Prolyl-Isomerase Pin1 Controls Key fMLP-Induced Neutrophil Functions
Abstract
:1. Introduction
2. Materials and Methods
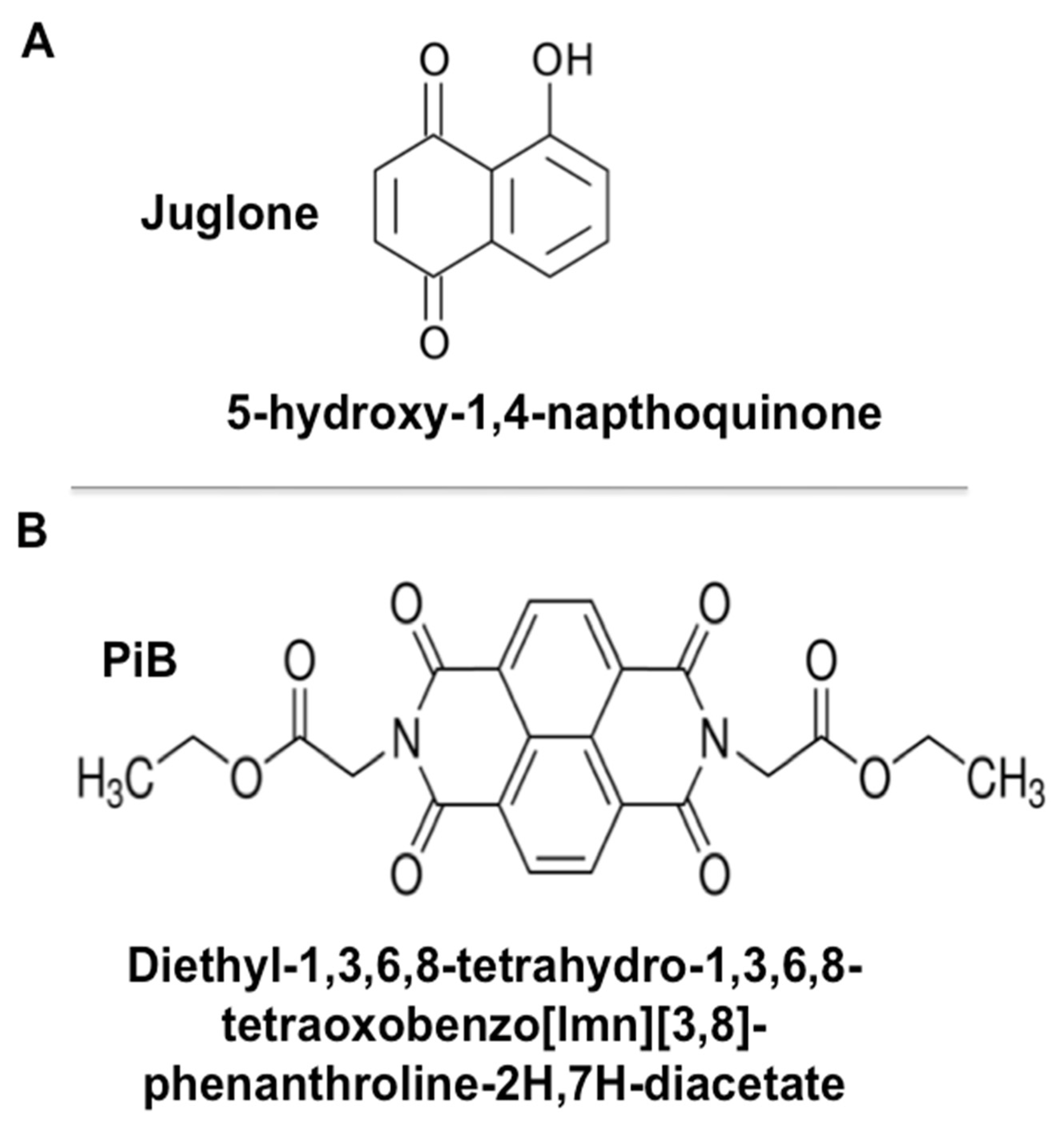
2.1. Chemicals and Reagents
2.2. Ethics Statement and Human Neutrophils Isolation
2.3. Recombinant Pin1 Expression
2.4. Pin1 Activity Assay
2.5. Under Agarose Migration Assay
2.6. Neutrophil Degranulation Assay
2.7. Total ROS Production Assay
2.8. Measurement of Superoxide Production by the Cytochrome C Reduction Assay
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
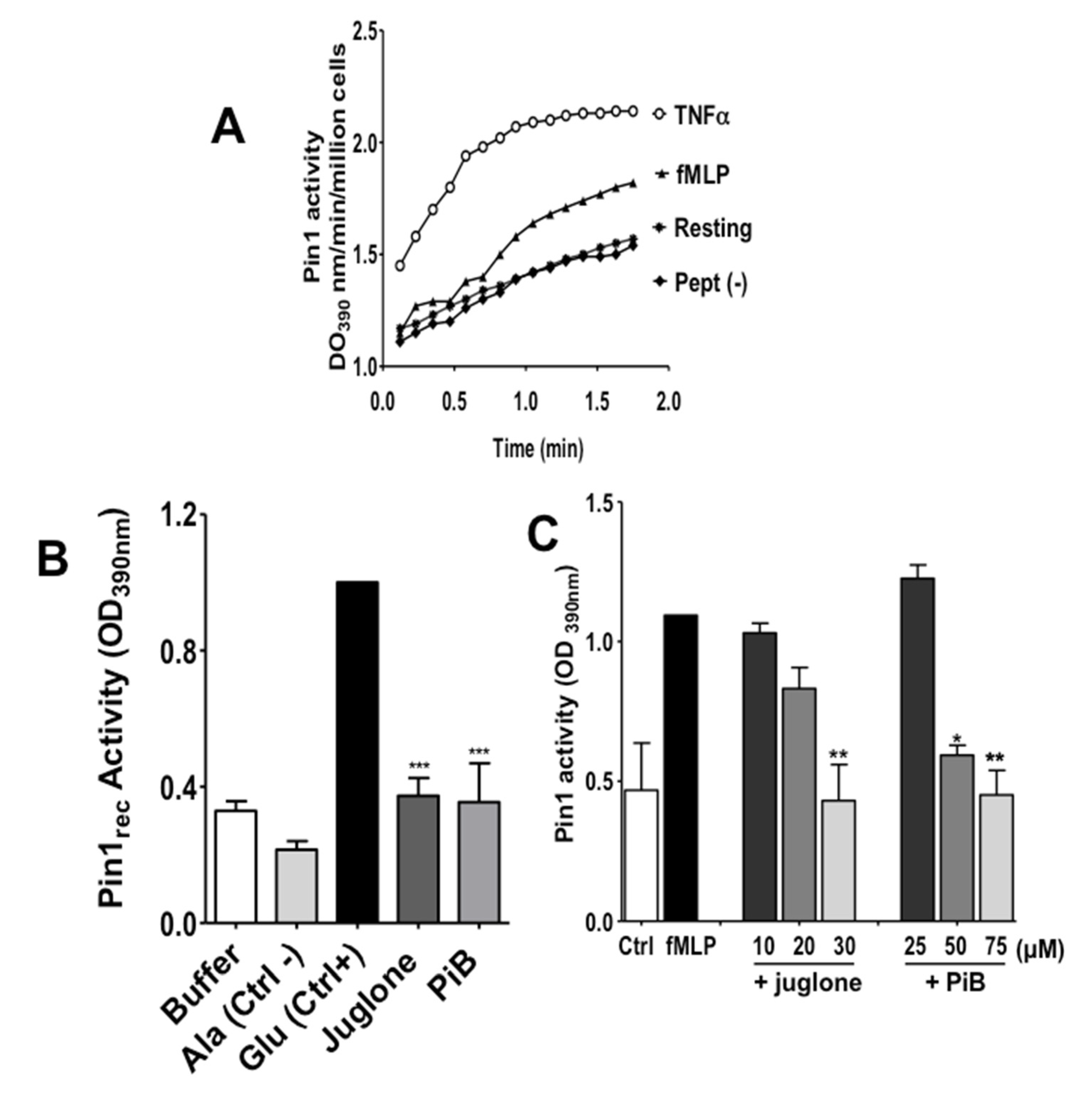
3.1. fMLP Induces an Increase of Pin1 Activity in Human Neutrophils which was Inhibited by Juglone and PiB
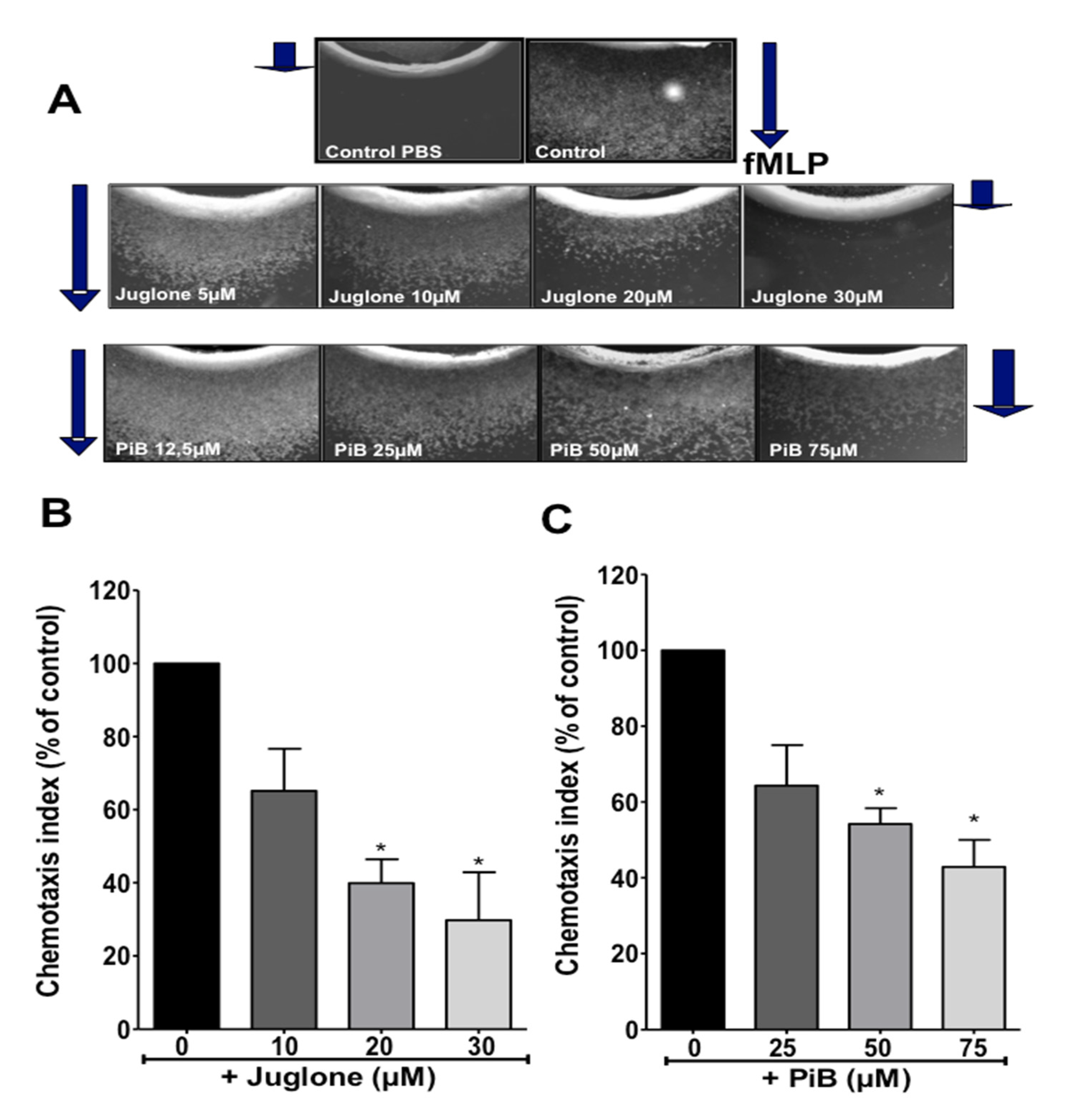
3.2. Juglone and PiB Inhibit Neutrophil Chemotaxis
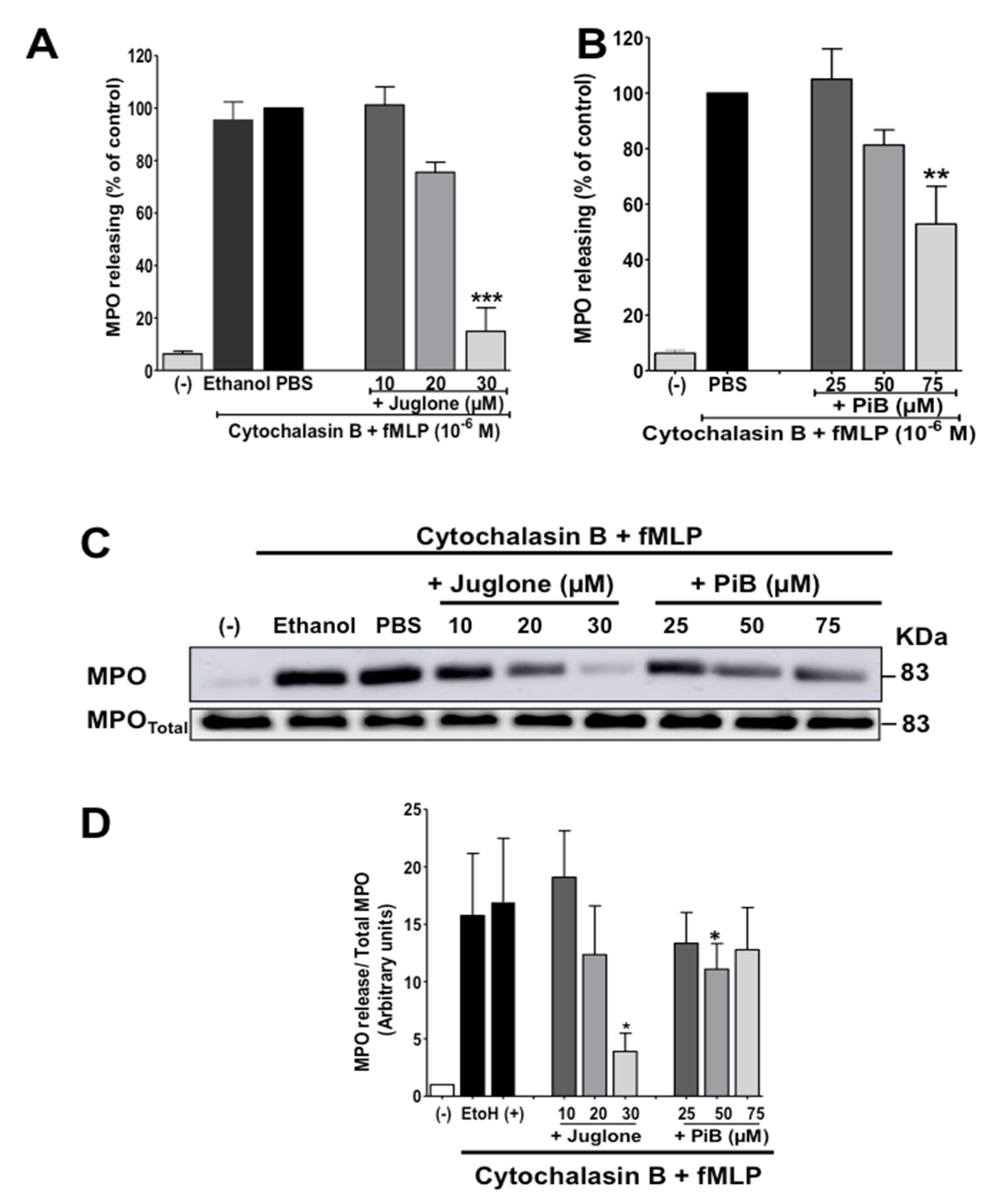
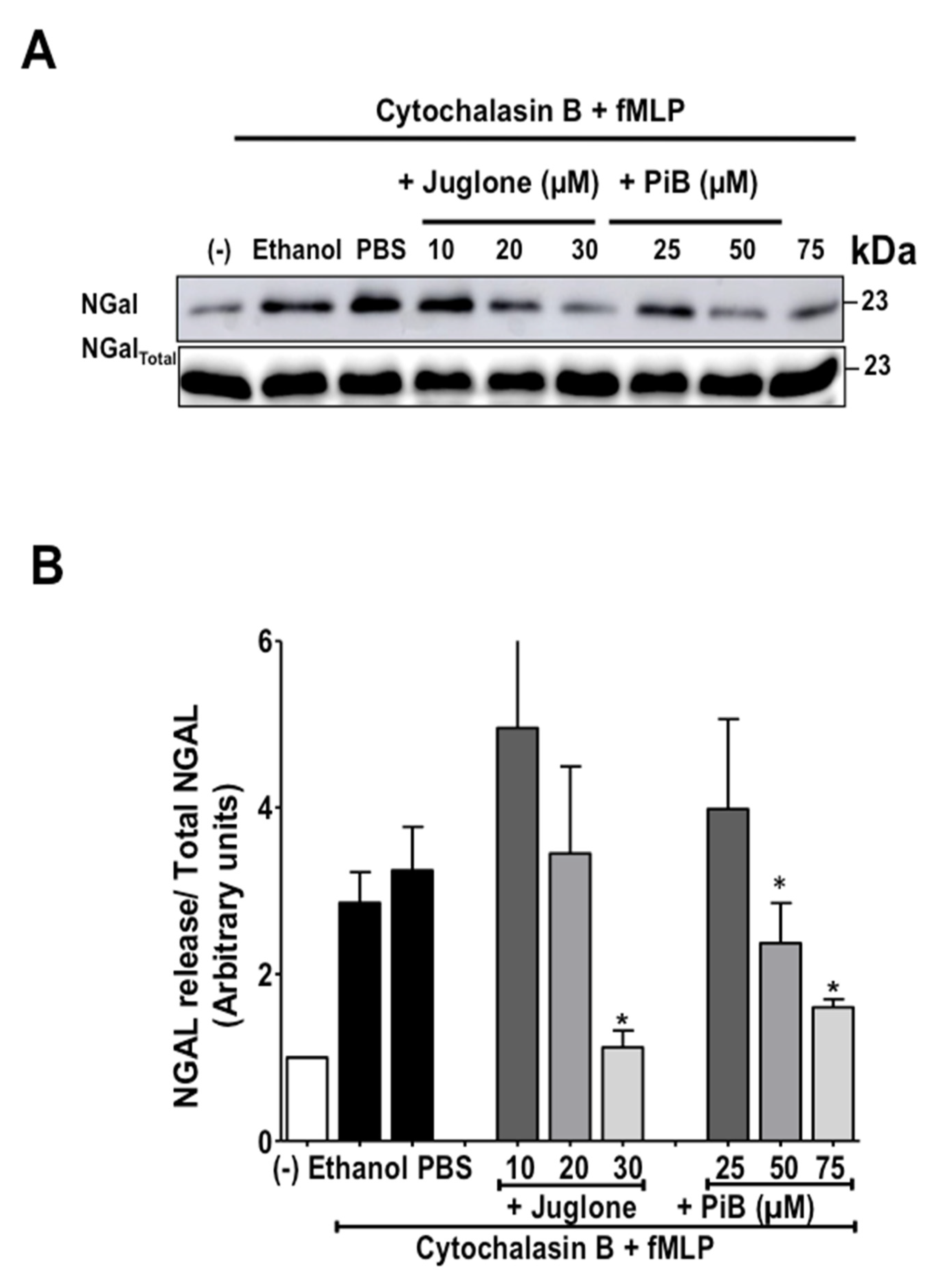
3.3. Juglone and PiB Inhibit fMLP-Induced Neutrophil Degranulation of Azurophil and Specific Granules
3.4. PiB Decreased Both Total ROS and Superoxide Anion Production
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.-C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-Y.; Weninger, W. Neutrophil migration in inflammation: Intercellular signal relay and crosstalk. Curr. Opin. Immunol. 