Cytoskeleton Response to Ionizing Radiation: A Brief Review on Adhesion and Migration Effects
Abstract
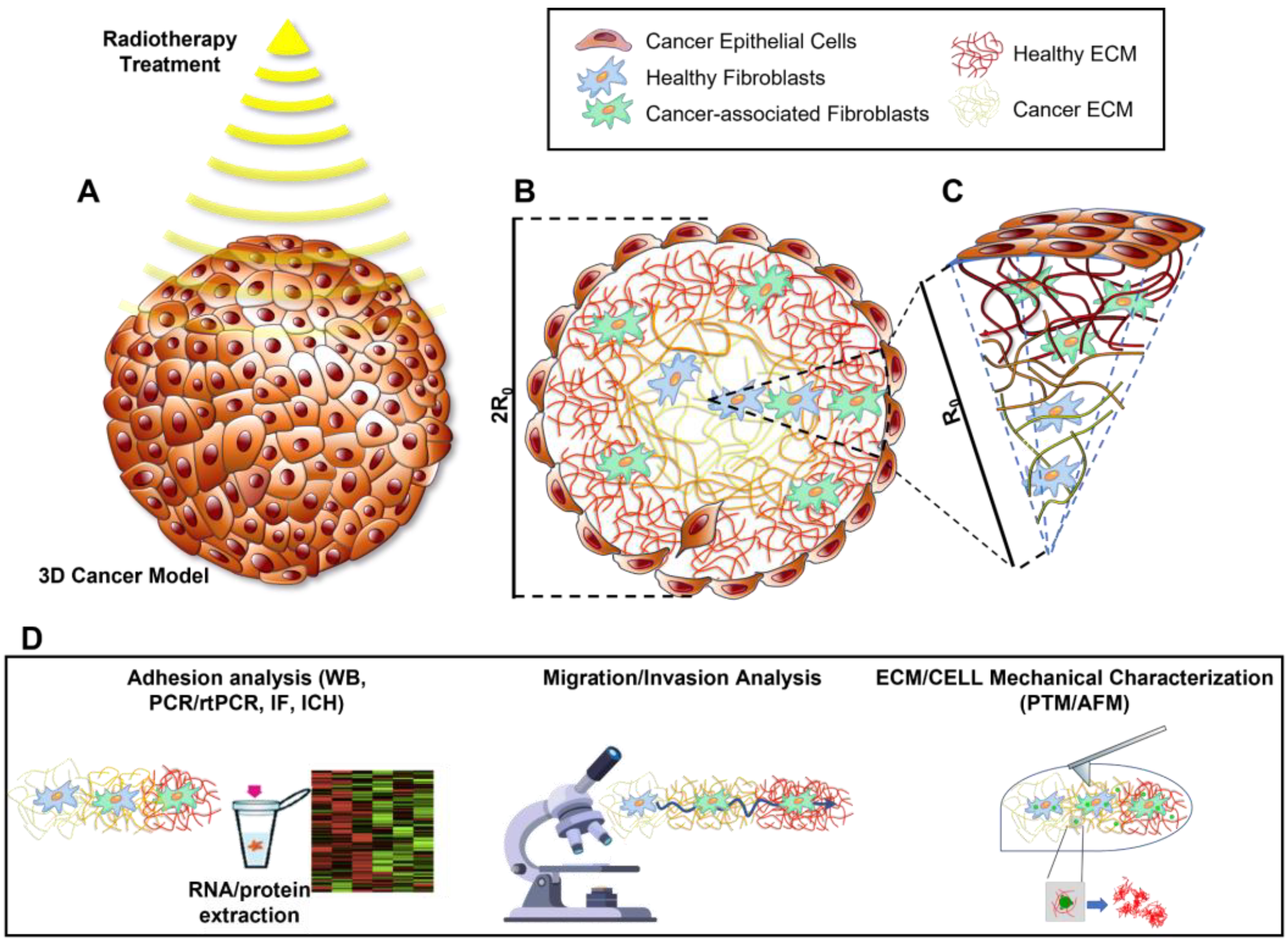
:1. Introduction
2. Radiation Effects on the Actin CSK
2.1. Ionizing Radiation and the Increase in the Polymerization of the Actin Filament
2.2. Ionizing Radiations and the Decrease in the Expression of the Actin Filament
3. Radiation Effects on Cell Adhesion
3.1. Radiation-Induced Changes in the Cell Adhesiveness through the Activation of Proteins Pathways
3.2. Alteration in Cells Adhesive Capabilities Stimulated by the Radiation-Induced Changes in the CSK
4. Radiation Effects on Cell Migration
4.1. Ionizing Radiation Increase Cell Motility through the Alteration of the CSK
4.2. Radiation-Induced Cell Migration through Protein Expression
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.L.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Ladoux, B.; Mège, R.M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef]
- Gefen, A.; Weihs, D. Mechanical cytoprotection: A review of cytoskeleton-protection approaches for cells. J. Biomech. 2016, 49, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Dehghani, F. The Cytoskeleton-A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [Green Version]
- Ohi, R.; Zanic, M. Ahead of the Curve: New Insights into Microtubule Dynamics. F1000Research 2016, 5, 314. [Google Scholar] [CrossRef] [PubMed]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef]
- Forth, S.; Kapoor, T.M. The mechanics of microtubule networks in cell division. J. Cell Biol. 2017, 216, 1525–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessels, M.M.; Qualmann, B. Interplay between membrane curvature and the actin cytoskeleton. Curr. Opin. Cell Biol. 2021, 68, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Porat-Shliom, N.; Milberg, O.; Masedunskas, A.; Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol. Life Sci. 2013, 70, 2099–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plastino, J.; Blanchoin, L. Dynamic stability of the actin ecosystem. J. Cell Sci. 2018, 132, jcs219832. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef]
- Goldmann, W.H. Intermediate filaments and cellular mechanics. Cell Biol. Int. 2018, 42, 132–138. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate filaments: Molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef]
- Wang, N.; Stamenović, D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am. J. Physiol. Cell Physiol. 2000, 279, C188–C194. [Google Scholar] [CrossRef] [Green Version]
- Nekrasova, O.E.; Mendez, M.G.; Chernoivanenko, I.S.; Tyurin-Kuzmin, P.A.; Kuczmarski, E.R.; Gelfand, V.I.; Goldman, R.D.; Minin, A.A. Vimentin intermediate filaments modulate the motility of mitochondria. Mol. Biol. Cell 2011, 22, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Goldman, R.D. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 2004, 5, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Biol. 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- De Pascalis, C.; Pérez-González, C.; Seetharaman, S.; Boëda, B.; Vianay, B.; Burute, M.; Leduc, C.; Borghi, N.; Trepat, X.; Etienne-Manneville, S. Intermediate filaments control collective migration by restricting traction forces and sustaining cell-cell contacts. J. Cell Biol. 2018, 217, 3031–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charrier, E.E.; Janmey, P.A. Mechanical Properties of Intermediate Filament Proteins. Methods Enzymol. 2016, 568, 35–57. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Brown, A.C.; Myers, D.R.; Sakurai, Y.; Mannino, R.G.; Tran, R.; Ahn, B.; Hardy, E.T.; Kee, M.F.; Kumar, S.; et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. USA 2014, 111, 14430–14435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paluch, E.K.; Aspalter, I.M.; Sixt, M. Focal adhesion-indipendent cell migration. Annu. Rev. Cell Dev. Biol. 2016, 32, 469–490. [Google Scholar] [CrossRef]
