Sex-Related Motor Deficits in the Tau-P301L Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Tests
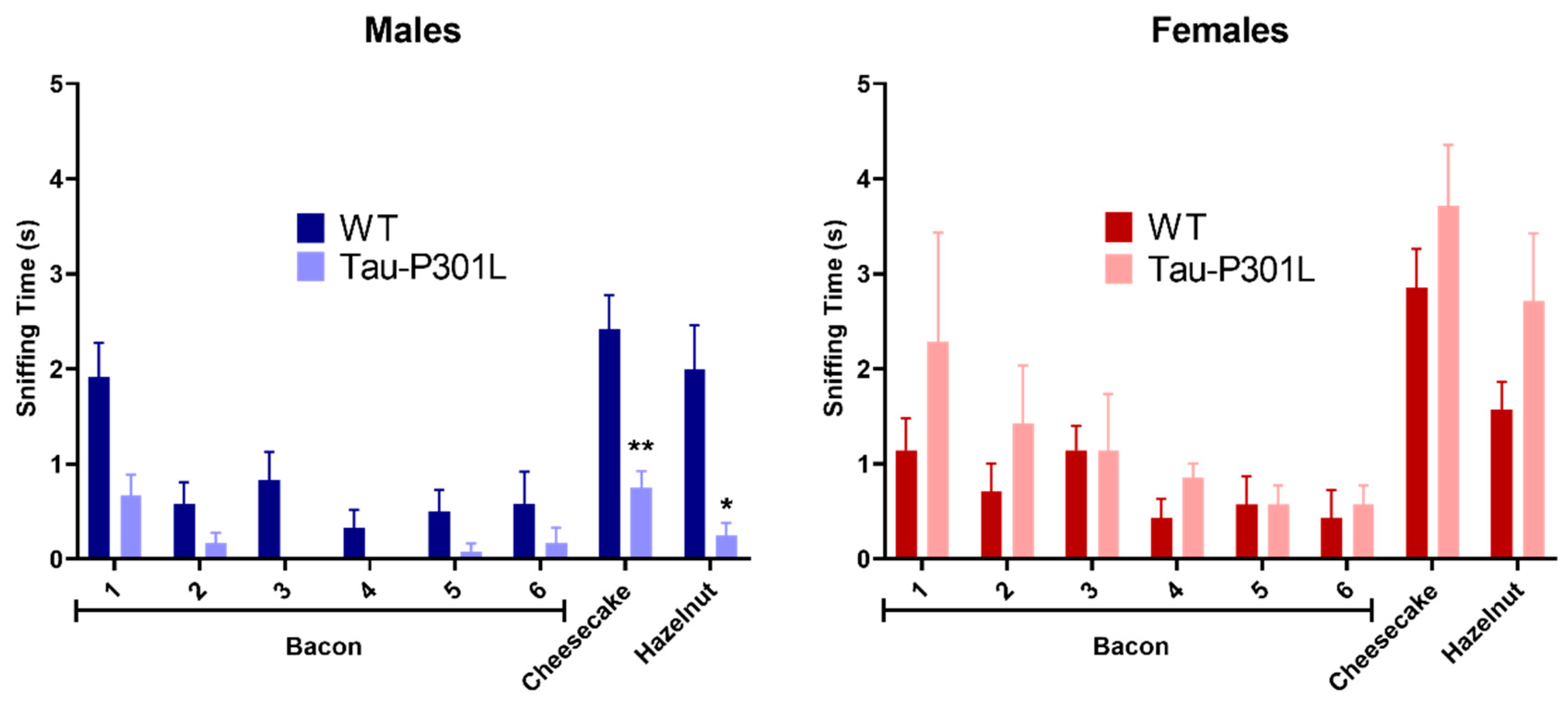
2.2.1. Habituation/Dishabituation Olfactory Test
2.2.2. Nesting Behavior Test
2.2.3. Marble Burying Test
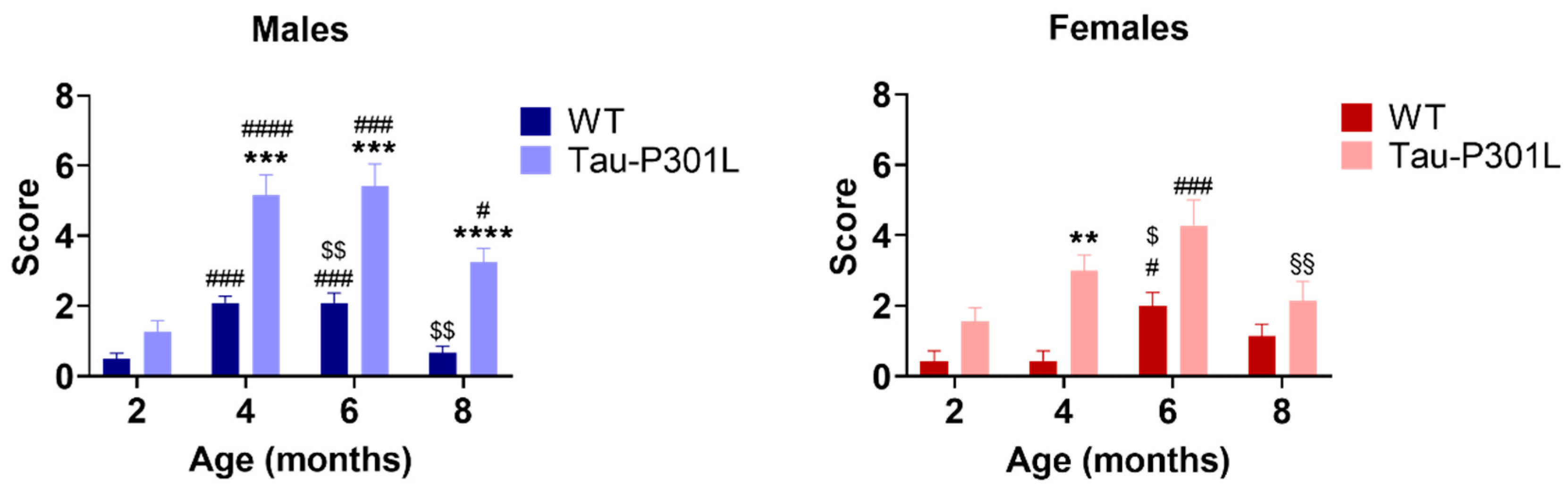
2.2.4. SHIRPA Test Battery
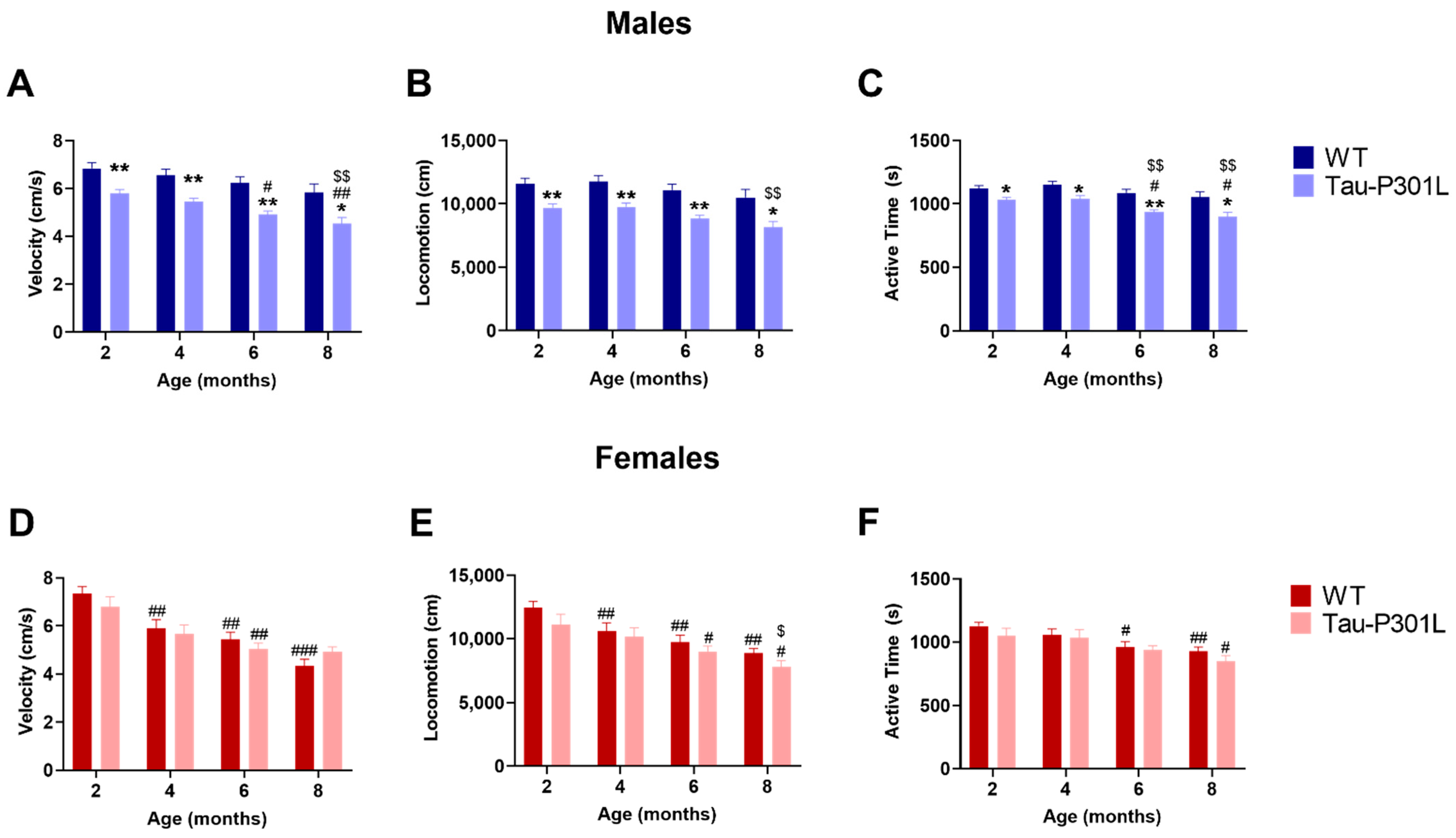
2.2.5. Open Field Test
2.2.6. Accelerating Rotarod
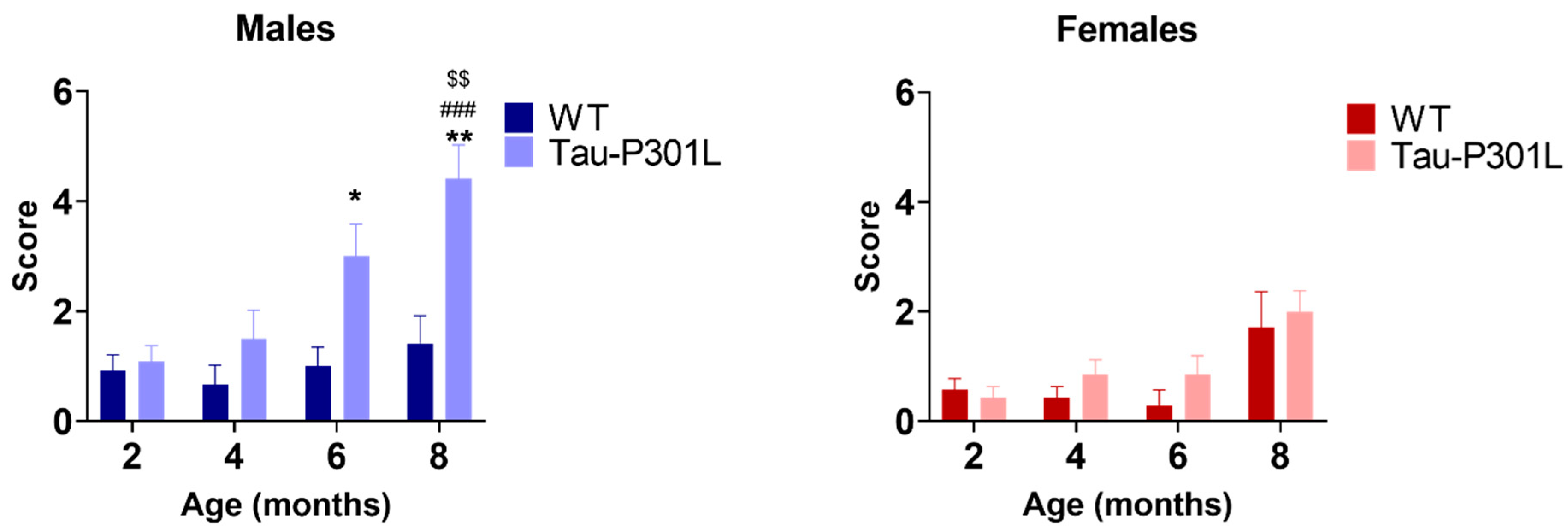
2.2.7. Modified Pole Test
2.2.8. Novel Object Recognition Test
2.2.9. T-Maze Spontaneous Alternation
2.2.10. Fear Conditioning Test
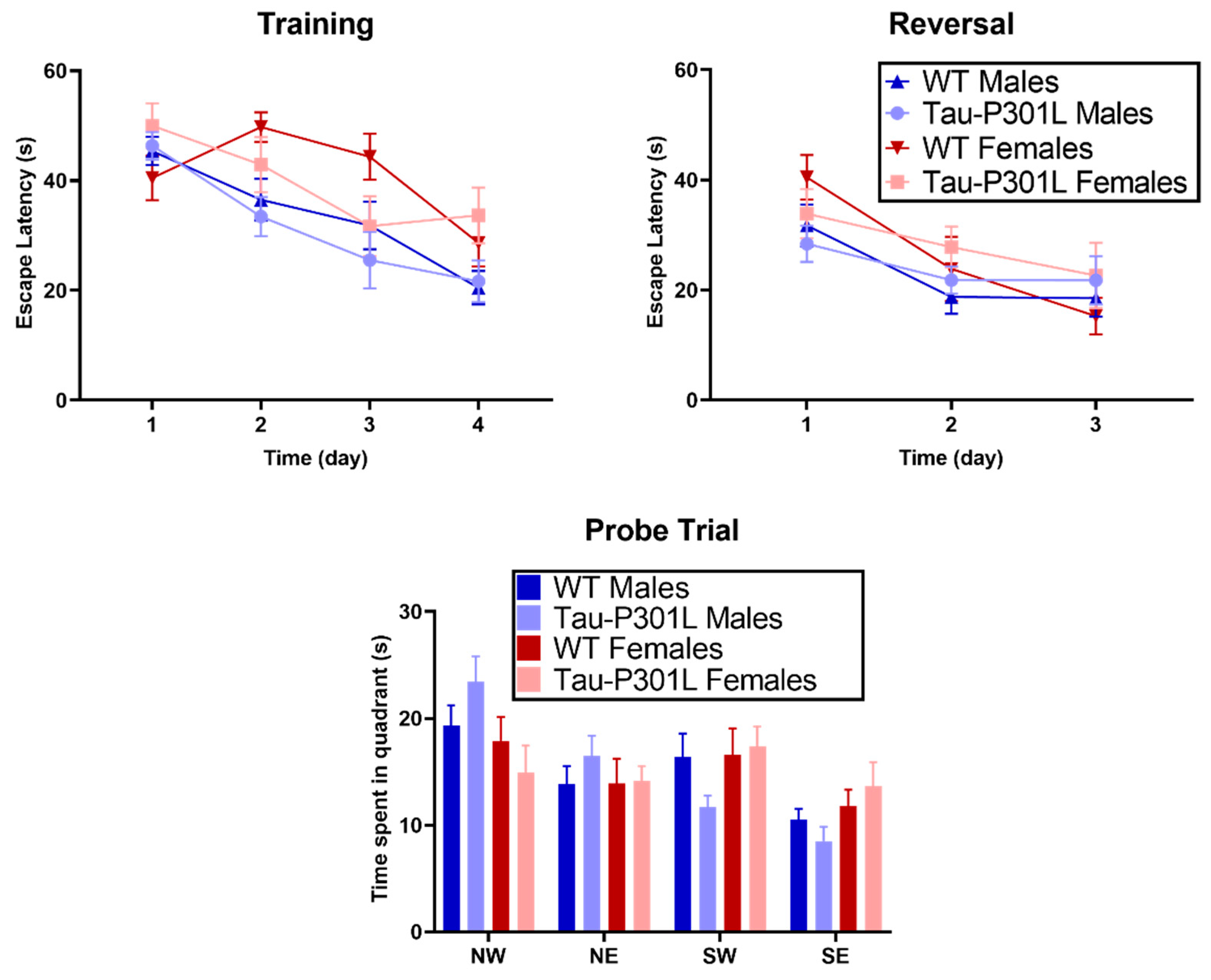
2.2.11. Morris Water Maze
2.3. Histology
2.4. Statistical Analysis
3. Results
3.1. Tau-P301L Male Mice Show Phenotypic Alterations Beginning at 4 Months of Age
3.2. Tau-P301L Male Mice Display Early Motor Deficits
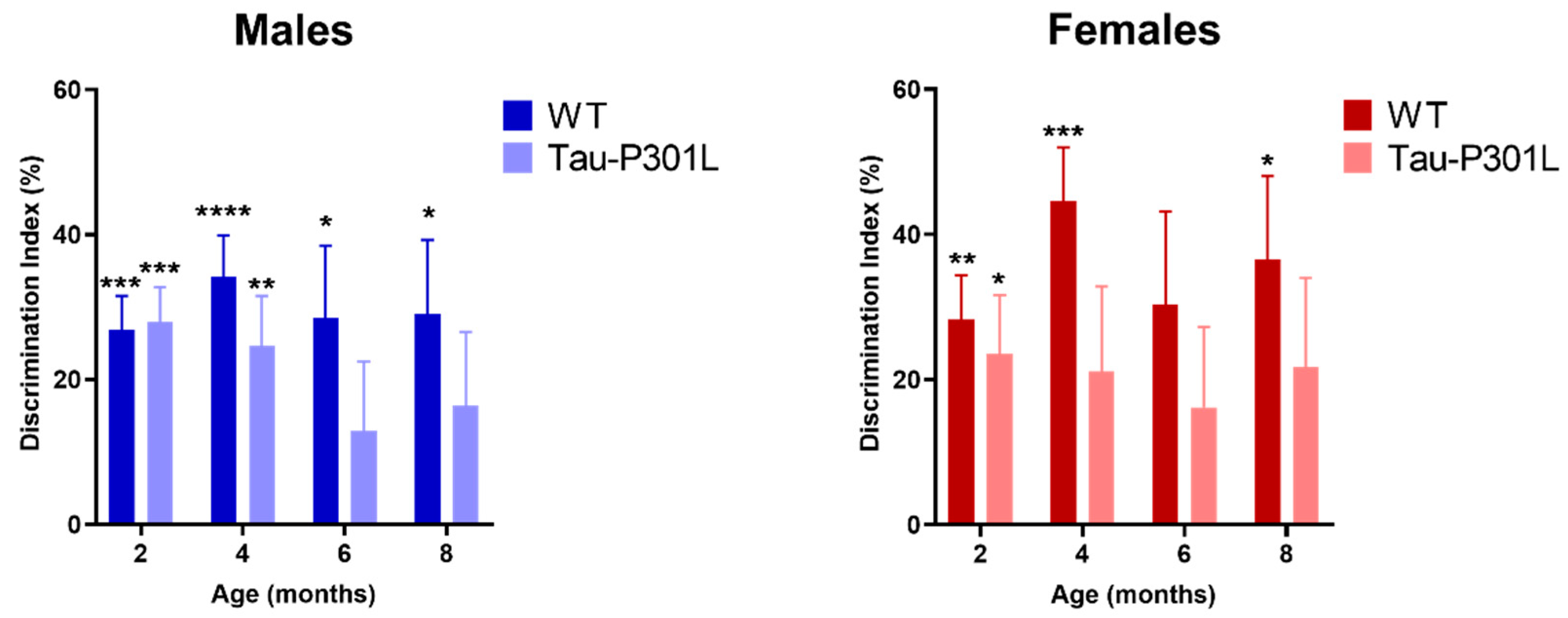
3.3. Tau-P301L Show Mild Cognitive Deficits in the NOR
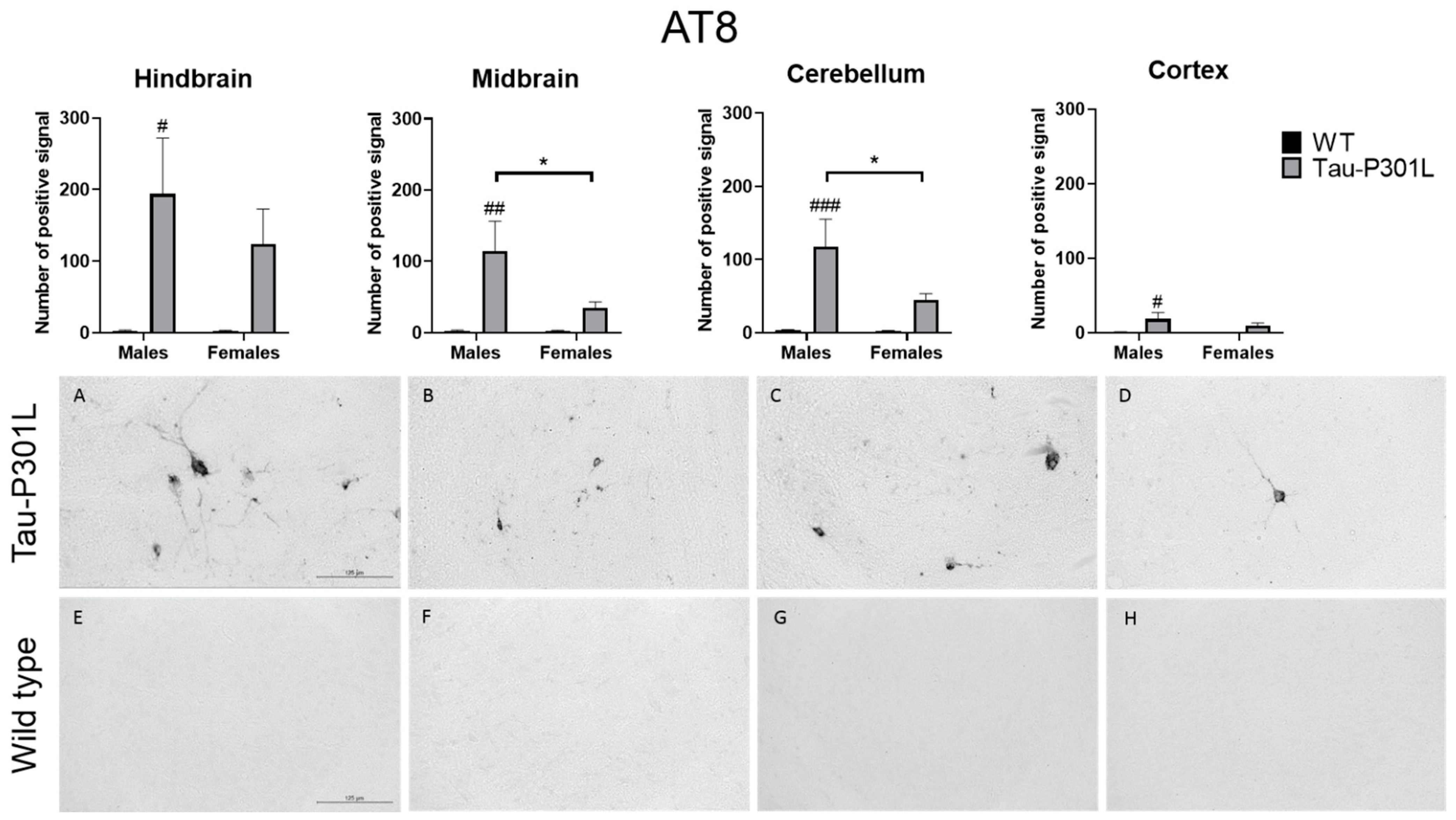
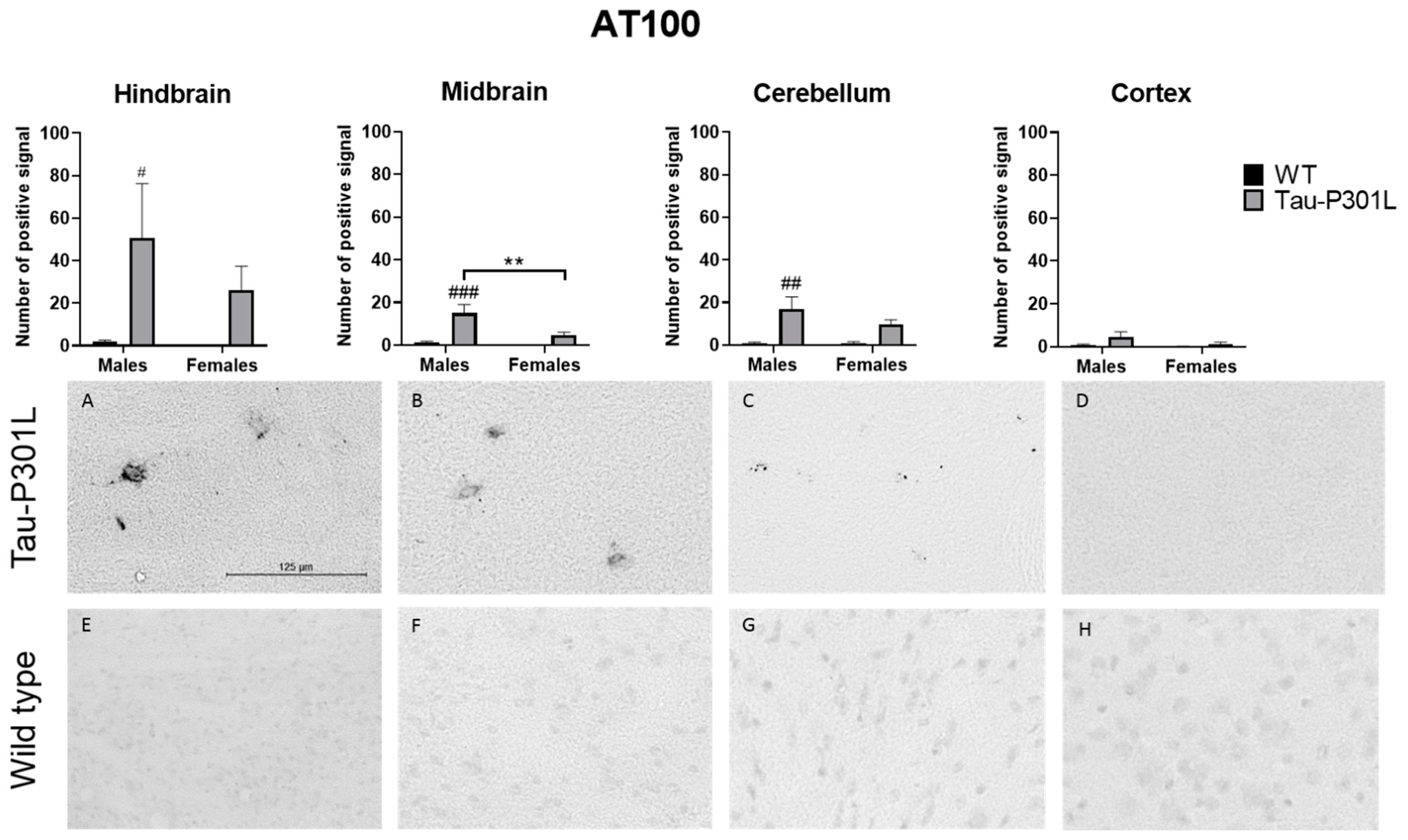
3.4. Tau-P301L Showed Distinct Tau Pathology in the Brain at 8 Months of Age
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jean, D.C.; Baas, P.W. It cuts two ways: Microtubule loss during Alzheimer disease. Embo J. 2013, 32, 2900–2902. [Google Scholar] [CrossRef]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L.F. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Combs, B.; Mueller, R.L.; Morfini, G.; Brady, S.T.; Kanaan, N.M. Tau and Axonal Transport. Misregulation in Tauopathies. Adv. Exp. Med. Biol. 2019, 1184, 81–95. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Cabezas-Opazo, F.; Deaton, C.A.; Vergara, E.H.; Johnson, G.V.W.; Quintanilla, R.A. It’s all about tau. Prog. Neurobiol. 2019, 175, 54–76. [Google Scholar] [CrossRef]
- Fischer, I.; Baas, P.W. Resurrecting the Mysteries of Big Tau. Trends Neurosci. 2020, 43, 493–504. [Google Scholar] [CrossRef]
- Mandelkow, E.M.; Biernat, J.; Drewes, G.; Gustke, N.; Trinczek, B.; Mandelkow, E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging 1995, 16, 355–362. [Google Scholar] [CrossRef]
- Götz, J.; Halliday, G.; Nisbet, R.M. Molecular Pathogenesis of the Tauopathies. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 239–261. [Google Scholar] [CrossRef]
