A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Primary Skin Cells
2.2. Generation of Epidermis Models
2.3. Burning of Epidermis Models
2.4. CEDEX Glucose Metabolism
2.5. Barrier Function Impedance
2.6. Viability Measurement (MTT Assay)
2.7. Measuring of the Burned Surface Area
2.8. Histological Staining and Immunofluorescence
2.9. Quantitative Analysis of Histological Sections Using a Scoring System
2.10. Cytometric Bead Assay
2.11. Statistical Analysis
3. Results
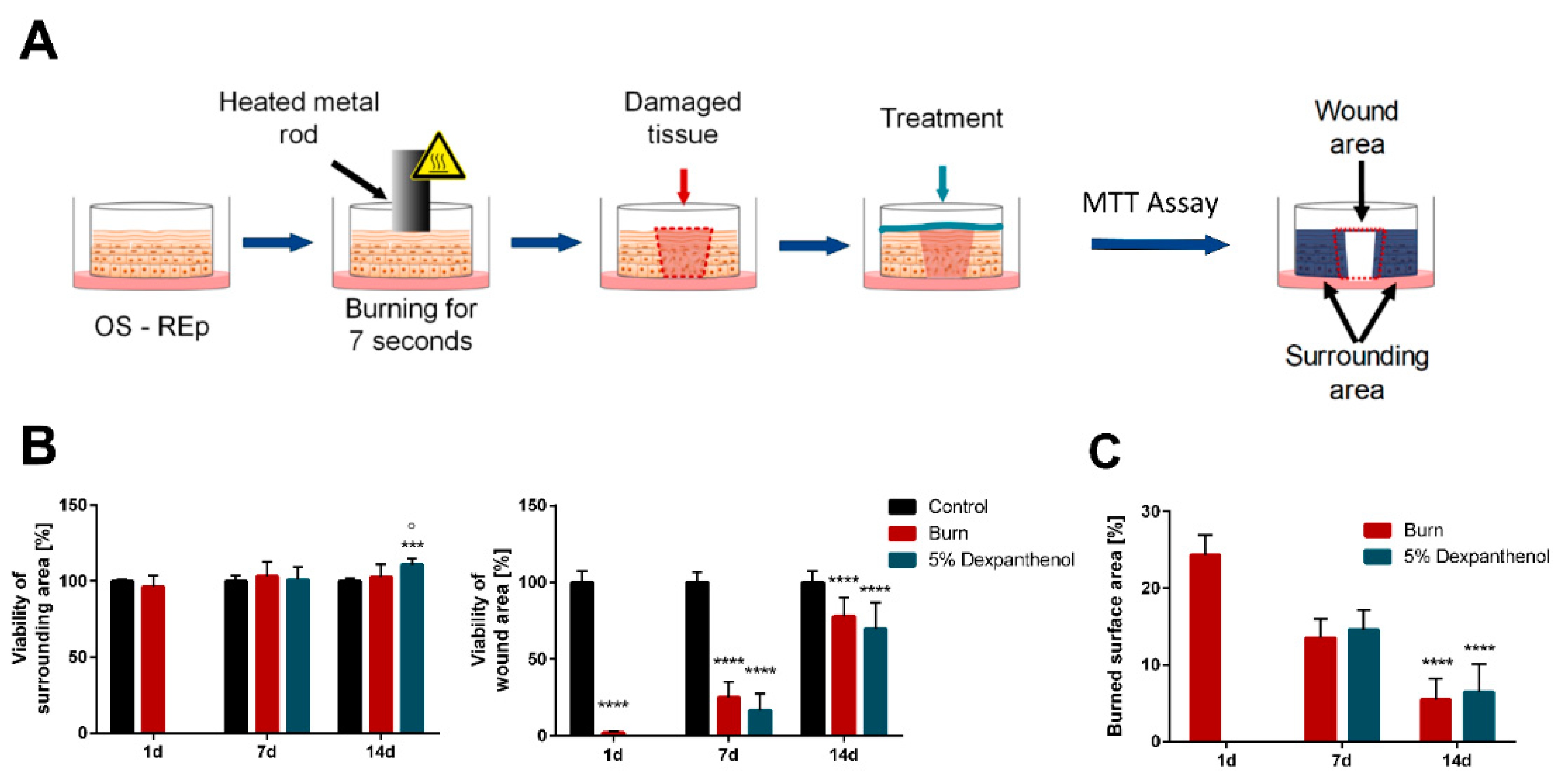
3.1. Burn Wounds Can Be Generated with a Heated Metal Rod and Regenerate over 14 Days
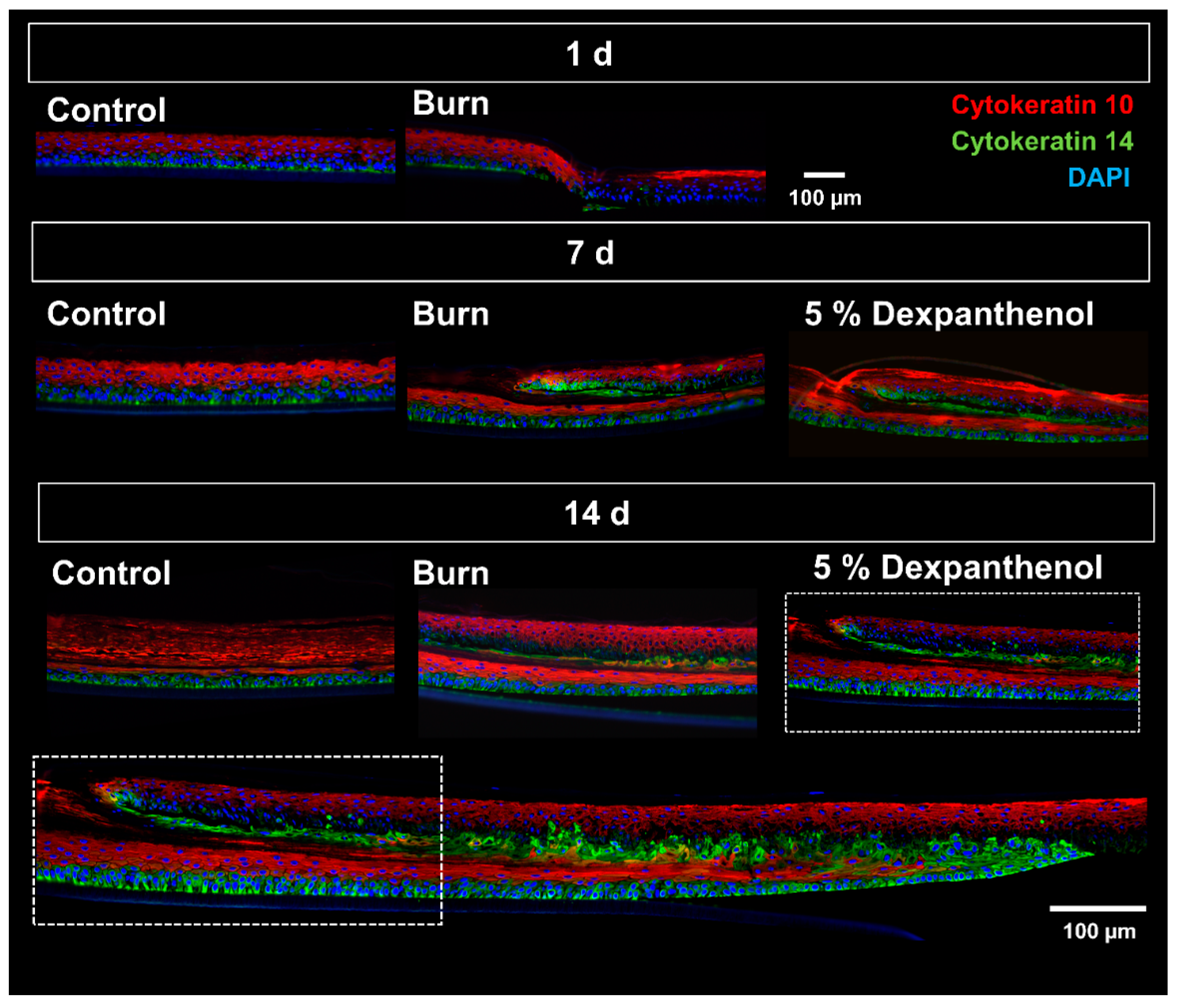
