Immunological Status of Bladder Cancer Patients Based on Urine Leukocyte Composition at Radical Cystectomy
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Isolation of Urine Cells
2.3. Staining Urine Cells
2.4. Peripheral Blood
2.5. Histology
2.6. Urine IL-6 and IL-8 Concentration
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Muscle-Invasive Bladder Cancer (MIBC) Patients and Urine Cell Composition
3.2. Neoadjuvant Chemotherapy Influences Urine Composition in MIBC Patients
3.3. Smoking Status Influences Urine Composition in MIBC Patients
3.4. The Affectation of Lymph Nodes at RC Time Influences Urine Cell Composition
3.5. Negative Correlation between Lymphocytes in Urine and in Bladder Biopsy at the Time of RC
3.6. The Composition of Urine at the Time of RC Time Is Associated with the Recurrence of LN (−) in Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Witjes, J.A.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; de Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; de Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; van der Heijden, A.G.; Sherif, A. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2013 Guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef]
- Cattaneo, F.; Motterle, G.; Zattoni, F.; Morlacco, A.; Moro, F.D. The Role of Lymph Node Dissection in the Treatment of Bladder Cancer. Front Surg. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, B.J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1 054 Patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leão, R.; Máximo, V.; Soares, P. Biomarkers for bladder cancer diagnosis and surveillance: A comprehensive review. Diagnostics 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Vlachostergios, P.J.; Faltas, B.M. The molecular limitations of biomarker research in bladder cancer. World J. Urol. 2019, 37, 837–848. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) 2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Minireview Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, E.C.; de Jong, W.H.; van der Meijden, A.P.M.; Steerenberg, P.A.; Witjes, F.; Vegt, P.D.J.; Debruyne, F.M.J.; Ruitenberg, E.J. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer—A flow cytofluorometric analysis. Urol. Res. 1991, 19, 45–50. [Google Scholar] [CrossRef]
- Black, A.J.; Sc, B.; Zargar, H.; Zargar-shoshtari, K.; Fairey, A.S.; Mertens, L.S.; Dinney, C.P.; Mir, M.C.; Krabbe, L.M.; Cookson, M.S.; et al. The prognostic value of the neutrophil-to-lymphocyte ratio in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy and radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 3–e17. [Google Scholar] [CrossRef]
- Guillamo, C.F.; Gimeno, L.; Server, G.; Martínez-Sánchez, M.V.; Escudero, J.F.; López-Cubillana, P.; Cabezas-Herrera, J.; Campillo, J.A.; Abellan, D.J.; Martínez-García, J.; et al. Immunological Risk Stratification of Bladder Cancer Based on Peripheral Blood Natural Killer Cell Biomarkers. Eur. Urol. Oncol. 2021, 4, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Shaul, M.E.; Fridlender, Z.G. Cancer-related circulating and tumor-associated neutrophils—Subtypes, sources and function. FEBS J. 2018, 285, 4316–4342. [Google Scholar] [CrossRef]
- Pfistershammer, K.; Majdic, O.; Stöckl, J.; Zlabinger, G.; Kirchberger, S.; Steinberger, P.; Knapp, W. CD63 as an Activation-Linked T Cell Costimulatory Element. J. Immunol. 2004, 173, 6000–6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Y.N.S.; Joshi, K.; Khetrapal, P.; Ismail, M.; Reading, J.L.; Sunderland, M.W.; Georgiou, A.; Furness, A.J.S.; Ben Aissa, A.; Ghorani, E.; et al. Urine-derived lymphocytes as a non-invasive measure of the bladder tumor immune microenvironment. J. Exp. Med. 2018, 215, 2748–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2020, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Sakatsume, M.; Xie, Y.; Ueno, M.; Obayashi, H.; Goto, S.; Narita, I.; Homma, N.; Tasaki, K.; Suzuki, Y.; Gejyo, F. Human glomerulonephritis accompanied by active cellular infiltrates shows effector T cells in urine. J. Am. Soc. Nephrol. 2001, 12, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [Green Version]