2017, 44, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Brazil, J.C.; Parkos, C.A. Pathobiology of neutrophil–epithelial interactions. Immunol. Rev. 2016, 273, 94–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauseef, W.M. How human neutrophils kill and degrade microbes: An integrated view. Immunol. Rev. 2007, 219, 88–102. [Google Scholar] [CrossRef]
- Nordenfelt, P.; Tapper, H. Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 2011, 90, 271–284. [Google Scholar] [CrossRef]
- Paige, L. Mechanisms of degranulation in neutrophils. Allergy Asthma Immunol. 2006, 2, 98–108. [Google Scholar]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Babior, B.M. Phagocytes and oxidative stress. Am. J. Med. 2000, 109, 33–44. [Google Scholar] [CrossRef]
- El-Benna, J.; Dang, P.M.-C.; Gougerot-Pocidalo, M.-A.; Elbim, C. Phagocyte NADPH oxidase: A multicomponent enzyme essential for host defenses. Arch. Immunol. Ther. Exp. 2005, 53, 199–206. [Google Scholar]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Bang, H.; Mech, C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed. Biochim. Acta 1984, 43, 1101–1111. [Google Scholar]
- Yaffe, M.B.; Schutkowski, M.; Shen, M.; Zhou, X.Z.; Stukenberg, P.T.; Rahfeld, J.U. Sequence specific and phosphorylation dependent proline isomerazation: A potential mitotic regulatory mechanism. Science 1997, 278, 1957–1960. [Google Scholar] [CrossRef]