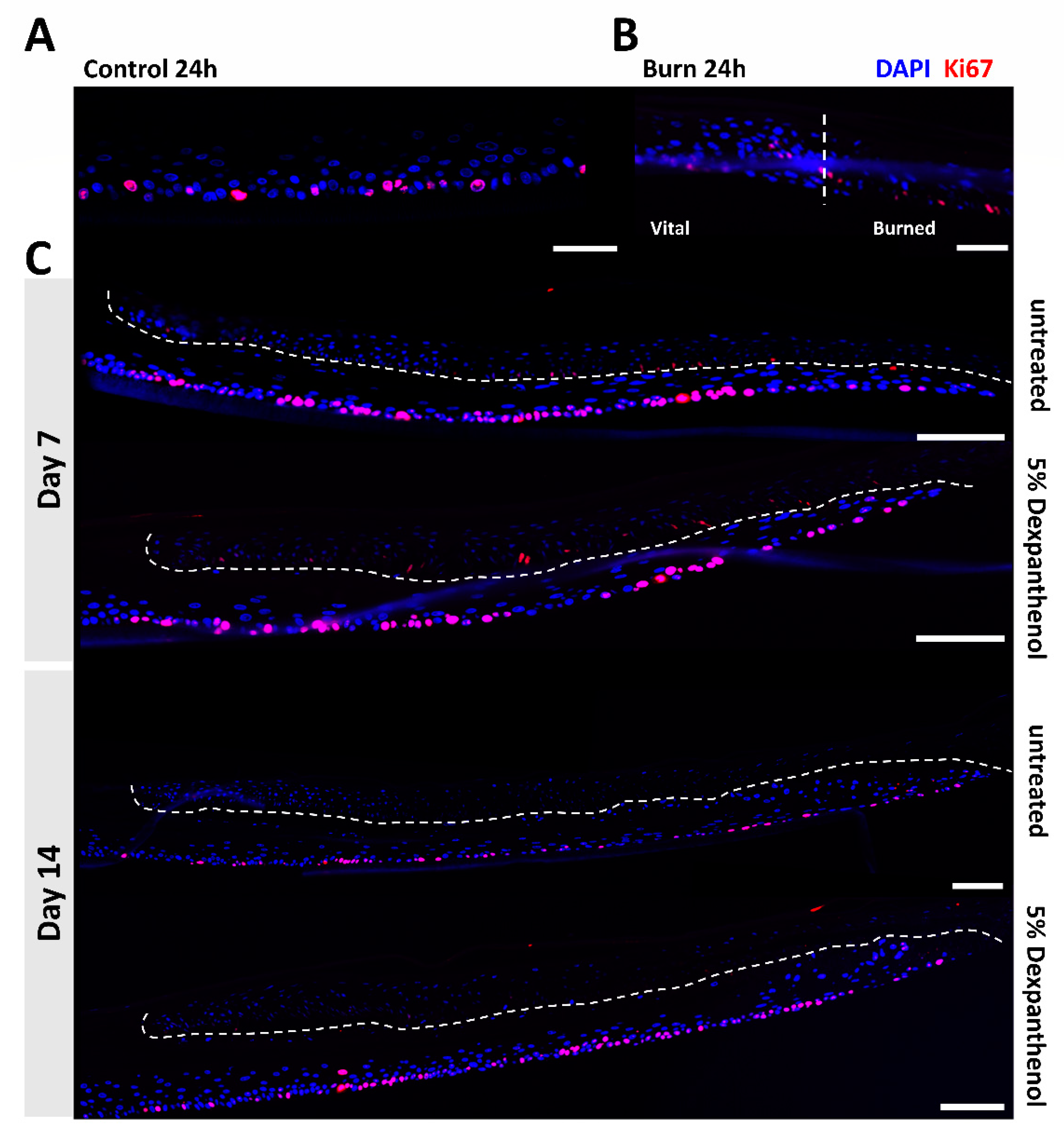
3.2. Wound Healing Can Be Monitored Using Histological and Immunohistological Analysis
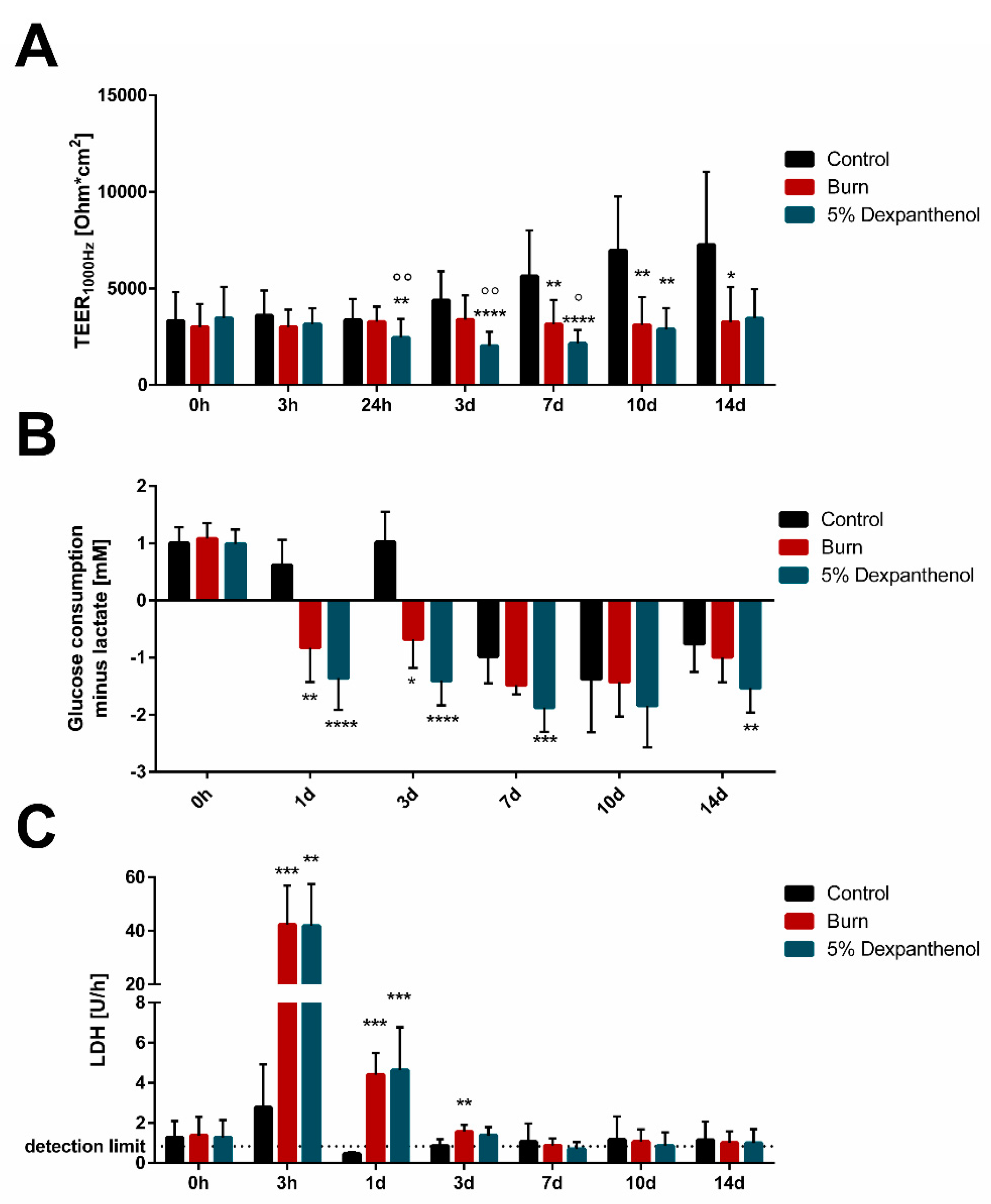
3.3. Barrier Integrity, LDH Release and Metabolic Changes Can Be Measured in Wound Models
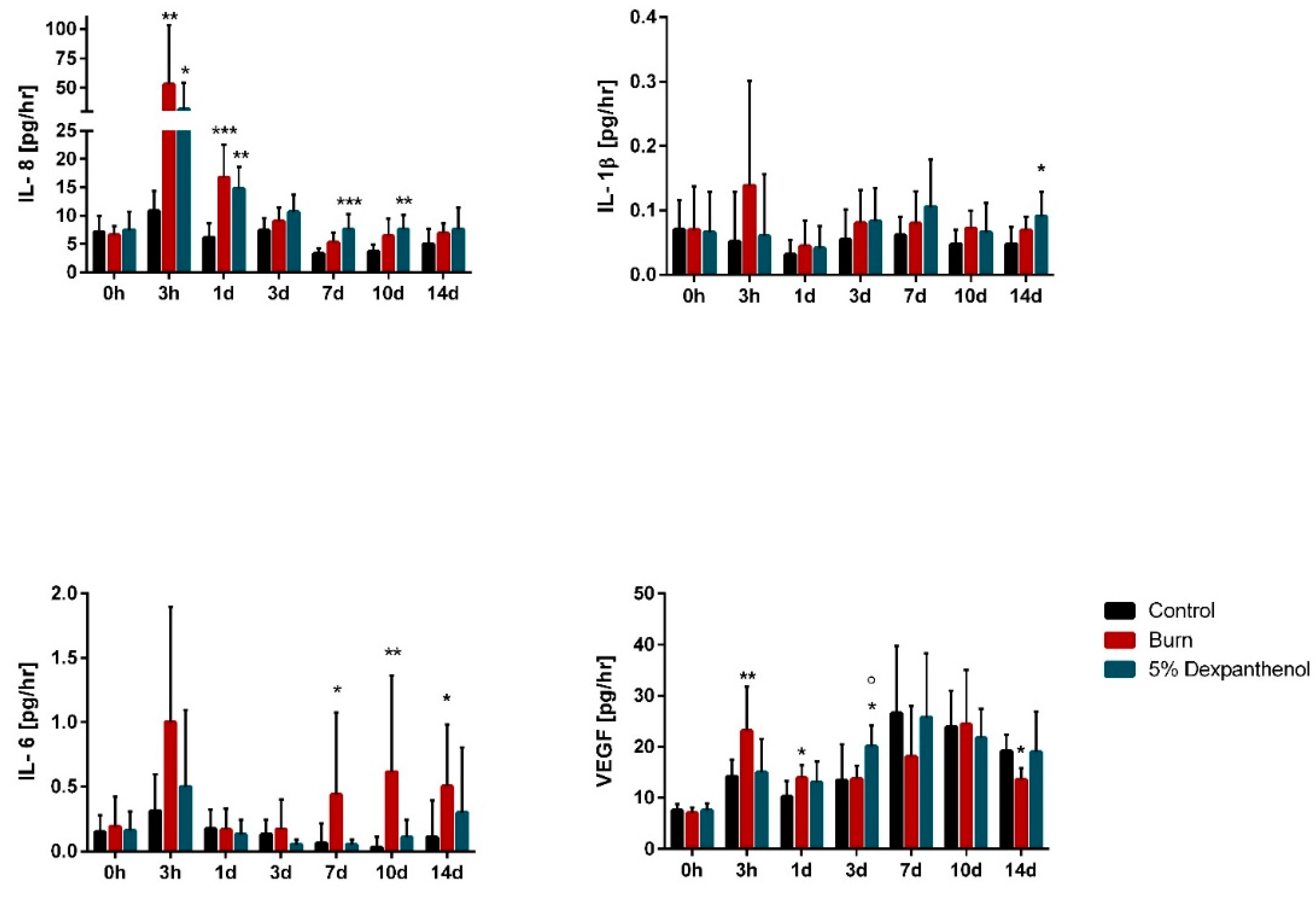
3.4. Burn Wounds Cause Inflammatory Activity in Reconstructed Human Epidermis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Burns. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 21 July 2021).
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.P. Recent trends in burn epidemiology worldwide: A systematic review. Burn. J. Int. Soc. Burn. Inj. 2017, 43, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell. Mol. Life Sci. CMLS 2014, 71, 3241–3255. [Google Scholar] [CrossRef] [Green Version]
- Our World in Data. Death Rates from Fires and Burns 1990–2017. Available online: https://ourworldindata.org/grapher/fire-death-rates (accessed on 21 July 2021).