- Seiler, R.; Gibb, E.A.; Wang, N.Q.; Oo, H.Z.; Lam, H.M.; van Kessel, K.E.; Voskuilen, C.S.; Winters, B.; Erho, N.; Takhar, M.M.; et al. Divergent biological response to neoadjuvant chemotherapy in muscle-invasive bladder cancer. Clin. Cancer Res. 2019, 25, 5082–5093. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.K.; Pham, A.; Vuong, W.; Liu, X.; Lin, Y.; Ruel, N.; Yuh, B.E.; Chan, K.; Wilson, T.; Lerner, S.P.; et al. Prognostic Significance of Neutrophilic Infiltration in Benign Lymph Nodes in Patients with Muscle-invasive Bladder Cancer. Eur. Urol. Focus 2017, 3, 130–135. [Google Scholar] [CrossRef]
- Suttmann, H.; Riemensberger, J.; Bentien, G.; Schmaltz, D.; Stöckle, M.; Jocham, D.; Böhle, A.; Brandau, S. Neutrophil granulocytes are required for effective Bacillus Calmette-Guérin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006, 66, 8250–8257. [Google Scholar] [CrossRef] [Green Version]
- De Franco, J.E. Neutrophils: Lifespan, Functions and Roles in Disease; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2010; pp. 235–253. [Google Scholar]
- Wang, T.-T.; Zhao, Y.-L.; Peng, L.-S.; Chen, N.; Chen, W.; Lv, Y.-P.; Mao, F.-Y.; Zhang, J.-Y.; Cheng, P.; Teng, Y.-S.; et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oki, Y.; Buglio, D.; Zhang, J.; Ying, Y.; Zhou, S.; Sureda, A.; Ben-Yehuda, D.; Zinzani, P.L.; Prince, H.M.; Harrison, S.J.; et al. Immune regulatory effects of panobinostat in patients with Hodgkin lymphoma through modulation of serum cytokine levels and T-cell PD1 expression. Blood Cancer J. 2014, 4, e236. [Google Scholar] [CrossRef]