- Muñoz-Lasso, D.C.; Romá-Mateo, C.; Pallardó, F.V.; Gonzalez-Cabo, P. Much More Than a Scaffold: Cytoskeletal Proteins in Neurological Disorders. Cells 2020, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Kounakis, K.; Tavernarakis, N. The Cytoskeleton as a Modulator of Aging and Neurodegeneration. Adv. Exp. Med. Biol. 2019, 1178, 227–245. [Google Scholar] [CrossRef]
- Sferra, A.; Nicita, F.; Bertini, E. Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7354. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Stumptner, C.; Zatloukal, K.; Denk, H. Intermediate filament cytoskeleton of the liver in health and disease. Histochem. Cell Biol. 2008, 129, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kräter, M.; Sapudom, J.; Bilz, N.C.; Pompe, T.; Guck, J.; Claus, C. Alterations in Cell Mechanics by Actin Cytoskeletal Changes Correlate with Strain-Specific Rubella Virus Phenotypes for Cell Migration and Induction of Apoptosis. Cells 2018, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporizzo, M.A.; Chen, C.Y.; Prosser, B.L. Cardiac microtubules in health and heart disease. Exp. Biol. Med. 2019, 244, 1255–1272. [Google Scholar] [CrossRef]
- Menachery, A.; Sapudom, J.; Vembadi, A.; Alatoom, A.; Teo, J.; Qasaimeh, M.A. Dielectrophoretic characterization of dendritic cell deformability upon maturation. Biotechniques 2021, 70, 29–36. [Google Scholar] [CrossRef]
- Alatoom, A.; Sapudom, J.; Soni, P.; Mohamed, W.K.E.; Garcia-Sabaté, A.; Teo, J. Artificial Biosystem for Modulation of Interactions between Antigen-Presenting Cells and T Cells. Adv. Biosyst. 2020, 4, e2000039. [Google Scholar] [CrossRef]
- Blumenthal, D.; Chandra, V.; Avery, L.; Burkhardt, J.K. Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex. Elife 2020, 9, e55995. [Google Scholar] [CrossRef]
- Hall, A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009, 28, 5–14. [Google Scholar] [CrossRef]
- Panzetta, V.; Musella, I.; Rapa, I.; Volante, M.; Netti, P.A.; Fusco, S. Mechanical phenotyping of cells and extracellular matrix as grade and stage markers of lung tumor tissues. Acta Biomater. 2017, 57, 334–341. [Google Scholar] [CrossRef]
- Rao, J.; Li, N. Microfilament actin remodeling as a potential target for cancer drug development. Curr. Cancer Drug Targets 2004, 4, 345–354. [Google Scholar] [CrossRef]
- Kumar, R.; Sanawar, R.; Li, X.; Li, F. Structure, biochemistry, and biology of PAK kinases. Gene 2017, 605, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hourwitz, M.J.; Campanello, L.; Fourkas, J.T.; Losert, W.; Parent, C.A. Actin Cytoskeleton and Focal Adhesions Regulate the Biased Migration of Breast Cancer Cells on Nanoscale Asymmetric Sawteeth. ACS Nano 2019, 13, 1454–1468. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J. Biomech. Eng. 2017, 139, 0210051-9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spatarelu, C.P.; Zhang, H.; Trung Nguyen, D.; Han, X.; Liu, R.; Guo, Q.; Notbohm, J.; Fan, J.; Liu, L.; Chen, Z. Biomechanics of Collective Cell Migration in Cancer Progression: Experimental and Computational Methods. ACS Biomater. Sci. Eng. 2019, 5, 3766–3787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.J.; Tang, Y.J.; Tang, Y.L.; Liang, X.H. What makes cells move: Requirements and obstacles for leader cells in collective invasion. Exp. Cell Res. 2019, 382, 111481. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.; Spill, F.; Kamm, R.D.; Zaman, M.H. Single-Cell Migration in Complex Microenvironments: Mechanics and Signaling Dynamics. J. Biomech. Eng. 2016, 138, 021004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascalis, C.; Etienne-Manneville, S. Single and collective cell migration: The mechanics of adhesions. Mol. Biol. Cell 2017, 28, 1833–1846. [Google Scholar] [CrossRef]