- Quintas-Neves, M.; Teylan, M.A.; Besser, L.; Soares-Fernandes, J.; Mock, C.N.; Kukull, W.A.; Crary, J.F.; Oliveira, T.G. Magnetic resonance imaging brain atrophy assessment in primary age-related tauopathy (PART). Acta Neuropathol. Commun. 2019, 7, 204. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Liao, D.; Miller, E.C.; Teravskis, P.J. Tau acts as a mediator for Alzheimer’s disease-related synaptic deficits. Eur. J. Neurosci. 2014, 39, 1202–1213. [Google Scholar] [CrossRef]
- Jadhav, S.; Cubinkova, V.; Zimova, I.; Brezovakova, V.; Madari, A.; Cigankova, V.; Zilka, N. Tau-mediated synaptic damage in Alzheimer’s disease. Transl. Neurosci. 2015, 6, 214–226. [Google Scholar] [CrossRef]
- Puvenna, V.; Engeler, M.; Banjara, M.; Brennan, C.; Schreiber, P.; Dadas, A.; Bahrami, A.; Solanki, J.; Bandyopadhyay, A.; Morris, J.K.; et al. Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res. 2016, 1630, 225–240. [Google Scholar] [CrossRef]
- Poorkaj, P.; Grossman, M.; Steinbart, E.; Payami, H.; Sadovnick, A.; Nochlin, D.; Tabira, T.; Trojanowski, J.Q.; Borson, S.; Galasko, D.; et al. Frequency of tau gene mutations in familial and sporadic cases of non-Alzheimer dementia. Arch. Neurol. 2001, 58, 383–387. [Google Scholar] [CrossRef]
- Forrest, S.L.; Kril, J.J.; Stevens, C.H.; Kwok, J.B.; Hallupp, M.; Kim, W.S.; Huang, Y.; McGinley, C.V.; Werka, H.; Kiernan, M.C.; et al. Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 2017, 141, 521–534. [Google Scholar] [CrossRef]
- Hasegawa, M.; Smith, M.J.; Goedert, M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 1998, 437, 207–210. [Google Scholar] [CrossRef]
- Roberson, E.D. Mouse models of frontotemporal dementia. Ann. Neurol. 2012, 72, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Medeiros, R.; Laferla, F.M. Transgenic mouse models of Alzheimer disease: Developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 2012, 18, 1131–1147. [Google Scholar] [CrossRef]
- Terwel, D.; Lasrado, R.; Snauwaert, J.; Vandeweert, E.; Van Haesendonck, C.; Borghgraef, P.; Van Leuven, F. Changed Conformation of Mutant Tau-P301L Underlies the Moribund Tauopathy, Absent in Progressive, Nonlethal Axonopathy of Tau-4R/2N Transgenic Mice*. J. Biol. Chem. 2005, 280, 3963–3973. [Google Scholar] [CrossRef]