- Sherif, A.; Winerdal, M.; Winqvist, O. Immune Responses to Neoadjuvant Chemotherapy in Muscle Invasive Bladder Cancer. Bladder Cancer 2018, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Chung, J.H.; Kim, S.H.; Lee, K.S.; Suh, K.J.; Lee, J.Y.; Kim, J.W.; Lee, J.O.; Kim, J.W.; Kim, Y.J.; et al. Effect of platinum-based chemotherapy on PD-L1 expression on tumor cells in non-small cell lung cancer. Cancer Res. Treat 2019, 51, 1086–1097. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κBto foster an immunosuppressive tumor microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.; Neuberger, M.; Daurkin, I.; Perrin, G.Q.; Algood, C.; Dahm, P.; Rosser, C.; Vieweg, J.; Gilbert, S.M.; Kusmartsev, S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int. J. Cancer 2012, 130, 1109–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, K.M.; Çolak, Y.; Ellervik, C.; Hasselbalch, H.C.; Bojesen, S.E.; Nordestgaard, B.G. Smoking and Increased White and Red Blood Cells: A Mendelian Randomization Approach in the Copenhagen General Population Study. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.; Bassi, A.; Gregg, E.O.; Nunziata, A.; Puntoni, R.; Corsini, E. Effects of cigarette smoking on circulating leukocytes and plasma cytokines in monozygotic twins. Clin. Chem. Lab. Med. 2015, 53, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerdidani, D.; Magkouta, S.; Chouvardas, P.; Karavana, V.; Glynos, K.; Roumelioti, F.; Zakynthinos, S.; Wauters, E.; Janssens, W.; Lambrechts, D.; et al. Cigarette Smoke–Induced Emphysema Exhausts Early Cytotoxic CD8 + T Cell Responses against Nascent Lung Cancer Cells. J. Immunol. 2018, 201, 1558–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- César-Neto, J.B.; Duarte, P.M.; De Oliveira, M.C.G.; Casati, M.Z.; Tambeli, C.H.; Parada, C.A.; Sallum, E.A.; Nociti, F.H. Smoking modulates interferon-γ expression in the gingival tissue of patients with chronic periodontitis. Eur. J. Oral Sci. 2006, 114, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.; Hu, X.; Gu, L.; Chen, B.; Khadaroo, P.A.; Shen, Z.; Dong, L.; Lv, Y.; Chitumba, M.N.; Liu, J. Smokers or non-smokers: Who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up-to-date meta-analysis. World J. Surg. Oncol. 