- van Helvert, S.; Storm, C.; Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018, 20, 8–20. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [Green Version]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [Green Version]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.; Heinrich, M.A.; Moreira Teixeira, L.; Prakash, J. 3D in vitro model (R) evolution: Unveiling tumor–stroma interactions. Trends Cancer 2021, 7, 249–264. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016, 38, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Karahalil, B.; Yardım-Akaydin, S.; Nacak Baytas, S. An overview of microtubule targeting agents for cancer therapy. Arh. Hig. Rada. Toksikol. 2019, 70, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Sapudom, J.; Pompe, T. Biomimetic tumor microenvironments based on collagen matrices. Biomater. Sci. 2018, 6, 2009–2024. [Google Scholar] [CrossRef]
- Riedel, S.; Hietschold, P.; Krömmelbein, C.; Kunschmann, T.; Konieczny, R.; Knolle, W.; Mierke, C.T.; Zink, M.; Mayr, S.G. Design of biomimetic collagen matrices by reagent-free electron beam induced crosslinking: Structure-property relationships and cellular response. Mater. Des. 2019, 168, 107606. [Google Scholar] [CrossRef]
- Sapudom, J.; Kalbitzer, L.; Wu, X.; Martin, S.; Pompe, T. Fibril bending stiffness of 3D collagen matrices instructs spreading and clustering of invasive and non-invasive breast cancer cells. Biomaterials 2019, 193, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Goldsmith, C.; Brada, M. Radiotherapy. Handb. Clin. Neurol. 2012, 104, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Blyth, B.J.; Cole, A.J.; MacManus, M.P.; Martin, O.A. Radiation therapy-induced metastasis: Radiobiology and clinical implications. Clin. Exp. Metastasis 2018, 35, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Bräuer-Krisch, E.; Hill, M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br. J. Radiol. 2018, 91, 20170628. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiaion in mammalian cells: Identities, mechanisms of formation and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [PubMed]
- Sinclair, W.K.; Fry, R.J.M. Mechanism of radiation interaction with DNA: Potential implication for radiation protection. Radial. Res. 1987, 112, 407–417. [Google Scholar] [CrossRef]
- Smith, C.A. DNA repair in specific sequences in mammalian cells. J. Cell Sci. Suppl. 1987, 6, 225–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkind, M.M. Repair process in radiation biology. Radiat. Res. 1984, 100, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Woloschack, G.E.; Chang-Liu, C.M.; Shearin Jones, P.; Jones, C.A. Modulation of gene expression in Syrian hamster embryo cells following ionizing radiation. Cancer Res. 1990, 50, 339–344. [Google Scholar]
- Woloschak, G.E.; Shearin-Jones, P.; Chang-Liu, C.M. Effects of ionizing radiation on expression of genes encoding cytoskeletal elements: Kinetics and dose effects. Mol. Carcinog. 1990, 3, 374–378. [Google Scholar] [CrossRef]
- Woloschak, G.E.; Chang-Liu, C.M. Expression of cytoskeletal elements in proliferating cells following radiation exposure. Int. J. Radiat. Biol. 1991, 59, 1173–1183. [Google Scholar] [CrossRef]
- Jasińska-Konior, K.; Wiecheć, O.; Sarna, M.; Panek, A.; Swakoń, J.; Michalik, M.; Urbańska, K.; Elas, M. Increased elasticity of melanoma cells after low-LET proton beam due to actin cytoskeleton rearrangements. Sci. Rep. 2019, 9, 7008. [Google Scholar] [CrossRef] [Green Version]