- Dutschmann, M.; Menuet, C.; Stettner, G.M.; Gestreau, C.; Borghgraef, P.; Devijver, H.; Gielis, L.; Hilaire, G.; Van Leuven, F. Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei. J. Neurosci. 2010, 30, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Boekhoorn, K.; Terwel, D.; Biemans, B.; Borghgraef, P.; Wiegert, O.; Ramakers, G.J.; de Vos, K.; Krugers, H.; Tomiyama, T.; Mori, H.; et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J. Neurosci. 2006, 26, 3514–3523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lehmkuhl, A.M.; Dirr, E.R.; Fleming, S.M. Olfactory assays for mouse models of neurodegenerative disease. J. Vis. Exp. JoVE 2014, e51804. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R. Assessing burrowing, nest construction, and hoarding in mice. J. Vis. Exp. JoVE 2012, e2607. [Google Scholar] [CrossRef]
- Deacon, R.M. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef]
- Rogers, D.C.; Fisher, E.M.; Brown, S.D.; Peters, J.; Hunter, A.J.; Martin, J.E. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 1997, 8, 711–713. [Google Scholar] [CrossRef]
- Alexandru, A.; Jagla, W.; Graubner, S.; Becker, A.; Bäuscher, C.; Kohlmann, S.; Sedlmeier, R.; Raber, K.A.; Cynis, H.; Rönicke, R.; et al. Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Aβ is induced by pyroglutamate-Aβ formation. J. Neurosci. 2011, 31, 12790–12801. [Google Scholar] [CrossRef]
- Dunkelmann, T.; Schemmert, S.; Honold, D.; Teichmann, K.; Butzküven, E.; Demuth, H.-U.; Shah, N.J.; Langen, K.-J.; Kutzsche, J.; Willbold, D.; et al. Comprehensive Characterization of the Pyroglutamate Amyloid-β Induced Motor Neurodegenerative Phenotype of TBA2.1 Mice. J. Alzheimer’s Dis. 2018, 63, 115–130. [Google Scholar] [CrossRef]
- Spowart-Manning, L.; Van der Staay, F. The T-maze continuous alternation task for assessing the effects of putative cognition enhancers in the mouse. Behav. Brain Res. 2004, 151, 37–46. [Google Scholar] [CrossRef]
