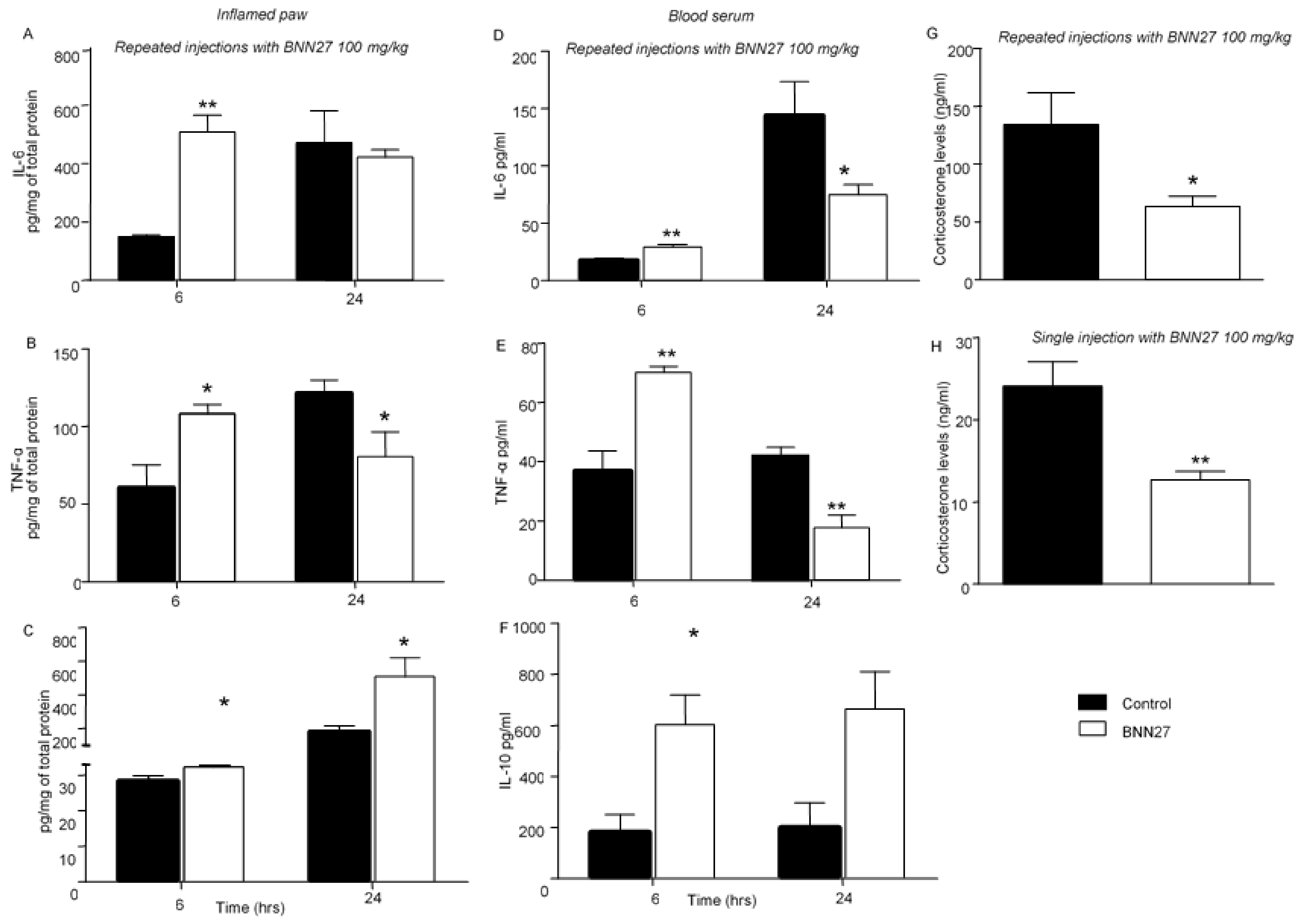
3.3. BNN27 Affected the Production and Release of Cytokines in the Inflamed Paw and Changed Their Plasma Levels
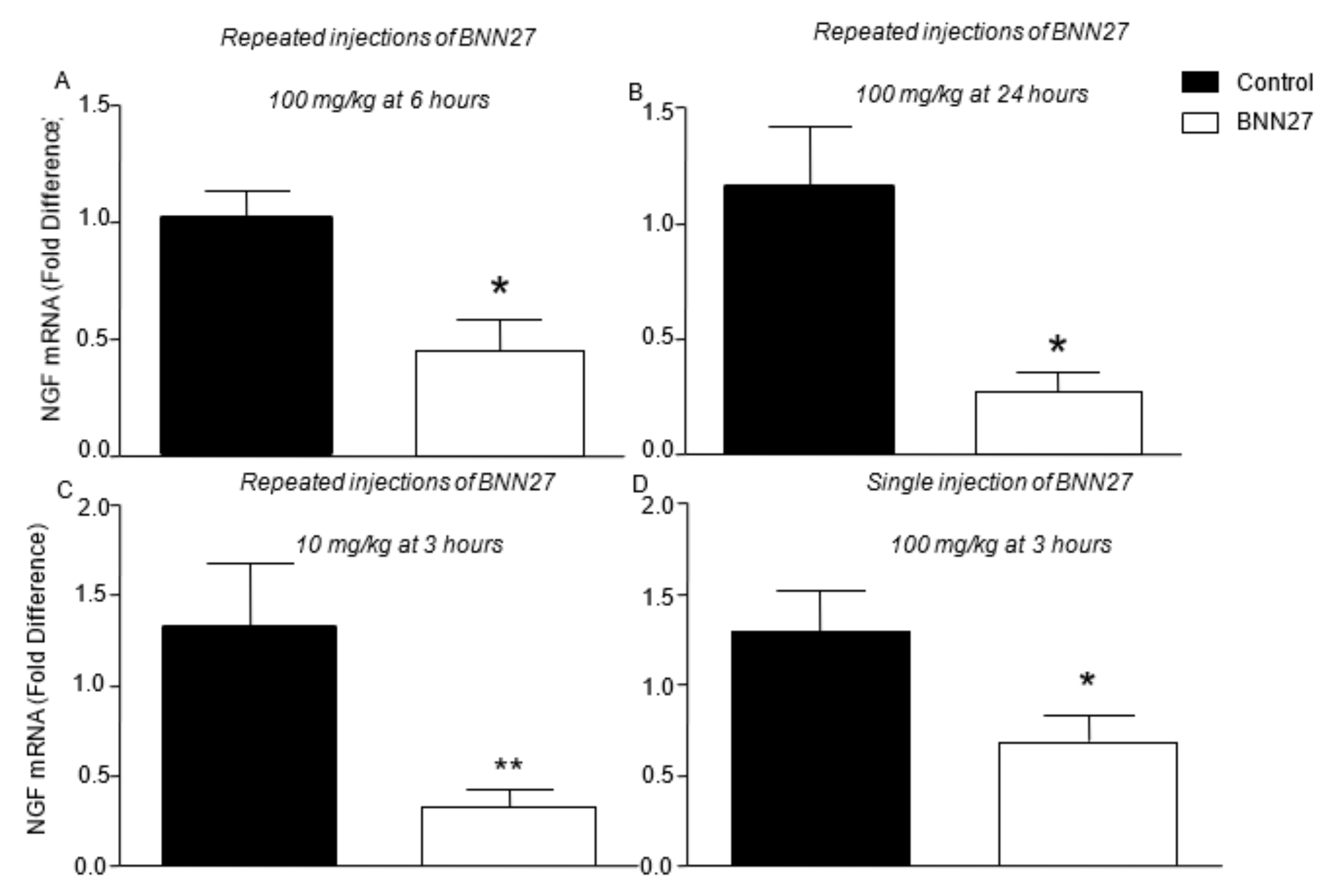
3.4. BNN27 Affects the Production of NGF and iNOS in the Inflamed Paw
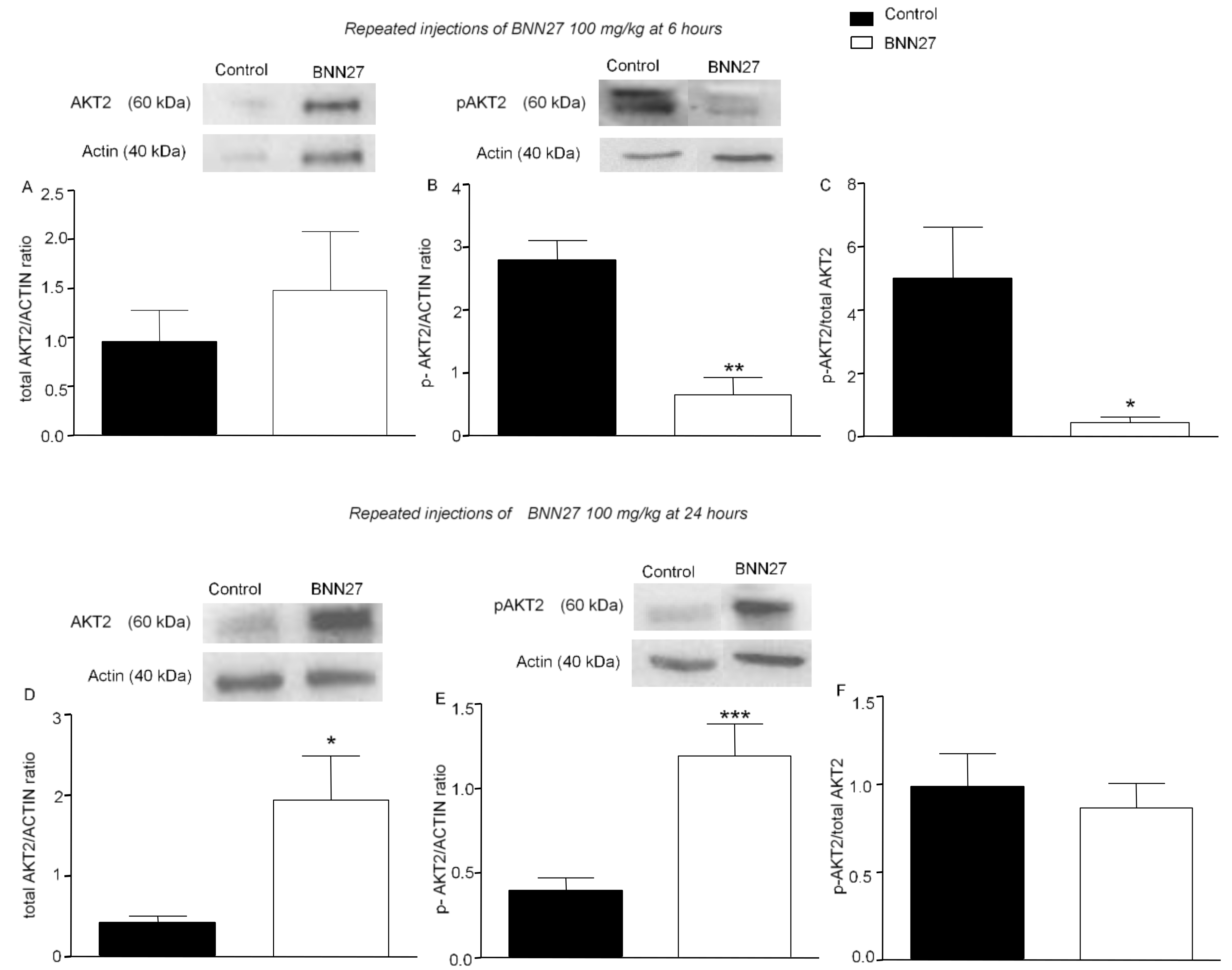
3.5. BNN27 Affects AKT Signaling Pathway
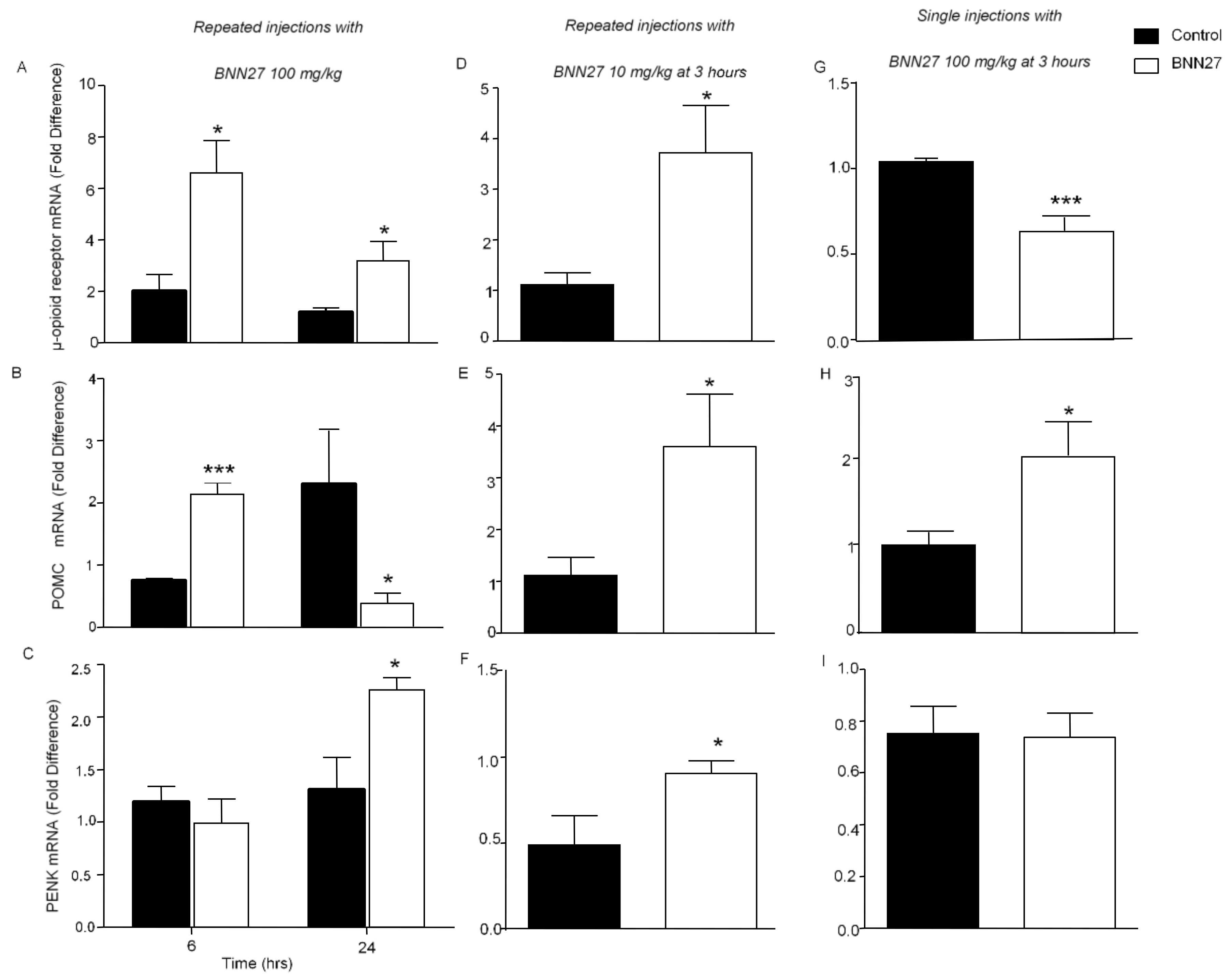
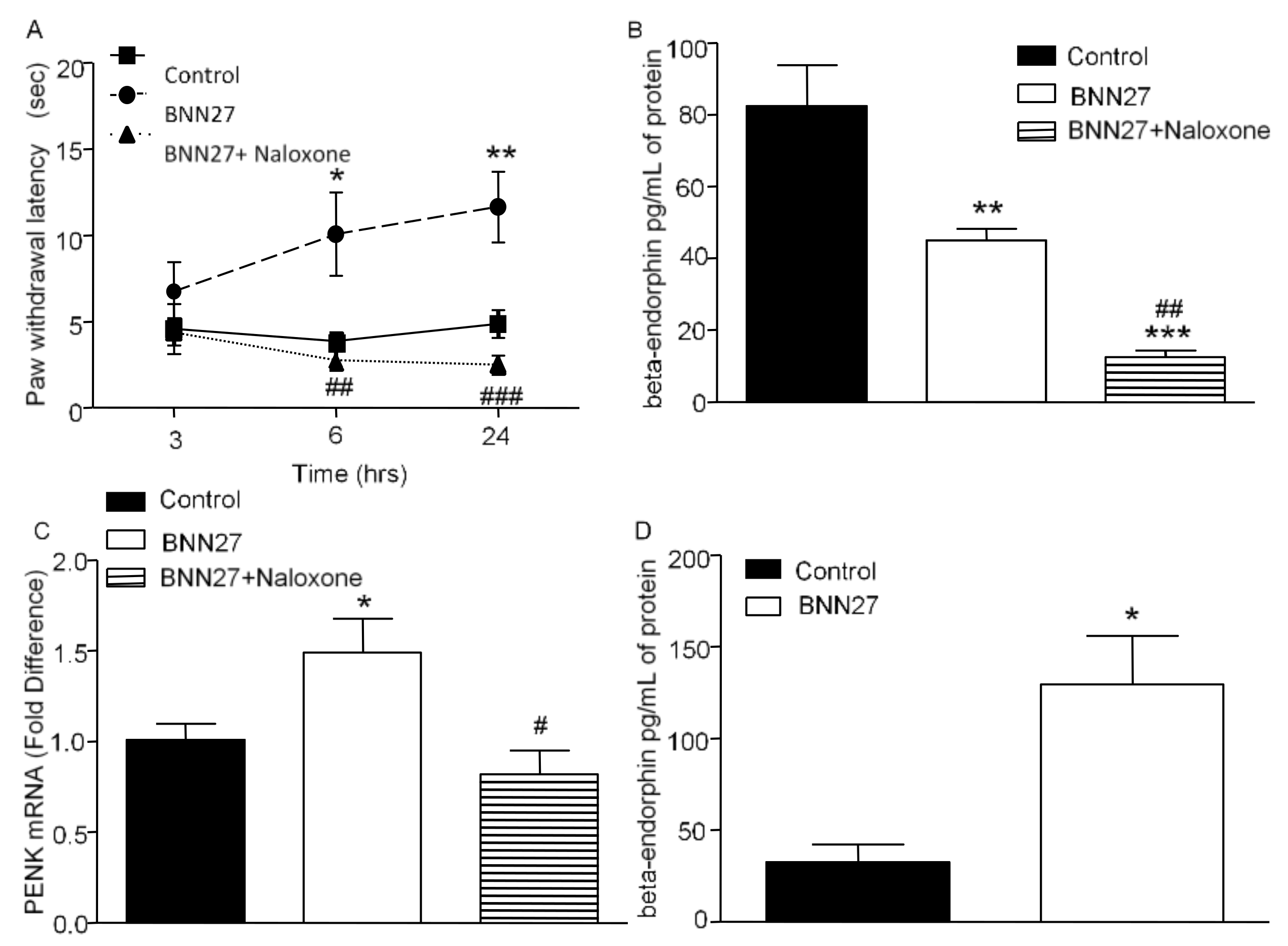
3.6. Effect of BNN27 on the Opioid System
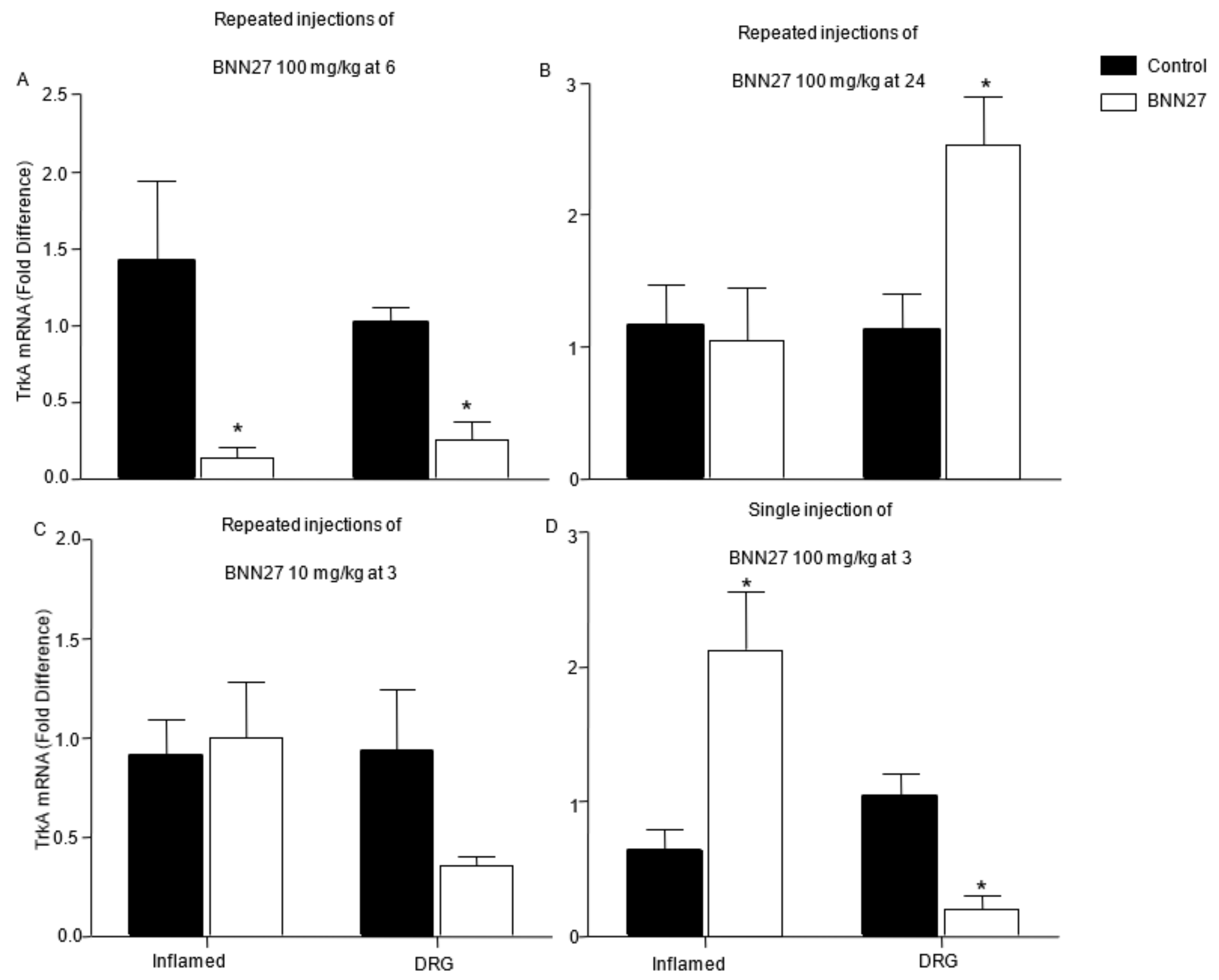
3.7. Effect of BNN27 on NGF and Its Receptors in Dorsal Root Ganglia (DRG)
3.8. Effect of BNN27 Following Inhibition of TrkA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, C.J. What is this thing called pain? J. Clin. Investig. 2010, 120, 3742–3744. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Leick, M.; Azcutia, V.; Newton, G.; Luscinskas, F.W. Leukocyte recruitment in inflammation: Basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 2014, 355, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Oka, K.; Hosoi, M.; Hori, T. Intracerebroventricular injection of interleukin-6 induces thermal hyperalgesia in rats. Brain Res. 1995, 692, 123–128. [Google Scholar] [CrossRef]
- Opree, A.; Kress, M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 2000, 20, 6289–6293. [Google Scholar] [CrossRef]
- Watkins, L.R.; Goehler, L.E.; Relton, J.; Brewer, M.T.; Maier, S.F. Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain Res. 1995, 692, 244–250. [Google Scholar] [CrossRef]
- Watkins, L.R.; Wiertelak, E.P.; Goehler, L.E.; Smith, K.P.; Martin, D.; Maier, S.F. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994, 654, 15–26. [Google Scholar] [CrossRef]
- Li, C.; Deng, T.; Shang, Z.; Wang, D.; Xiao, Y. Blocking TRPA1 and TNF-alpha Signal Improves Bortezomib-Induced Neuropathic Pain. Cell Physiol. Biochem. 2018, 51, 2098–2110. [Google Scholar] [CrossRef]