- Amano, M.; Masanori, N.; Kozo, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Gabryś, D.; Greco, O.; Patel, G.; Prise, K.M.; Tozer, G.M.; Kanthou, C. Radiation effects on the cytoskeleton of endothelial cells and endothelial monolayer permeability. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1553–1562. [Google Scholar] [CrossRef]
- Panzetta, V.; Musella, I.; Pugliese, M.; Piccolo, C.; Pasqua, G.; Netti, P.; Fusco, S. Effects of High Energy X-Rays on Cell Morphology and Functions. In Proceedings of the 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG), Coimbra, Portugal, 16–18 February 2017; pp. 1–4. [Google Scholar]
- Panzetta, V.; De Menna, M.; Musella, I.; Pugliese, M.; Quarto, M.; Netti, P.A.; Fusco, S. X-rays effects on cytoskeleton mechanics of healthy and tumor cells. Cytoskeleton 2017, 74, 40–52. [Google Scholar] [CrossRef]
- Fusco, S.; Panzetta, V.; Embrione, V.; Netti, P.A. Crosstalk between focal adhesions and material mechanical properties governs cell mechanics and functions. Acta Biomater. 2015, 23, 63–71. [Google Scholar] [CrossRef]
- Mohammadkarim, A.; Tabatabaei, M.; Parandakh, A.; Mokhtari-Dizaji, M.; Tafazzoli-Shadpour, M.; Khani, M.M. Radiation therapy affects the mechanical behavior of human umbilical vein endothelial cells. J. Mech. Behav. Biomed. Mater. 2018, 85, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Franchitto, A.; Pichierri, P.; Piergentili, R.; Crescenzi, M.; Bignami, M.; Palitti, F. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 2003, 22, 2110–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, M.; Witkin, K.L.; Cohen-Fix, O. Sizing up the nucleus: Nuclear shape, size and nuclear-envelope assembly. J. Cell Sci. 2009, 22 Pt 10, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Pennacchio, F.A.; Nastaly, P.; Poli, A.; Maiuri, P. Tailoring cellular function: The contribution of the nucleus in mechanotransduction. Front. Bioeng. Biotechnol. 2020, 8, 596746. [Google Scholar] [CrossRef] [PubMed]
- Vishavkarma, R.; Raghavan, S.; Kuyyamudi, C.; Majumder, A.; Dhawan, J.; Pullarkat, P.A. Role of actin filaments in correlating nuclear shape and cell spreading. PLoS ONE 2014, 9, e107895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmann, T.; Grabiec, U.; Vogel, C.; Ghadban, C.; Ensminger, S.; Bache, M.; Vordermark, D.; Dehghani, F. The Impact of Non-Lethal Single-Dose Radiation on Tumor Invasion and Cytoskeletal Properties. Int. J. Mol. Sci. 2017, 18, 2001. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Ras-related GTPases and the cytoskeleton. Mol. Biol. Cell 1992, 3, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhang, J.; Zheng, Q.; Li, M.; Liu, Y.; Zhang, B.; Liu, B.; Zhang, H.; Miao, G. Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons. Neural Regen. Res. 2014, 9, 1129–1137. [Google Scholar] [CrossRef]
- Gordon-Weeks, P.R.; Fournier, A.E. Neuronal cytoskeleton in synaptic plasticity and regeneration. J. Neurochem. 2014, 129, 206–212. [Google Scholar] [CrossRef]
- Moss, D.K.; Betin, V.M.; Malesinski, S.D.; Lane, J.D. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 2006, 119, 2362–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramojanee, S.N.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Low-dose dental irradiation decreases oxidative stress in osteoblastic MC3T3-E1 cells without any changes in cell viability, cellular proliferation and cellular apoptosis. Arch. Oral Biol. 2012, 57, 252–256. [Google Scholar] [CrossRef]
- Sangsuwan, T.; Haghdoost, S. The nucleotide pool, a target for low-dose gamma-ray-induced oxidative stress. Radiat. Res. 2008, 170, 776–783. [Google Scholar] [CrossRef]