- Toon, M.H.; Maybauer, D.M.; Arceneaux, L.L.; Fraser, J.F.; Meyer, W.; Runge, A.; Maybauer, M.O. Children with burn injuries—Assessment of trauma, neglect, violence and abuse. J. Inj. Violence Res. 2011, 3, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Jensen, J.-M.; Crichton-Smith, A.; Fowler, A.; Clitherow, J. Rationale Behandlung von Patienten mit Verbrennungen 1. Grades [Rational treatment of first-degree burns]. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2007, 58, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Sadasivam, M.; Gupta, A.; De Melo, W.C.; Huang, Y.Y.; Yin, R.; Chandran, R.; Kumar, R.; Otufowora, A.; Nyame, T.; et al. Animal models of skin disease for drug discovery. Expert Opin. Drug Discov. 2013, 8, 331–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [Google Scholar] [CrossRef]
- The European Parliament and the Council Directive 2010/63/EU of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/eli/dir/2010/63 (accessed on 21 July 2021).
- Kurosawa, T.M. Japanese Regulation of Laboratory Animal Care with 3Rs. Special Issue: Proc. 6th World Congress on Alternatives & Animal Use in the Life Sciences. Altern. Anim. Test. EXperimentation (AATEX) 2007, 2007, 317–321. [Google Scholar]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Parliament and the Council Directive 2003/15/EC of 27 February 2003 on the Approximation of the Laws of the Member States Relating to Cosmetic Products. Available online: https://eur-lex.europa.eu/eli/dir/2003/15 (accessed on 21 July 2021).
- Coolen, N.A.; Vlig, M.; van den Bogaerdt, A.J.; Middelkoop, E.; Ulrich, M.M.W. Development of an in vitro burn wound model. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2008, 16, 559–567. [Google Scholar] [CrossRef]
- Emanuelsson, P.; Kratz, G. Characterization of a new in vitro burn wound model. Burn. J. Int. Soc. Burn. Inj. 1997, 23, 32–36. [Google Scholar] [CrossRef]
- Marques, A.P. (Ed.) Skin Tissue Models; Academic Press: London, UK, 2018. [Google Scholar]
- Fernandes, A.C.M.; França, J.P.D.; Gaiba, S.; Aloise, A.C.; Oliveira, A.F.D.; Moraes, A.A.D.F.S.; França, L.P.D.; Ferreira, L.M. Development of experimental in vitro burn model. Acta Cir. Bras. 2014, 29, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hettler, A.; Werner, S.; Eick, S.; Laufer, S.; Weise, F. A new in vitro model to study cellular responses after thermomechanical damage in monolayer cultures. PLoS ONE 2013, 8, e82635. [Google Scholar] [CrossRef]
- Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4. Available online: https://doi.org/10.1787/9789264242845-en (accessed on 21 July 2021).
- Test No. 406: Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4. Available online: https://doi.org/10.1787/9789264070660-en (accessed on 21 July 2021).
- Test No. 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method. Available online: https://doi.org/10.1787/9789264242753-en (accessed on 21 July 2021).