- Curzon, P.; Rustay, N.R.; Browman, K.E. Frontiers in Neuroscience. Cued and Contextual Fear Conditioning for Rodents. In Methods of Behavior Analysis in Neuroscience; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis. Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2009. [Google Scholar]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Augustinack, J.C.; Schneider, A.; Mandelkow, E.-M.; Hyman, B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002, 103, 26–35. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Vanmechelen, E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 1995, 189, 167–170. [Google Scholar] [CrossRef]
- Zheng-Fischhöfer, Q.; Biernat, J.; Mandelkow, E.-M.; Illenberger, S.; Godemann, R.; Mandelkow, E. Sequential phosphorylation of Tau by glycogen synthase kinase-3β and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur. J. Biochem. 1998, 252, 542–552. [Google Scholar] [CrossRef]
- Scearce-Levie, K.; Sanchez, P.E.; Lewcock, J.W. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat. Rev. Drug Discov. 2020, 19, 447–462. [Google Scholar] [CrossRef]
- Samaey, C.; Schreurs, A.; Stroobants, S.; Balschun, D. Early Cognitive and Behavioral Deficits in Mouse Models for Tauopathy and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 335. [Google Scholar] [CrossRef]
- Polydoro, M.; Acker, C.M.; Duff, K.; Castillo, P.E.; Davies, P. Age-Dependent Impairment of Cognitive and Synaptic Function in the htau Mouse Model of Tau Pathology. J. Neurosci. 2009, 29, 10741–10749. [Google Scholar] [CrossRef]
- Bodea, L.-G.; Evans, H.T.; Van der Jeugd, A.; Ittner, L.M.; Delerue, F.; Kril, J.; Halliday, G.; Hodges, J.; Kiernan, M.C.; Götz, J. Accelerated aging exacerbates a pre-existing pathology in a tau transgenic mouse model. Aging Cell 2017, 16, 377–386. [Google Scholar] [CrossRef]
- Van der Jeugd, A.; Vermaercke, B.; Halliday, G.M.; Staufenbiel, M.; Götz, J. Impulsivity, decreased social exploration, and executive dysfunction in a mouse model of frontotemporal dementia. Neurobiol. Learn. Mem. 2016, 130, 34–43. [Google Scholar] [CrossRef]