- Lippens, G.; Landrieu, I.; Smet, C. Molecular mechanisms of the phospho-dependentprolylcis/trans isomerase Pin1. FEBS J. 2007, 274, 5211–5222. [Google Scholar] [CrossRef]
- Lee, Y.M.; Liou, Y.-C. Gears-In-Motion: The Interplay of WW and PPIase Domains in Pin1. Front. Oncol. 2018, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.J.; Esnault, S.; Schinzel, A.; Borner, C.; Malter, J. The peptidyl-prolylisomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax-activation. Nat. Immunol. 2009, 10, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Que, J.; Chen, Y.C.; Lin, J.T.; Liou, Y.C.; Liao, P.C. Pin1 acts as a negative regulator of the G2/M transition by in-teracting with the Aurora-A Bora complex. J. Cell. Sci. 2013, 126, 4862–4872. [Google Scholar] [PubMed] [Green Version]
- Nakatsu, Y.; Yamamotoya, T.; Ueda, K.; Ono, H.; Inoue, M.K.; Matsunaga, Y. Prolyl isomerase Pin1 in metabolic reprogram-ming of cancer cells. Cancer. Lett. 2020, 470, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-J.; Wulf, G.; Zhou, X.Z.; Davies, P.; Lu, K.P. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999, 399, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Ryo, A.; Uemura, H.; Ishiguro, H.; Saitoh, T.; Yamaguchi, A.; Perrem, K.; Kubota, Y.; Lu, K.P.; Aoki, I. Stable Suppression of Tumorigenicity by Pin1-Targeted RNA Interference in Prostate Cancer. Clin. Cancer Res. 2005, 11, 7523–7531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussetta, T.; Gougerot-Pocidalo, M.A.; Hayem, G.; Ciappelloni, S.; Raad, H.; Arabi, D. Theprolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils. Blood 2010, 116, 5795–5802. [Google Scholar] [CrossRef] [Green Version]
- Makni-Maalej, K.; Boussetta, T.; Hurtado-Nedelec, M.; Belambri, S.A.; Gougerot-Pocidalo, M.A.; El-Benna, J. The TLR7/8 agonist CL097 primes N-formyl-methionyl-leucyl-phenylalanine-stimulated NADPH oxidase activation in human neutrophils: Critical role of p47phox phosphorylation and the proline isomerase Pin1. J. Immunol. 2012, 189, 4657–4665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makni-Maalej, K.; Marzaioli, V.; Boussetta, T.; Belambri, S.A.; Gougerot-Pocidalo, M.-A.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. TLR8, but not TLR7, induces the priming of the NADPH oxidase activation in human neutrophils. J. Leukoc. Biol. 2015, 97, 1081–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Bedouhene, S.; Hurtado-Nedelec, M.; Pintard, C.; Dang, P.M.-C.; Yu, S.; El-Benna, J. The Prolyl Isomerase Pin1 Controls Lipopolysaccharide-Induced Priming of NADPH Oxidase in Human Neutrophils. Front. Immunol. 2019, 10, 2567. [Google Scholar] [CrossRef]