- Hao, Y.; Niu, H.; An, S.; Wang, M.; Wang, Z. Downregulation of iNOS, IL-1beta, and P2X7 Expression in Mast Cells via Activation of PAR4 Contributes to the Inhibition of Visceral Hyperalgesia in Rats. J. Immunol. Res. 2018, 2018, 3256908. [Google Scholar] [CrossRef]
- Jeanjean, A.P.; Moussaoui, S.M.; Maloteaux, J.M.; Laduron, P.M. Interleukin-1 beta induces long-term increase of axonally transported opiate receptors and substance P. Neuroscience 1995, 68, 151–157. [Google Scholar] [CrossRef]
- Uceyler, N.; Valenza, R.; Stock, M.; Schedel, R.; Sprotte, G.; Sommer, C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006, 54, 2656–2664. [Google Scholar] [CrossRef]
- Stein, C. Targeting pain and inflammation by peripherally acting opioids. Front. Pharmacol. 2013, 4, 123. [Google Scholar] [CrossRef]
- Rittner, H.L.; Brack, A.; Machelska, H.; Mousa, S.A.; Bauer, M.; Schafer, M.; Stein, C. Opioid peptide-expressing leukocytes: Identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology 2001, 95, 500–508. [Google Scholar] [CrossRef]
- Machelska, H. Functional evidence of pain control by the immune system. Adv. Exp. Med. Biol. 2003, 521, 88–97. [Google Scholar]
- Machelska, H.; Brack, A.; Mousa, S.A.; Schopohl, J.K.; Rittner, H.L.; Schafer, M.; Stein, C. Selectins and integrins but not platelet-endothelial cell adhesion molecule-1 regulate opioid inhibition of inflammatory pain. Br. J. Pharmacol. 2004, 142, 772–780. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Tsatsanis, C.; Dermitzaki, E.; Alexaki, V.I.; Castanas, E.; Margioris, A.N.; Gravanis, A. Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 8209–8214. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Remboutsika, E.; Margioris, A.N.; Gravanis, A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol. Metab. 2008, 19, 300–307. [Google Scholar] [CrossRef]