- Takenokuchi, M.; Kadoyama, K.; Chiba, S.; Sumida, M.; Matsuyama, S.; Saigo, K.; Taniguchi, T. SJLB mice develop tauopathy-induced parkinsonism. Neurosci. Lett. 2010, 473, 182–185. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, W.; Zhu, X.; Chen, R.; Wang, X. Olfactory Dysfunctions and Decreased Nitric Oxide Production in the Brain of Human P301L Tau Transgenic Mice. Neurochem. Res. 2016, 41, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; McGowan, E.; Rockwood, J.; Melrose, H.; Nacharaju, P.; Van Slegtenhorst, M.; Gwinn-Hardy, K.; Murphy, M.P.; Baker, M.; Yu, X.; et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000, 25, 402–405. [Google Scholar] [CrossRef]
- Götz, J.; Chen, F.; Barmettler, R.; Nitsch, R.M. Tau Filament Formation in Transgenic Mice Expressing P301L Tau*. J. Biol. Chem. 2001, 276, 529–534. [Google Scholar] [CrossRef]
- You, Y.; Botros, M.B.; Enoo, A.A.V.; Bockmiller, A.; Herron, S.; Delpech, J.C.; Ikezu, T. Cre-inducible Adeno Associated Virus-mediated Expression of P301L Mutant Tau Causes Motor Deficits and Neuronal Degeneration in the Substantia Nigra. Neuroscience 2019, 422, 65–74. [Google Scholar] [CrossRef]
- Mirra, S.S.; Murrell, J.R.; Gearing, M.; Spillantini, M.G.; Goedert, M.; Crowther, R.A.; Levey, A.I.; Jones, R.; Green, J.; Shoffner, J.M.; et al. Tau Pathology in a Family with Dementia and a P301L Mutation in Tau. J. Neuropathol. Exp. Neurol. 1999, 58, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Dayton, R.D.; Lin, W.L.; Dickson, D.W. Tau gene transfer, but not alpha-synuclein, induces both progressive dopamine neuron degeneration and rotational behavior in the rat. Neurobiol. Dis. 2005, 20, 64–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kovacs, G.G. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Dickson, D.W.; Ahmed, Z.; Algom, A.A.; Tsuboi, Y.; Josephs, K.A. Neuropathology of variants of progressive supranuclear palsy. Curr. Opin. Neurol. 2010, 23, 394–400. [Google Scholar] [CrossRef]
- Armstrong, R.A.; McKee, A.C.; Cairns, N.J. Pathology of the Superior Colliculus in Chronic Traumatic Encephalopathy. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2017, 94, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Tu, M.; Murray, M.E.; Dickson, D.W. Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer’s disease and progressive supranuclear palsy. Neurosci. Lett. 2011, 491, 122–126. [Google Scholar] [CrossRef]