- Kiesewetter, L.; Littau, L.; Walles, H.; Boccaccini, A.R.; Groeber-Becker, F. Reepithelialization in focus: Non-invasive monitoring of epidermal wound healing in vitro. Biosens. Bioelectron. 2019, 142, 111555. [Google Scholar] [CrossRef]
- Groeber, F.; Schober, L.; Schmid, F.F.; Traube, A.; Kolbus-Hernandez, S.; Daton, K.; Hoffmann, S.; Petersohn, D.; Schaefer-Korting, M.; Walles, H.; et al. Catch-up validation study of an in vitro skin irritation test method based on an open source reconstructed epidermis (phase II). Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2016, 36, 254–261. [Google Scholar] [CrossRef]
- Groeber, F.; Engelhardt, L.; Egger, S.; Werthmann, H.; Monaghan, M.; Walles, H.; Hansmann, J. Impedance spectroscopy for the non-destructive evaluation of in vitro epidermal models. Pharm. Res. 2015, 32, 1845–1854. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, T.; Schweinlin, M.; Kollhoff, R.T.; Engelhardt, L.; Lotz, C.; Groeber-Becker, F.; Walles, H.; Metzger, M.; Hansmann, J. Nanostructured TiN-Coated Electrodes for High-Sensitivity Noninvasive Characterization of In Vitro Tissue Models. ACS Appl. Nano Mater. 2018, 1, 2284–2293. [Google Scholar] [CrossRef]
- Phypers, B.; Pierce, J.T. Lactate physiology in health and disease. Contin. Educ. Anaesth. Crit. Care Pain 2006, 6, 128–132. [Google Scholar] [CrossRef]
- Gravante, G.; Filingeri, V.; Delogu, D.; Santeusanio, G.; Palmieri, M.B.; Esposito, G.; Montone, A.; Sconocchia, G. Apoptotic cell death in deep partial thickness burns by coexpression analysis of TUNEL and Fas. Surgery 2006, 139, 854–855. [Google Scholar] [CrossRef]
- Cotran, R.S.; Kumar, V.; Robbins, S.L.; Saunders, W.B. Robbins’ Pathologic Basis of Disease, 4th ed.; WB Saunders Company: Philadelphia, PA, USA, 1989. [Google Scholar]
- Jackson, D.M. The diagnosis of the depth of burning. Br. J. Surg. 1953, 40, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.L.F.; Bennett, D.D.; Taylor, L.J. Improving the histologic characterization of burn depth. J. Cutan. Pathol. 2017, 44, 998–1004. [Google Scholar] [CrossRef]
- Sangita, C.; Garima, G.; Jayanthi, Y.; Arneet, A.; Neelkamal, K. Histological indicators of cutaneous lesions caused by electrocution, flame burn and impact abrasion. Med. Sci. Law 2018, 58, 216–221. [Google Scholar] [CrossRef]
- Breetveld, M.; Richters, C.D.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Comparison of wound closure after burn and cold injury in human skin equivalents. J. Investig. Dermatol. 2006, 126, 1918–1921. [Google Scholar] [CrossRef] [Green Version]
- Iljas, J.D.; Röhl, J.; McGovern, J.A.; Moromizato, K.H.; Parker, T.J.; Cuttle, L. A human skin equivalent burn model to study the effect of a nanocrystalline silver dressing on wound healing. Burns 2021, 47, 417–429. [Google Scholar] [CrossRef]
- Ando, M.; Kawashima, T.; Kobayashi, H.; Ohkawara, A. Immunohistological detection of proliferating cells in normal and psoriatic epidermis using Ki-67 monoclonal antibody. J. Dermatol. Sci. 1990, 1, 441–446. [Google Scholar] [CrossRef]
- Petrovic, A.; Petrovic, V.; Milojkovic, B.; Nikolic, I.; Jovanovic, D.; Antovic, A.; Milic, M. Immunohistochemical distribution of Ki67 in epidermis of thick glabrous skin of human digits. Arch. Dermatol. Res. 2018, 310, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γ and potential clinical implications. Exp. Ther. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, S.; Kono, T.; Sauder, D.N.; McKenzie, R.C. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J. Investig. Dermatol. 1993, 101, 690–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.-H.; Yim, H.; Kim, D. Changes in the levels of interleukins 6, 8, and 10, tumor necrosis factor alpha, and granulocyte-colony stimulating factor in Korean burn patients: Relation to burn size and postburn time. Ann. Lab. Med. 2012, 32, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.L.; Lin, W.L.; Shen, H.D.; Fang, R.H. Changes in levels of serum IL-8 in burned patients. Burns 1997, 23, 555–559. [Google Scholar] [CrossRef]