- Mensah-Nyagan, A.G.; Kibaly, C.; Schaeffer, V.; Venard, C.; Meyer, L.; Patte-Mensah, C. Endogenous steroid production in the spinal cord and potential involvement in neuropathic pain modulation. J. Steroid Biochem. Mol. Biol. 2008, 109, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Poisbeau, P.; Patte-Mensah, C.; Keller, A.F.; Barrot, M.; Breton, J.D.; Luis-Delgado, O.E.; Freund-Mercier, M.J.; Mensah-Nyagan, A.G.; Schlichter, R. Inflammatory pain upregulates spinal inhibition via endogenous neurosteroid production. J. Neurosci. 2005, 25, 11768–11776. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, I.; Charalampopoulos, I.; Alexaki, V.I.; Avlonitis, N.; Pediaditakis, I.; Efstathopoulos, P.; Calogeropoulou, T.; Castanas, E.; Gravanis, A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol. 2011, 9, e1001051. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, I.; Iliopoulos, I.; Theologidis, I.; Delivanoglou, N.; Margioris, A.N.; Charalampopoulos, I.; Gravanis, A. Dehydroepiandrosterone: An ancestral ligand of neurotrophin receptors. Endocrinology 2015, 156, 16–23. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Fodelianaki, G.; Neuwirth, A.; Mund, C.; Kourgiantaki, A.; Ieronimaki, E.; Lyroni, K.; Troullinaki, M.; Fujii, C.; Kanczkowski, W.; et al. DHEA inhibits acute microglia-mediated inflammation through activation of the TrkA-Akt1/2-CREB-Jmjd3 pathway. Mol. Psychiatry 2018, 23, 1410–1420. [Google Scholar] [CrossRef]
- Woolf, C.J.; Safieh-Garabedian, B.; Ma, Q.P.; Crilly, P.; Winter, J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994, 62, 327–331. [Google Scholar] [CrossRef]
- Jing, Y.Y.; Wang, J.Y.; Li, X.L.; Wang, Z.H.; Pei, L.; Pan, M.M.; Dong, X.P.; Fan, G.X.; Yuan, Y.K. Nerve growth factor of red nucleus involvement in pain induced by spared nerve injury of the rat sciatic nerve. Neurochem. Res. 2009, 34, 1612–1618. [Google Scholar] [CrossRef]