- Dennison, J.L.; Ricciardi, N.R.; Lohse, I.; Volmar, C.-H.; Wahlestedt, C. Sexual Dimorphism in the 3xTg-AD Mouse Model and Its Impact on Pre-Clinical Research. J. Alzheimer’s Dis. 2021, 80, 41–52. [Google Scholar] [CrossRef]
- Grudzien, A.; Shaw, P.; Weintraub, S.; Bigio, E.; Mash, D.C.; Mesulam, M.M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging 2007, 28, 327–335. [Google Scholar] [CrossRef]
- Kelly, S.C.; He, B.; Perez, S.E.; Ginsberg, S.D.; Mufson, E.J.; Counts, S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017, 5, 8. [Google Scholar] [CrossRef]
- Tanila, H. Wading pools, fading memories—Place navigation in transgenic mouse models of Alzheimer’s disease. Front. Aging Neurosci. 2012, 4, 11. [Google Scholar] [CrossRef]
- Izquierdo, I.; Furini, C.R.; Myskiw, J.C. Fear Memory. Physiol. Rev. 2016, 96, 695–750. [Google Scholar] [CrossRef]
- Dye, R.V.; Miller, K.J.; Singer, E.J.; Levine, A.J. Hormone replacement therapy and risk for neurodegenerative diseases. Int. J. Alzheimers Dis. 2012, 2012, 258454. [Google Scholar] [CrossRef]
- Phung, T.K.; Waltoft, B.L.; Laursen, T.M.; Settnes, A.; Kessing, L.V.; Mortensen, P.B.; Waldemar, G. Hysterectomy, oophorectomy and risk of dementia: A nationwide historical cohort study. Dement. Geriatr. Cogn. Disord. 2010, 30, 43–50. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Feng, X.; Jia, M.; Ai, N.; Dong, Y.; Zheng, Y.; Fu, L.; Yu, B.; Zhang, H.; et al. The behavioural and neuropathologic sexual dimorphism and absence of MIP-3α in tau P301S mouse model of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 72. [Google Scholar] [CrossRef]
- Yang, J.-T.; Wang, Z.-J.; Cai, H.-Y.; Yuan, L.; Hu, M.-M.; Wu, M.-N.; Qi, J.-S. Sex. Differences in Neuropathology and Cognitive Behavior in APP/PS1/tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 736–746. [Google Scholar] [CrossRef]
- Clinton, L.K.; Billings, L.M.; Green, K.N.; Caccamo, A.; Ngo, J.; Oddo, S.; McGaugh, J.L.; LaFerla, F.M. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol. Dis. 2007, 28, 76–82. [Google Scholar] [CrossRef]
- Goodman, Y.; Bruce, A.J.; Cheng, B.; Mattson, M.P. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J. Neurochem. 1996, 66, 1836–1844. [Google Scholar] [CrossRef]