- Ozbalkan, Z.; Aslar, A.K.; Yildiz, Y.; Aksaray, S. Investigation of the course of proinflammatory and anti-inflammatory cytokines after burn sepsis. Int. J. Clin. Pract. 2004, 58, 125–129. [Google Scholar] [CrossRef]
- Gragnani, A.; Cezillo, M.V.; da Silva, I.D.; de Noronha, S.M.R.; Correa-Noronha, S.A.; Ferreira, L.M. Gene expression profile of cytokines and receptors of inflammation from cultured keratinocytes of burned patients. Burn. J. Int. Soc. Burn. Inj. 2014, 40, 947–956. [Google Scholar] [CrossRef]
- Yeh, F.L.; Lin, W.L.; Shen, H.D.; Fang, R.H. Changes in circulating levels of interleukin 6 in burned patients. Burns 1999, 25, 131–136. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. Chem. Med. Chem. 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Chang, K.C.; Ma, H.; Liao, W.C.; Lee, C.K.; Lin, C.Y.; Chen, C.C. The optimal time for early burn wound excision to reduce pro-inflammatory cytokine production in a murine burn injury model. Burn. J. Int. Soc. Burn. Inj. 2010, 36, 1059–1066. [Google Scholar] [CrossRef]
- Hübner, G.; Brauchle, M.; Smola, H.; Madlener, M.; Fässler, R.; Werner, S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996, 8, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Hayashi, T.; Ago, K.; Ago, M.; Kanekura, T.; Ogata, M. Temporal expression of wound healing-related genes in skin burn injury. Leg. Med. 2014, 16, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, C.; Zimmer, S.; Kremer, J.P.; Walz, A.; Rordorf-Adam, C.; Henckel von Donnersmarck, G.; Faist, E. Comparative analysis of transcription and protein release of the inflammatory cytokines interleukin-1 beta (IL-1 beta) and interleukin-8 (IL-8) following major burn and mechanical trauma. Shock 1995, 4, 241–246. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Wallace, R.G.; Kenealy, M.R.; Brady, A.J.; Twomey, L.; Duffy, E.; Degryse, B.; Caballero-Lima, D.; Moyna, N.M.; Custaud, M.A.; Meade-Murphy, G.; et al. Development of dynamic cell and organotypic skin models, for the investigation of a novel visco-elastic burns treatment using molecular and cellular approaches. Burn. J. Int. Soc. Burn. Inj. 2020, 46, 1585–1602. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, Y.; Amann, P.M.; Heise, R.; Czaja, K.; Steiner, T.; Merk, H.F.; Skazik-Voogt, C.; Baron, J.M. Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg. Med. 2015, 47, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.; Proksch, E.; Baron, J.M.; Schmid, D.; Zhang, L. Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals 2020, 13, 138. [Google Scholar] [CrossRef]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical use of dexpanthenol in skin disorders. Am. J. Clin. Dermatol. 2002, 3, 427–433. [Google Scholar] [CrossRef]
- Gehring, W.; Gloor, M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study. Arzneim. Forsch. 2000, 50, 659–663. [Google Scholar] [CrossRef]
- Parnell, L.K.S.; Volk, S.W. The Evolution of Animal Models in Wound Healing Research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, V.; Kruse, D.; de Mattos, I.B.; Zöphel, S.; Tiltmann, K.-K.; Reigl, A.; Khan, S.; Funk, M.; Bodenschatz, K.; Groeber-Becker, F. A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level. Biomedicines 2021, 9, 1153. https://doi.org/10.3390/biomedicines9091153
Schneider V, Kruse D, de Mattos IB, Zöphel S, Tiltmann K-K, Reigl A, Khan S, Funk M, Bodenschatz K, Groeber-Becker F. A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level. Biomedicines. 2021; 9(9):1153. https://doi.org/10.3390/biomedicines9091153
Chicago/Turabian StyleSchneider, Verena, Daniel Kruse, Ives Bernardelli de Mattos, Saskia Zöphel, Kendra-Kathrin Tiltmann, Amelie Reigl, Sarah Khan, Martin Funk, Karl Bodenschatz, and Florian Groeber-Becker. 2021. "A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level" Biomedicines 9, no. 9: 1153. https://doi.org/10.3390/biomedicines9091153
APA StyleSchneider, V., Kruse, D., de Mattos, I. B., Zöphel, S., Tiltmann, K.-K., Reigl, A., Khan, S., Funk, M., Bodenschatz, K., & Groeber-Becker, F. (2021). A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level. Biomedicines, 9(9), 1153. https://doi.org/10.3390/biomedicines9091153