- Asaithamby, A.; Chen, D.J. Cellular responses to DNA double-strand breaks after low-dose gamma-irradiation. Nucleic Acids Res. 2009, 37, 3912–3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, C.; Li, P.; Li, S.; Zhao, Z.; Chen, Y.; Huo, B.; Zhang, D. Connexin 43 is a potential regulator in fluid shear stress-induced signal transduction in osteocytes. J. Orthop. Res. 2013, 31, 1959–1965. [Google Scholar] [CrossRef]
- Moorer, M.C.; Stains, J.P. Connexin43 and the Intercellular Signaling Network Regulating Skeletal Remodeling. Curr. Osteoporos. Rep. 2017, 15, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Onoda, J.M.; Kantak, S.S.; Diglio, C.A. Radiation induced endothelial cell retraction in vitro: Correlation with acute pulmonary edema. Pathol. Oncol. Res. 1999, 5, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Savla, U.; Waters, C.M. Barrier function of airway epithelium: Effects of radiation and protection by keratinocyte growth factor. Radiat. Res. 1998, 150, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhou, Z.; Yan, F.; Dong, Q.; Wang, L.; Sha, W.; Xu, Q.; Zhu, X.; Zhao, L. Low-dose X-ray irradiation induces morphological changes and cytoskeleton reorganization in osteoblasts. Exp. Ther. Med. 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Vardouli, L.; Moustakas, A.; Stournaras, C. LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-beta. J. Biol. Chem. 2005, 280, 11448–11457. [Google Scholar] [CrossRef] [Green Version]
- Somosy, Z.; Sass, M.; Bognár, G.; Kovács, J.; Köteles, G.J. X-irradiation-induced disorganization of cytoskeletal filaments and cell contacts in HT29 cells. Scanning Microsc. 1995, 9, 763–770. [Google Scholar] [PubMed]
- Lamers, M.L.; Padilha, D.M.; Bernardi, L.; Da Silveira, H.E.; Fossati, A.C. X-ray irradiation alters the actin cytoskeleton in murine lacrimal glands. Acta Odontol. Scand. 2014, 72, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, Y.; Zhou, H.J.; Du, Y.T.; Zhang, B.P.; Zhang, J.; Miao, G.Y.; Liu, B.; Zhang, H. X-ray radiation promotes the metastatic potential of tongue squamous cell carcinoma cells via modulation of biomechanical and cytoskeletal properties. Hum. Exp. Toxicol. 2015, 34, 894–903. [Google Scholar] [CrossRef]
- Stroka, K.M.; Aranda-Espinoza, H. Effects of Morphology vs. Cell-Cell Interactions on Endothelial Cell Stiffness. Cell Mol. Bioeng. 2011, 4, 9–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, M.J. Cell adhesion assays. Methods Mol. Biol. 2009, 522, 203–210. [Google Scholar] [CrossRef]
- Cavalcanti-Adam, E.A.; Volberg, T.; Micoulet, A.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007, 92, 2964–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margadant, C.; Monsuur, H.N.; Norman, J.C.; Sonnenberg, A. Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 2011, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cheng, H.; Yuan, Y.; Wu, S. Regulation of ionizing radiation-induced adhesion of breast cancer cells to fibronectin by alpha5beta1 integrin. Radiat. Res. 2014, 181, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Meineke, V.; Gilbertz, K.P.; Schilperoort, K.; Cordes, N.; Sendler, A.; Moede, T.; Van Beuningen, D. Ionizing radiation modulates cell surface integrin expression and adhesion of COLO-320 cells to collagen and fibronectin in vitro. Strahlenther Onkol. 2002, 178, 709–714. [Google Scholar] [CrossRef]
- Schaller, M.D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 2001, 1540, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef] [Green Version]
- Huveneers, S.; Danen, E.H. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Hauck, C.R.; Hsia, D.A.; Ilic, D.; Schlaepfer, D.D. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 2002, 277, 12487–12490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornberg, L.J. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck 1998, 20, 745–752. [Google Scholar] [CrossRef]