- Luine, V.N. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp. Neurol. 1985, 89, 484–490. [Google Scholar] [CrossRef]
- Behl, C.; Widmann, M.; Trapp, T.; Holsboer, F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem. Biophys. Res. Commun. 1995, 216, 473–482. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Keeling, J.L.; Keller, J.N.; Huang, F.F.; Camondola, S.; Mattson, M.P. Antiinflammatory effects of estrogen on microglial activation. Endocrinology 2000, 141, 3646–3656. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Description |
|---|---|
| Restlessness | Difficulty staying in one body position for an extended period of time |
| Apathy | Motionless and lowered head |
| Stereotyped behaviour | |
| Convulsion | |
| Abnormal body carriage | Body posture |
| Alertness | Response to object proximity |
| Abnormal gait | Uncommon walk, e.g., paddling, waddling, running |
| Startle response | Response to an acoustic signal |
| Loss of righting reflex | Time when the mouse return to standing position when turned on its back |
| Touch response | |
| Pinna reflex | |
| Cornea reflex | |
| Forelimb placing reflex | Response to stretch their front paws when hanged in proximity to the surface |
| Hanging behaviour | Mouse stays on the rod or falls |
| Pain response | Response to tail pinch |
| Grooming | Overall fur condition |
| Parameters | Phenotypic Alterations |
|---|---|
| Restlessness | No alterations |
| Apathy | No alterations |
| Stereotyped behavior | No alterations |
| Convulsion | No alterations |
| Abnormal body carriage | Hunchback |
| Alertness | No alterations |
| Abnormal gait | Waddling walk and slower compared to WT |
| Startle response | No alterations |
| Loss of righting reflex | Some Tau-P301L mice have light loss of righting reflex |
| Touch response | Less responsive to touch than WT |
| Pinna reflex | No alterations |
| Cornea reflex | No alterations |
| Forelimb placing reflex | Paralysis (“Clasping”) of the limbs |
| Hanging behavior | Tau-P301L male mice fall faster from the rod than WT male mice |
| Pain response | No alterations |
| Grooming | The Tau-P301L male mice have very good fur condition compared to WT |