- Hennig, L.; Christner, C.; Kipping, M.; Schelbert, B.; Rücknagel, K.P.; Grabley, S.; Küllertz, G.; Fischer, G. Selective inactivation of par-vulin-likepeptidyl-prolylcis/transisomerases by juglone. Biochemistry 1998, 37, 5953–5960. [Google Scholar] [CrossRef]
- Uchida, T.; Takamiya, M.; Takahashi, M.; Miyashita, H.; Ikeda, H.; Terada, T.; Matsuo, Y.; Shirouzu, M.; Yokoyama, S.; Fujimori, F.; et al. Pin1 and Par14 Peptidyl Prolyl Isomerase Inhibitors Block Cell Proliferation. Chem. Biol. 2003, 10, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.M.-C.; Stensballe, A.; Boussetta, T.; Raad, H.; Dewas, C.; Kroviarski, Y.; Hayem, G.; Jensen, O.N.; Gougerot-Pocidalo, M.-A.; El-Benna, J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Investig. 2006, 116, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Raad, H.; Paciet, M.-H.; Boussetta, T.; Kroviarski, Y.; Morel, F.; Quinn, M.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C.; El-Benna, J. Regulation of the phagocyte NADPH oxidase activity: Phosphorylation of gp91 phox /NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67 phox, and p47 phox. FASEB J. 2008, 23, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.D.; Quie, P.G.; Simmons, R.L. Chemotaxis under agarose: A new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J. Immunol. 