- Cance, W.G.; Harris, J.E.; Iacocca, M.V.; Roche, E.; Yang, X.; Chang, J.; Simkins, S.; Xu, L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: Correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 2000, 6, 2417–2423. [Google Scholar] [PubMed]
- Golubovskaya, V.M.; Kweh, F.A.; Cance, W.G. Focal adhesion kinase and cancer. Histol. Histopathol. 2009, 24, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.A.; Mitra, S.K.; Hauck, C.R.; Streblow, D.N.; Nelson, J.A.; Ilic, D.; Huang, S.; Li, E.; Nemerow, G.R.; Leng, J.; et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003, 160, 753–767. [Google Scholar] [CrossRef]
- Nguemgo Kouam, P.; Bühler, H.; Hero, T.; Adamietz, I.A. The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition. Radiat. Oncol. 2019, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Nalla, A.K.; Asuthkar, S.; Bhoopathi, P.; Gujrati, M.; Dinh, D.H.; Rao, J.S. Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin β1/FAK signaling in medulloblastoma. PLoS ONE 2010, 5, e13006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rofstad, E.K.; Mathiesen, B.; Galappathi, K. Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: Radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res. 2004, 64, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubash, A.D.; Menold, M.M.; Samson, T.; Boulter, E.; García-Mata, R.; Doughman, R.; Burridge, K. Focal adhesions: New angles on an old structure. Int. Rev. Cell Mol. Biol. 2009, 277, 1–65. [Google Scholar] [CrossRef]
- Cox, E.A.; Sastry, S.K.; Huttenlocher, A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol. Biol. Cell 2001, 12, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.; Gaugler, M.H.; Rodallec, A.; Bonnaud, S.; Paris, F.; Corre, I. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem. Biophys. Res. Commun. 2011, 414, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, V.; Pugliese, M.G.; Musella, I.; De Menna, M.; Netti, P.A.; Fusco, S. A biophysical analysis to assess x-ray sensitivity of healthy and tumour cells. Radiat. Prot. Dosim. 2019, 183, 116–120. [Google Scholar] [CrossRef]
- Panzetta, V.; Menna, M.; Bucci, D.; Giovannini, V.; Pugliese, M.; Quarto, M.; Fusco, S.; Netti, P. X-ray irradiation affects morphology, proliferation and migration rate of healthy and cancer cells. J. Mech. Med. Biol. 2015, 15, 1540022. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.; Campbell, J.; Williams, J. Transformation by Polyoma Virus affects Adhesion of Fibroblasts. Nat. New Biol. 1971, 231, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, V.; La Verde, G.; Pugliese, M.; Artiola, V.; Arrichiello, C.; Muto, P.; Commara, M.; Netti, P.A.; Fusco, S. Adhesion and Migration Response to Radiation Therapy of Mammary Epithelial and Adenocarcinoma Cells Interacting with Different Stiffness Substrates. Cancers (Basel) 2020, 12, 1170. [Google Scholar] [CrossRef]
- Panciera, T.; Citron, A.; Di Biagio, D.; Battilana, G.; Gandin, A.; Giulitti, S.; Forcato, M.; Bicciato, S.; Panzetta, V.; Fusco, S.; et al. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat. Mater. 2020, 19, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; He, Y.; Zhao, M.; Jiang, J. Collective cell migration: Implications for wound healing and cancer invasion. Burn. Trauma 2013, 1, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Kai, F.; Laklai, H.; Weaver, V.M. Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease. Trends Cell Biol. 2016, 26, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, A.R.; Parsons, J.T. Cell migration--movin’ on. Science 1999, 286, 1102–1103. [Google Scholar] [CrossRef]