- Ugolini, G.; Marinelli, S.; Covaceuszach, S.; Cattaneo, A.; Pavone, F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 2985–2990. [Google Scholar] [CrossRef]
- Khodorova, A.; Nicol, G.D.; Strichartz, G. The TrkA receptor mediates experimental thermal hyperalgesia produced by nerve growth factor: Modulation by the p75 neurotrophin receptor. Neuroscience 2017, 340, 384–397. [Google Scholar] [CrossRef]
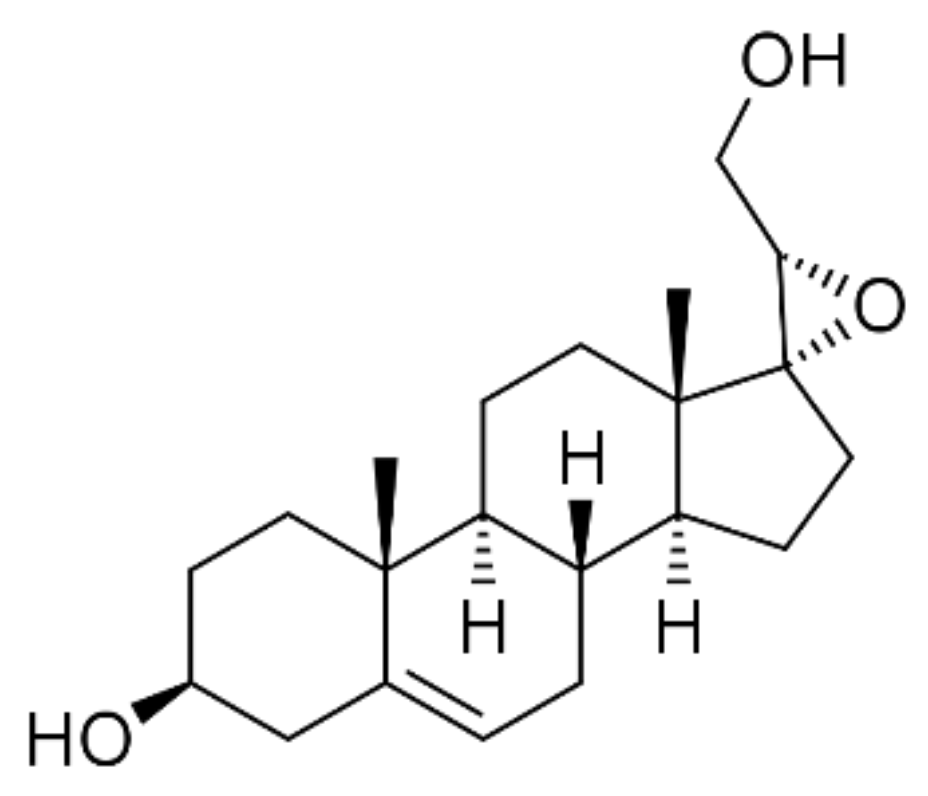
- Pediaditakis, I.; Efstathopoulos, P.; Prousis, K.C.; Zervou, M.; Arevalo, J.C.; Alexaki, V.I.; Nikoletopoulou, V.; Karagianni, E.; Potamitis, C.; Tavernarakis, N.; et al. Selective and differential interactions of BNN27, a novel C17-spiroepoxy steroid derivative, with TrkA receptors, regulating neuronal survival and differentiation. Neuropharmacology 2016, 111, 266–282. [Google Scholar] [CrossRef]
- Calogeropoulou, T.; Avlonitis, N.; Minas, V.; Alexi, X.; Pantzou, A.; Charalampopoulos, I.; Zervou, M.; Vergou, V.; Katsanou, E.S.; Lazaridis, I.; et al. Novel dehydroepiandrosterone derivatives with antiapoptotic, neuroprotective activity. J. Med. Chem. 2009, 52, 6569–6587. [Google Scholar] [CrossRef]
- Iban-Arias, R.; Lisa, S.; Mastrodimou, N.; Kokona, D.; Koulakis, E.; Iordanidou, P.; Kouvarakis, A.; Fothiadaki, M.; Papadogkonaki, S.; Sotiriou, A.; et al. The Synthetic Microneurotrophin BNN27 Affects Retinal Function in Rats with Streptozotocin-Induced Diabetes. Diabetes 2018, 67, 321–333. [Google Scholar] [CrossRef]
- Bonetto, G.; Charalampopoulos, I.; Gravanis, A.; Karagogeos, D. The novel synthetic microneurotrophin BNN27 protects mature oligodendrocytes against cuprizone-induced death, through the NGF receptor TrkA. Glia 2017, 65, 1376–1394. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999, 40, 111–118. [Google Scholar] [CrossRef]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef]