2020, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.H.; de la Iglesia, J.V.; Wang, X.; Song, F.; Chaudhary, R.; Masannat, J.; Conejo-Garcia, J.; Hernandez-Prera, J.; Slebos, R. Tobacco smoking is associated with the immune suppressive microenvironment in head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2019, 30, v772. [Google Scholar] [CrossRef]
- Hautmann, R.E.; De Petriconi, R.C.; Pfeiffer, C.; Volkmer, B.G. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: Long-term results in 1100 patients. Eur. Urol. 2012, 61, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Meng, L.; Wang, X.; Diao, T.; Hu, M.; Wang, M.; Liu, M.; Wang, J. Young age increases the risk of lymph-node metastasis in patients with muscle-invasive bladder urothelial carcinoma. BMC Cancer 2020, 20, 851. [Google Scholar] [CrossRef]
- Li, F.; Hong, X.; Hou, L.; Lin, F.; Chen, P.; Pang, S.; Du, Y.; Huang, H.; Tan, W. A greater number of dissected lymph nodes is associated with more favorable outcomes in bladder cancer treated by radical cystectomy: A meta-analysis. Oncotarget 2016, 7, 61284–61294. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Zhan, Y.; Chen, X.; Wu, B.; Liu, B. Bladder cancer exhibiting high immune infiltration shows the lowest response rate to immune checkpoint inhibitors. Front. Oncol. 2019, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, G.; Lövgren, K.; Lauss, M.; Chebil, G.; Patschan, O.; Gudjonsson, S.; Månsson, W.; Fernö, M.; Leandersson, K.; Lindgren, D.; et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. 2014, 32, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, S.M.A.; Lee, A.H.S.; Paish, E.C.; MacMillan, R.D.; Ellis, I.O.; Green, A.R. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat. 2012, 132, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Takakura, K.; Ito, Z.; Suka, M.; Kanai, T.; Matsumoto, Y.; Odahara, S.; Matsudaira, H.; Haruki, K.; Fujiwara, Y.; Saito, R.; et al. Comprehensive assessment of the prognosis of pancreatic cancer: Peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand. J. Gastroenterol. 2016, 51, 610–617. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Nakano, O.; Naito, Y.; Nagura, H.; Ohtani, H.; Nakano, O.; Sato, M.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; et al. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopath-ologic demonstration of antitumor immunity. Cancer Res. 2001, 61, 5132–5136. [Google Scholar]
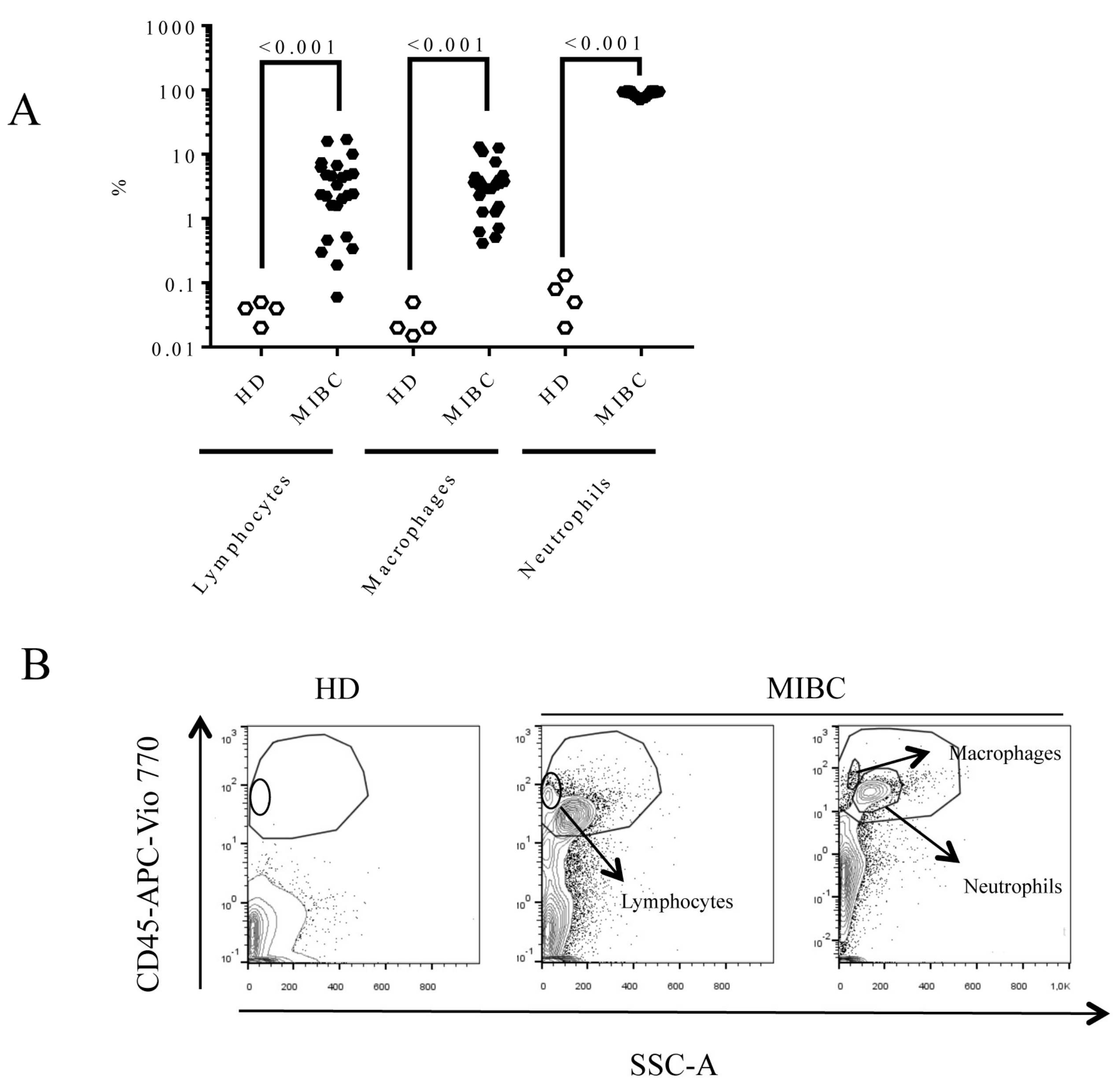

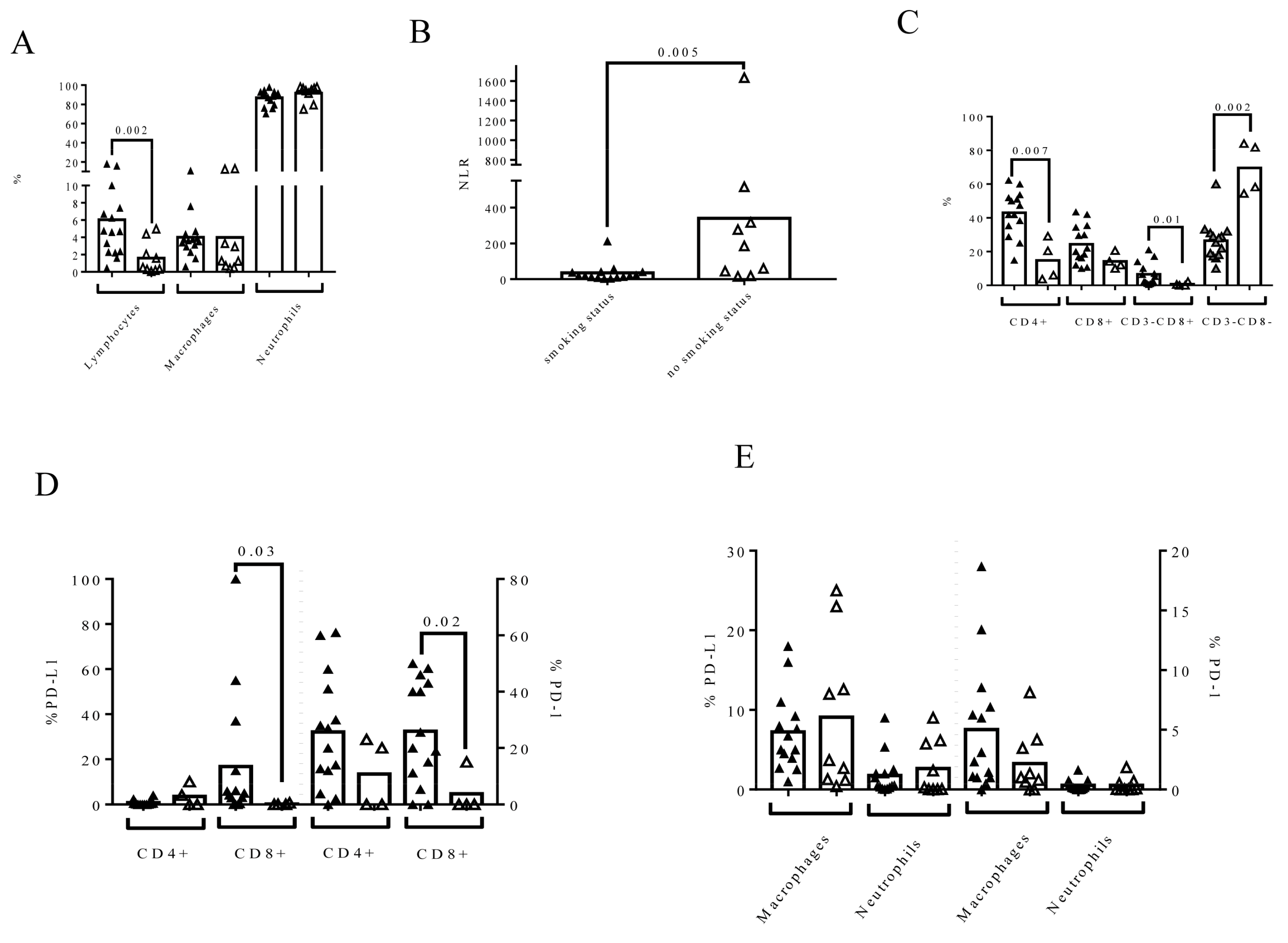

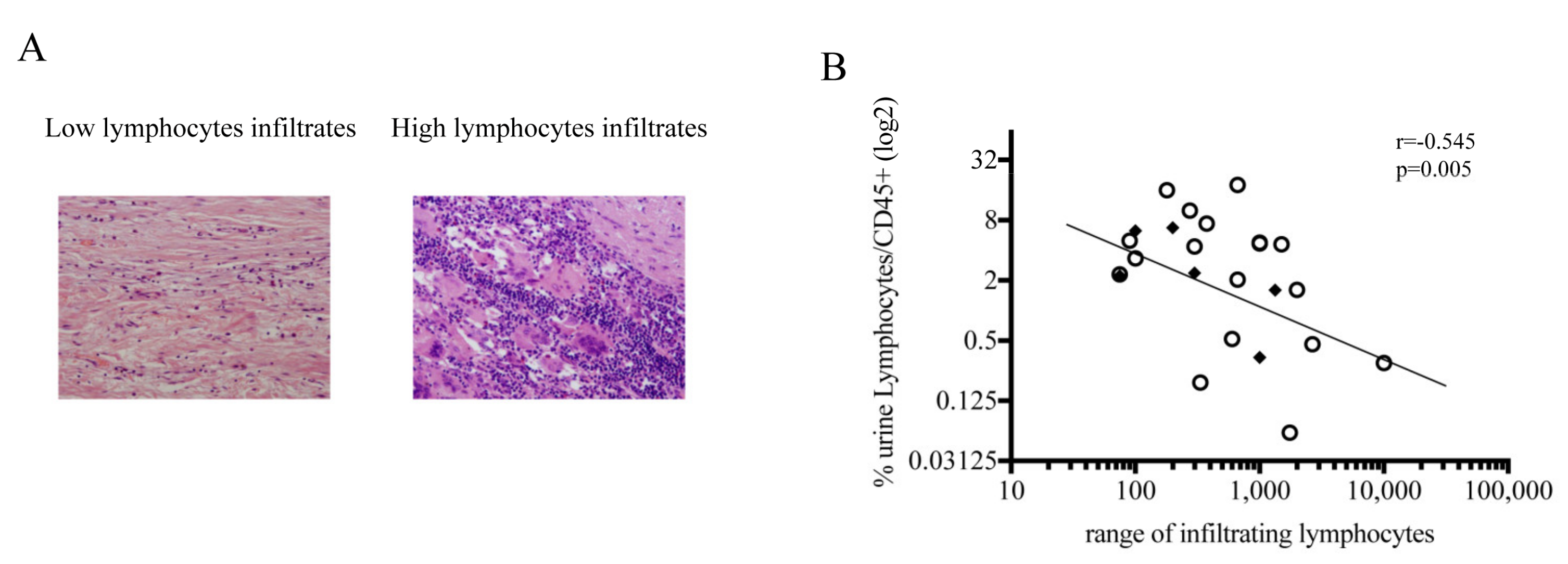
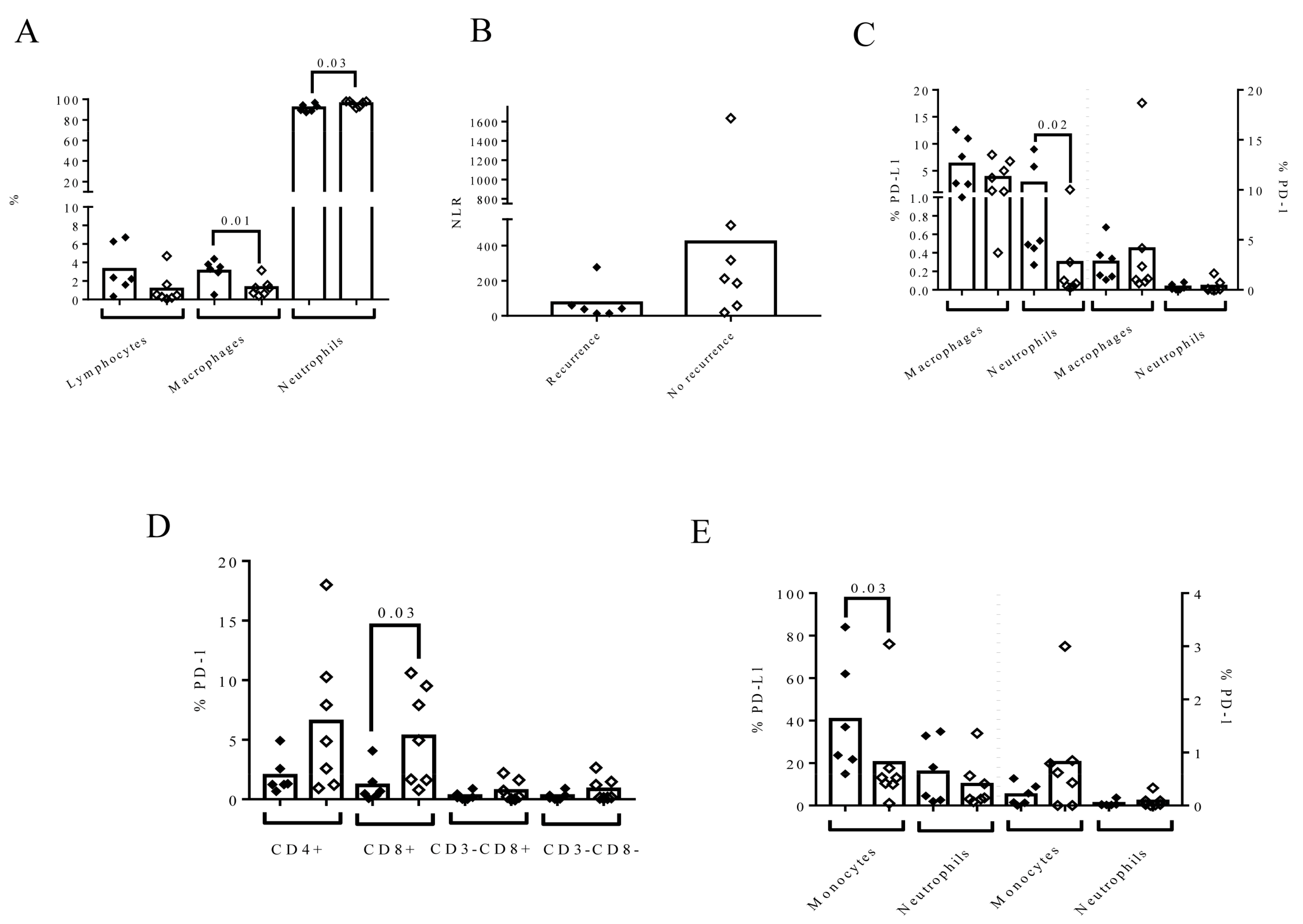
| MIBC (n = 24) | |
|---|---|
| Age (years, mean ± SD) | 67.96 ± 10.58 |
| Male, n (%) | 18 (75) |
| Smoking status, n (%) | |
| Yes | 15 (62.5) |
| No | 6 (37.5) |
| Previous treatment to RTU, n (%) | |
| BCG | 7 (29.16) |
| Mitomycin | 2 (8.34) |
| no treatment | 15 (62.5) |
| Neoadjuvant chemotherapy, n (%) | |
| Yes | 9 (37.5) |
| No | 15 (62.5) |
| Lymph node, n (%) | |
| Positive | 5 (20.8) |
| Negative | 19 (79.2) |
| Carcinoma in situ, n (%) | |
| Positive | 13 (54.16) |
| Negative | 11 (45.84) |
| Urine | Blood | |
|---|---|---|
| %Lymphocytes/CD45+ | 2.84 (0.79–5.94) | 27.91 (20.7–31.22) |
| %Macrophages-monocytes/CD45+ | 3.35 (1.34–4.27) | 5.06 (3.09–6.97) |
| %Neutrophils/CD45+ | 90.75 (80.86–94.95) | 62.34 (56.88–67.21) |
| Neutrophil/Lymphocyte | 30.27 (14.22–154.6) | 2.13 (1.81–3.38) |
| Lymphocyte subpopulations | (n = 18) | (n = 24) |
| % CD4+ | 40.24 (23.87–51.24) | 52.91 (43.21–58.28) |
| CD4+PD-L1+/CD4+ | 0.3 (0–1.33) | 0.045(0.01–0.30) |
| CD4+PD1+/CD4+ | 20 (3.6–30) | 2.03 (1.03–4.91) |
| %CD8+ | 19.23 (12–31.08) | 23.37 (19.57–32.68) |