- Hauck, C.R.; Hsia, D.A.; Puente, X.S.; Cheresh, D.A.; Schlaepfer, D.D. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 2002, 21, 6289–6302. [Google Scholar] [CrossRef] [Green Version]
- Lark, A.L.; Livasy, C.A.; Dressler, L.; Moore, D.T.; Millikan, R.C.; Geradts, J.; Iacocca, M.; Cowan, D.; Little, D.; Craven, R.J.; et al. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod. Pathol. 2005, 18, 1289–1294. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Camphausen, K.; Moses, M.A.; Beecken, W.D.; Khan, M.K.; Folkman, J.; O’Reilly, M.S. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001, 61, 2207–2211. [Google Scholar] [PubMed]
- Cheng, J.C.; Chou, C.H.; Kuo, M.L.; Hsieh, C.Y. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 2006, 25, 7009–7018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.W.; Mizumoto, K.; Urashima, T.; Nagai, E.; Maehara, N.; Sato, N.; Nakajima, M.; Tanaka, M. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin. Cancer Res. 2002, 8, 1223–1227. [Google Scholar] [PubMed]
- Fusco, S.; Panzetta, V.; Netti, P.A. Mechanosensing of substrate stiffness regulates focal adhesions dynamics in cell. Meccanica 2017, 52, 3389–3398. [Google Scholar] [CrossRef]
- Kraning-Rush, C.M.; Reinhart-King, C.A. Controlling matrix stiffness and topography for the study of tumor cell migration. Cell Adh. Migr. 2012, 6, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Haage, A.; Schneider, I.C. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J. 2014, 28, 3589–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.M.; Bird, D.; Lang, G.; Cox, T.R.; Erler, J.T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 2013, 32, 1863–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peela, N.; Sam, F.S.; Christenson, W.; Truong, D.; Watson, A.W.; Mouneimne, G.; Ros, R.; Nikkhah, M. A three dimensional micropatterned tumor model for breast cancer cell migration studies. Biomaterials 2016, 81, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, H.; Huang, J.; Xiong, C. Substrate Stiffness Coupling TGF-β1 Modulates Migration and Traction Force of MDA-MB-231 Human Breast Cancer Cells in Vitro. ACS Biomater. Sci. Eng. 2018, 4, 1337–1345. [Google Scholar] [CrossRef]
- Cordes, N.; Hansmeier, B.; Beinke, C.; Meineke, V.; Van Beuningen, D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br. J. Cancer 2003, 89, 2122–2132. [Google Scholar] [CrossRef] [Green Version]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp. Dermatol. 2014, 23, 813–818. [Google Scholar] [CrossRef]
- Lekka, M. Discrimination Between Normal and Cancerous Cells Using AFM. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Weaver, V.M. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Bökel, C.; Brown, N.H. Integrins in development: Moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 2002, 3, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Danen, E.H.; Sonnenberg, A. Integrins in regulation of tissue development and function. J. Pathol. 2003, 201, 632–641. [Google Scholar] [CrossRef]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, 018267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetze, K.; Scholz, M.; Taucher-Scholz, G.; Mueller-Klieser, W. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int. J. Radiat. Biol. 2007, 83, 889–896. [Google Scholar] [CrossRef]
- Onoda, J.M.; Piechocki, M.P.; Honn, K.V. Radiation-induced increase in expression of the alpha IIb beta 3 integrin in melanoma cells: Effects on metastatic potential. Radiat. Res. 1992, 130, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Taverna, D.; Ullman-Culleré, M.; Rayburn, H.; Bronson, R.T.; Hynes, R.O. A test of the role of alpha5 integrin/fibronectin interactions in tumorigenesis. Cancer Res. 1998, 58, 848–853. [Google Scholar]