1975, 115, 1650–1656. [Google Scholar]
- Bedouhene, S.; Moulti-Mati, F.; Dang, P.M.-C.; El-Benna, J. Oleuropein and hydroxytyrosol inhibit the N-formyl-methionyl-leucyl-phenylalanine-induced neutrophil degranulation and chemotaxis via AKT, p38, and ERK1/2 MAP-Kinase inhibition. Inflammopharmacology 2017, 25, 673–680. [Google Scholar] [CrossRef]
- Bedouhène, S.; Dang, P.M.-C.; Hurtado-Nedelec, M.; El-Benna, J. Neutrophil Degranulation of Azurophil and Specific Granules. Methods Mol. Biol. 2019, 2087, 215–222. [Google Scholar] [CrossRef]
- Yurewicz, E.C.; Zimmerman, M. Cytochalasin B-dependent release of azurophil granule enzymes from human polymorpho-nuclear leukocytes. Inflammation 1977, 2, 259–264. [Google Scholar] [CrossRef]
- Smith, R.J.; Iden, S.S. Properties of concanavalin A-elicited granule exocytosis from human polymorphonuclear neutrophils. Inflammation 1980, 4, 343–358. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, J.T.; Lees, C.J.; Miller, C.H.; McCall, E.C.; Lewis, J.C.; Love, S.H.; Wykle, R.L. Selective desensitization of neutrophils: Further studies with 1-O-alkyl-sn-glycero-3-phosphocholine analogues. J. Immunol. 1981, 127, 731–737. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]