| CD8+PD-L1+/CD8+ | 3.16 (0–15) | 0.79 (0.06–30.87) |
| CD8+ PD1+/CD8+ | 15 (0–40) | 1.98 (0.65–4.74) |
| % CD3-CD8+ | 2.25 (0.92–7.75) | 3.77 (1.94–5.5) |
| % CD3-CD8- | 29 (18.78–55.44) | 18.83 (16.06–21.24) |
| %Macrophages-monocytes | ||
| PD-L1+/macrophages | 5 (2.68–11.75) | 18.85 (13.03–34.98) |
| PD1+/macrophages | 1.39 (0.68–6.18) | 0.10 (0–0.59) |
| %Neutrophils | ||
| PD-L1+/neutrophils | 0.51 (0.10–3.64) | 16 (3.08–32.18) |
| PD1+/neutrophils | 0.13 (0.03–0.51) | 0.02 (0.01–0.09) |
| Lymphocytes/CD326+ | 1.48 (0.48–9.02) | |
| % PD-L1+ CD326+ | 6.87 (1.87–14.73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantó, E.; Faba, Ó.R.; Zamora, C.; Mulet, M.; Garcia-Cuerva, M.S.; Palomino, A.; Anguera, G.; Breda, A.; Maroto, P.; Vidal, S. Immunological Status of Bladder Cancer Patients Based on Urine Leukocyte Composition at Radical Cystectomy. Biomedicines 2021, 9, 1125. https://doi.org/10.3390/biomedicines9091125
Cantó E, Faba ÓR, Zamora C, Mulet M, Garcia-Cuerva MS, Palomino A, Anguera G, Breda A, Maroto P, Vidal S. Immunological Status of Bladder Cancer Patients Based on Urine Leukocyte Composition at Radical Cystectomy. Biomedicines. 2021; 9(9):1125. https://doi.org/10.3390/biomedicines9091125
Chicago/Turabian StyleCantó, Elisabet, Óscar Rodríguez Faba, Carlos Zamora, Maria Mulet, Maria Soledad Garcia-Cuerva, Ana Palomino, Georgia Anguera, Alberto Breda, Pablo Maroto, and Sílvia Vidal. 2021. "Immunological Status of Bladder Cancer Patients Based on Urine Leukocyte Composition at Radical Cystectomy" Biomedicines 9, no. 9: 1125. https://doi.org/10.3390/biomedicines9091125
APA StyleCantó, E., Faba, Ó. R., Zamora, C., Mulet, M., Garcia-Cuerva, M. S., Palomino, A., Anguera, G., Breda, A., Maroto, P., & Vidal, S. (2021). Immunological Status of Bladder Cancer Patients Based on Urine Leukocyte Composition at Radical Cystectomy. Biomedicines, 9(9), 1125. https://doi.org/10.3390/biomedicines9091125