- Fehrenbacher, J.C.; Vasko, M.R.; Duarte, D.B. Models of inflammation: Carrageenan- or complete Freund’s Adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr. Protoc. Pharmacol. 2012. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.; Zhao, Z.Q. Resiniferatoxin reversibly blocks adjuvant-induced thermal hyperalgesia in the rat. Eur. J. Pharmacol. 2003, 481, 301–304. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Mizokami, S.S.; Hohmann, M.S.; Staurengo-Ferrari, L.; Carvalho, T.T.; Zarpelon, A.C.; Possebon, M.I.; de Souza, A.R.; Veneziani, R.C.; Arakawa, N.S.; Casagrande, R.; et al. Pimaradienoic Acid Inhibits Carrageenan-Induced Inflammatory Leukocyte Recruitment and Edema in Mice: Inhibition of Oxidative Stress, Nitric Oxide and Cytokine Production. PLoS ONE 2016, 11, e0149656. [Google Scholar] [CrossRef]
- Lewin, G.R.; Rueff, A.; Mendell, L.M. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur. J. Neurosci. 1994, 6, 1903–1912. [Google Scholar] [CrossRef]
- Rueff, A.; Mendell, L.M. Nerve growth factor NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J. Neurophysiol. 1996, 76, 3593–3596. [Google Scholar] [CrossRef]
- Afrazi, S.; Esmaeili-Mahani, S. Allopregnanolone suppresses diabetes-induced neuropathic pain and motor deficit through inhibition of GABAA receptor down-regulation in the spinal cord of diabetic rats. Iran J. Basic Med. Sci. 2014, 17, 312–317. [Google Scholar]
- Gasinska, E.; Bujalska-Zadrozny, M.; Sar, M.; Makulska-Nowak, H. Influence of acute and subchronic oral administration of dehydroepiandrosterone (DHEA) on nociceptive threshold in rats. Pharmacol. Rep. 2012, 64, 965–969. [Google Scholar] [CrossRef]