| Staining | Brain Region | WT | Tau-P301L | Significance | ||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||
| NeuN (Spot Count) | Hindbrain | 1434.7 ± 111.3 | 1307.6 ± 208.9 | 817.1 ± 181.7 | 1286.0 ± 246.4 | WT males vs. Tau-P301L males (p = 0.012) |
| Midbrain | 1376.8 ± 184.5 | 1487.8 ± 108.9 | 1191.9± 139.9 | 1474.3 ± 237.6 | n.s | |
| Cortex | 3545.1 ± 197.0 | 2879.1 ± 262.8 | 4141.1 ± 210.7 | 3651.3 ± 285.7 | n.s | |
| Cerebellum | 1834.0 ± 83.6 | 1259.0 ± 123.5 | 2060.1 ± 158.2 | 1461.1 ± 187.0 | n.s | |
| GFAP (Stained Area) | Hindbrain | 27.0 ± 1.0 | 29.3 ± 1.5 | 31.5 ± 3.6 | 33.4 ± 1.1 | n.s |
| Midbrain | 18.6 ± 2.8 | 21.0 ± 3.3 | 19.9 ± 2.8 | 31.2 ± 2.1 | n.s | |
| Cortex | 15.7 ± 2.7 | 23.4 ± 3.1 | 21.7 ± 4.4 | 31.8 ± 1.7 | n.s | |
| Cerebellum | 8.5 ± 1.3 | 11.0 ± 1.8 | 11.8 ± 1.6 | 15.4 ± 1.0 | n.s | |
| CD11b (Stained Area) | Hindbrain | 6.7 ± 0.9 | 5.6 ± 0.4 | 6.5 ± 0.6 | 5.7 ± 0.8 | n.s |
| Midbrain | 6.0 ± 0.4 | 5.2 ± 0.6 | 5.5 ± 0.7 | 5.1 ± 0.5 | n.s | |
| Cortex | 6.0 ± 0.5 | 5.9 ± 0.6 | 6.9 ± 0.9 | 6.0 ± 0.7 | n.s | |
| Cerebellum | 6.5 ± 0.4 | 4.9 ± 0.7 | 6.6 ± 0.3 | 5.8 ± 0.3 | n.s | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, L.C.; Honold, D.; Bauer, R.; Shah, N.J.; Langen, K.-J.; Willbold, D.; Kutzsche, J.; Willuweit, A.; Schemmert, S. Sex-Related Motor Deficits in the Tau-P301L Mouse Model. Biomedicines 2021, 9, 1160. https://doi.org/10.3390/biomedicines9091160
Camargo LC, Honold D, Bauer R, Shah NJ, Langen K-J, Willbold D, Kutzsche J, Willuweit A, Schemmert S. Sex-Related Motor Deficits in the Tau-P301L Mouse Model. Biomedicines. 2021; 9(9):1160. https://doi.org/10.3390/biomedicines9091160
Chicago/Turabian StyleCamargo, Luana Cristina, Dominik Honold, Robert Bauer, N. Jon Shah, Karl-Josef Langen, Dieter Willbold, Janine Kutzsche, Antje Willuweit, and Sarah Schemmert. 2021. "Sex-Related Motor Deficits in the Tau-P301L Mouse Model" Biomedicines 9, no. 9: 1160. https://doi.org/10.3390/biomedicines9091160
APA StyleCamargo, L. C., Honold, D., Bauer, R., Shah, N. J., Langen, K.-J., Willbold, D., Kutzsche, J., Willuweit, A., & Schemmert, S. (2021). Sex-Related Motor Deficits in the Tau-P301L Mouse Model. Biomedicines, 9(9), 1160. https://doi.org/10.3390/biomedicines9091160