- Bedouhène, S.; Moulti-Mati, F.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils. Am. J. Blood Res. 2017, 7, 41–48. [Google Scholar] [PubMed]
- Chao, S.H.; Greenleaf, A.L.; Price, D.H. Juglone, an inhibitor of the peptidyl-prolylisomerase Pin1, also directly blocks tran-scription. Nucleic Acids Res. 2001, 29, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fila, C.; Metz, C.; van der Sluijs, P. Juglone Inactivates Cysteine-rich Proteins Required for Progression through Mitosis. J. Biol. Chem. 2008, 283, 21714–21724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.-J.; Hu, J.; Kashi, V.P.; Kelly, E.; Denlinger, L.C.; Lutchman, K.; McDonald, J.G.; Jarjour, N.N.; Malter, J.S. Epstein-Barr Virus–induced Gene 2 Mediates Allergen-induced Leukocyte Migration into Airways. Am. J. Respir. Crit. Care Med. 2017, 195, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Heit, B.; Tavener, S.; Raharjo, E.; Kubes, P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 2002, 159, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Futosi, K.; Mócsai, A. Tyrosine kinase signaling pathways in neutrophils. Immunol. Rev. 2016, 273, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Mc Leish, K.R.; Uriarte, S.M.; Tandon, S.; Creed, T.M.; Le, J.; Ward, A.R. Exocytosis of neutrophil granule subsets and activation of prolylisomerase 1 are required for respiratory burst priming. J. Innate Immun. 2013, 5, 277–289. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedouhene, S.; Liu, M.; Senani, N.; Boussetta, T.; Pintard, C.; Dang, P.M.-C.; El-Benna, J. Prolyl-Isomerase Pin1 Controls Key fMLP-Induced Neutrophil Functions. Biomedicines 2021, 9, 1130. https://doi.org/10.3390/biomedicines9091130
Bedouhene S, Liu M, Senani N, Boussetta T, Pintard C, Dang PM-C, El-Benna J. Prolyl-Isomerase Pin1 Controls Key fMLP-Induced Neutrophil Functions. Biomedicines. 2021; 9(9):1130. https://doi.org/10.3390/biomedicines9091130
Chicago/Turabian StyleBedouhene, Samia, Min Liu, Nassima Senani, Tarek Boussetta, Coralie Pintard, Pham My-Chan Dang, and Jamel El-Benna. 2021. "Prolyl-Isomerase Pin1 Controls Key fMLP-Induced Neutrophil Functions" Biomedicines 9, no. 9: 1130. https://doi.org/10.3390/biomedicines9091130
APA StyleBedouhene, S., Liu, M., Senani, N., Boussetta, T., Pintard, C., Dang, P. M.-C., & El-Benna, J. (2021). Prolyl-Isomerase Pin1 Controls Key fMLP-Induced Neutrophil Functions. Biomedicines, 9(9), 1130. https://doi.org/10.3390/biomedicines9091130