- Kibaly, C.; Meyer, L.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Biochemical and functional evidence for the control of pain mechanisms by dehydroepiandrosterone endogenously synthesized in the spinal cord. FASEB J. 2008, 22, 93–104. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Margioris, A.N.; Gravanis, A. Neurosteroid dehydroepiandrosterone exerts anti-apoptotic effects by membrane-mediated, integrated genomic and non-genomic pro-survival signaling pathways. J. Neurochem. 2008, 107, 1457–1469. [Google Scholar] [CrossRef]
- Rittner, H.L.; Stein, C. Involvement of cytokines, chemokines and adhesion molecules in opioid analgesia. Eur. J. Pain 2005, 9, 109–112. [Google Scholar] [CrossRef]
- Cunha, F.Q.; Poole, S.; Lorenzetti, B.B.; Ferreira, S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992, 107, 660–664. [Google Scholar] [CrossRef]
- Danenberg, H.D.; Alpert, G.; Lustig, S.; Ben-Nathan, D. Dehydroepiandrosterone protects mice from endotoxin toxicity and reduces tumor necrosis factor production. Antimicrob. Agents Chemother. 1992, 36, 2275–2279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, G.F.; Tseng, J. Regulation of murine interleukin-10 production by dehydroepiandrosterone. J. Interferon Cytokine Res. 2000, 20, 471–478. [Google Scholar] [CrossRef]
- Straub, R.H.; Konecna, L.; Hrach, S.; Rothe, G.; Kreutz, M.; Scholmerich, J.; Falk, W.; Lang, B. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: Possible link between endocrinosenescence and immunosenescence. J. Clin. Endocrinol. Metab. 1998, 83, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.B.; Basso, P.J.; Nardini, V.; Silva, A.; Chica, J.E.; Cardoso, C.R. Dehydroepiandrosterone (DHEA) restrains intestinal inflammation by rendering leukocytes hyporesponsive and balancing colitogenic inflammatory responses. Immunobiology 2016, 221, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Cecconello, A.L.; Torres, I.L.; Oliveira, C.; Zanini, P.; Niches, G.; Ribeiro, M.F. DHEA administration modulates stress-induced analgesia in rats. Physiol. Behav. 2016, 157, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Gramsch, C.; Hassan, A.H.; Przewlocki, R.; Parsons, C.G.; Peter, K.; Herz, A. Local opioid receptors mediating antinociception in inflammation: Endogenous ligands. Prog. Clin. Biol. Res. 1990, 328, 425–427. [Google Scholar]
- Hassan, A.H.; Ableitner, A.; Stein, C.; Herz, A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience 1993, 55, 185–195. [Google Scholar] [CrossRef]
- Stein, C.; Hassan, A.H.; Lehrberger, K.; Giefing, J.; Yassouridis, A. Local analgesic effect of endogenous opioid peptides. Lancet 1993, 342, 321–324. [Google Scholar] [CrossRef]
- Rittner, H.L.; Labuz, D.; Schaefer, M.; Mousa, S.A.; Schulz, S.; Schafer, M.; Stein, C.; Brack, A.; Rittner, H.L.; Labuz, D.; et al. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J. 2006, 20, 2627–2629. [Google Scholar] [CrossRef]
- Sitte, N.; Busch, M.; Mousa, S.A.; Labuz, D.; Rittner, H.; Gore, C.; Krause, H.; Stein, C.; Schäfer, M. Lymphocytes upregulate signal sequence-encoding proopiomelanocortin mRNA and beta-endorphin during painful inflammation in vivo. J. Neuroimmunol. 2007, 183, 133–145. [Google Scholar] [CrossRef]
- McMahon, S.B. NGF as a mediator of inflammatory pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 431–440. [Google Scholar]
- Vergadi, E.; Vaporidi, K.; Theodorakis, E.E.; Doxaki, C.; Lagoudaki, E.; Ieronymaki, E.; Alexaki, V.I.; Helms, M.; Kondili, E.; Soennichsen, B.; et al. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J. Immunol. 2014, 192, 394–406. [Google Scholar] [CrossRef]
- Buttgereit, F.; Burmester, G.R.; Simon, L.S. Gastrointestinal toxic side effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2-specific inhibitors. Am. J. Med. 2001, 110, 13S–19S. [Google Scholar] [CrossRef]
- Contet, C.; Kieffer, B.L.; Befort, K. Mu opioid receptor: A gateway to drug addiction. Curr. Opin. Neurobiol. 2004, 14, 370–378. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| beta-actin | tctctttgatgtcacgcacg | tcagaaggactcctatgtgg |
| Pomc | gctgcttcagacctccatagatgtg | cagcgagaggtcgagtttgc |
| Penk | cgacatcaatttcctggcgt | agatccttgcaggtctccca |
| μ-opioid receptor | acgctcagacgttccattct | tccaaagaggcccactacac |
| Ngf | cacccacccagtcttcc | ctcggcacttggtctcaaa |
| Trka | cctgcaacgcttggagtttg | cactcttcacgatggttaggct |
| iNOS | tcctggaggaagtgggccaag | cctccacgggcccggtactc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulaki, S.; Rassouli, O.; Liapakis, G.; Gravanis, A.; Venihaki, M. Analgesic and Anti-Inflammatory Effects of the Synthetic Neurosteroid Analogue BNN27 during CFA-Induced Hyperalgesia. Biomedicines 2021, 9, 1185. https://doi.org/10.3390/biomedicines9091185
Poulaki S, Rassouli O, Liapakis G, Gravanis A, Venihaki M. Analgesic and Anti-Inflammatory Effects of the Synthetic Neurosteroid Analogue BNN27 during CFA-Induced Hyperalgesia. Biomedicines. 2021; 9(9):1185. https://doi.org/10.3390/biomedicines9091185
Chicago/Turabian StylePoulaki, Smaragda, Olga Rassouli, George Liapakis, Achille Gravanis, and Maria Venihaki. 2021. "Analgesic and Anti-Inflammatory Effects of the Synthetic Neurosteroid Analogue BNN27 during CFA-Induced Hyperalgesia" Biomedicines 9, no. 9: 1185. https://doi.org/10.3390/biomedicines9091185
APA StylePoulaki, S., Rassouli, O., Liapakis, G., Gravanis, A., & Venihaki, M. (2021). Analgesic and Anti-Inflammatory Effects of the Synthetic Neurosteroid Analogue BNN27 during CFA-Induced Hyperalgesia. Biomedicines, 9(9), 1185. https://doi.org/10.3390/biomedicines9091185