- Wu, C.; Hughes, P.E.; Ginsberg, M.H.; McDonald, J.A. Identification of a new biological function for the integrin alpha v beta 3: Initiation of fibronectin matrix assembly. Cell Adhes. Commun. 1996, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Park, M.J.; Kwak, H.J.; Lee, H.C.; Kim, M.S.; Lee, S.H.; Park, I.C.; Rhee, C.H.; Hong, S.I. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006, 66, 8511–8519. [Google Scholar] [CrossRef] [Green Version]
- Yoshimasu, T.; Sakurai, T.; Oura, S.; Hirai, I.; Tanino, H.; Kokawa, Y.; Naito, Y.; Okamura, Y.; Ota, I.; Tani, N.; et al. Increased expression of integrin alpha3beta1 in highly brain metastatic subclone of a human non-small cell lung cancer cell line. Cancer Sci. 2004, 95, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Pumiglia, K.; DiPersio, C.M. Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: A novel mechanism of integrin-mediated MMP gene expression. J. Cell Sci. 2005, 118, 1185–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogineni, V.R.; Nalla, A.K.; Gupta, R.; Gujrati, M.; Klopfenstein, J.D.; Mohanam, S.; Rao, J.S. α3β1 integrin promotes radiation-induced migration of meningioma cells. Int. J. Oncol. 2011, 38, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Wild-Bode, C.; Weller, M.; Rimner, A.; Dichgans, J.; Wick, W. Sublethal irradiation promotes migration and invasiveness of glioma cells: Implications for radiotherapy of human glioblastoma. Cancer Res. 2001, 61, 2744–2750. [Google Scholar]
- Johnson, D.S.; Chen, Y.H. Ras family of small GTPases in immunity and inflammation. Curr. Opin. Pharmacol. 2012, 12, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Grosshans, B.L.; Ortiz, D.; Novick, P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 2006, 103, 11821–11827. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.A.; Shattil, S.J. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 2000, 25, 388–391. [Google Scholar] [CrossRef]
- Rolfe, B.E.; Worth, N.F.; World, C.J.; Campbell, J.H.; Campbell, G.R. Rho and vascular disease. Atherosclerosis 2005, 183, 1–16. [Google Scholar] [CrossRef]
- Cardama, G.A.; Alonso, D.F.; Gonzalez, N.; Maggio, J.; Gomez, D.E.; Rolfo, C.; Menna, P.L. Relevance of small GTPase Rac1 pathway in drug and radio-resistance mechanisms: Opportunities in cancer therapeutics. Crit. Rev. Oncol. Hematol. 2018, 124, 29–36. [Google Scholar] [CrossRef]
- Bid, H.K.; Roberts, R.D.; Manchanda, P.K.; Houghton, P.J. RAC1: An emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol. Cancer Ther. 2013, 12, 1925–1934. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, A.A.; Govek, E.E.; Böttner, B.; Van Aelst, L. Rho GTPases: Signaling, migration, and invasion. Exp. Cell Res. 2000, 261, 1–12. [Google Scholar] [CrossRef]
- Aznar, S.; Lacal, J.C. Rho signals to cell growth and apoptosis. Cancer Lett. 2001, 165, 1–10. [Google Scholar] [CrossRef]
- Fritz, G.; Brachetti, C.; Bahlmann, F.; Schmidt, M.; Kaina, B. Rho GTPases in human breast tumours: Expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer 2002, 87, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Denoyelle, C.; Vasse, M.; Körner, M.; Mishal, Z.; Ganné, F.; Vannier, J.P.; Soria, J.; Soria, C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: An in vitro study. Carcinogenesis 2001, 22, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Arias-Romero, L.E.; Chernoff, J. Targeting Cdc42 in cancer. Expert. Opin. Ther. Targets 2013, 17, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapon, N.; Hall, A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 1997, 9, 86–92. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Cdc42--the centre of polarity. J. Cell Sci. 2004, 117, 1291–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Kuehn, H.S.; Rådinger, M.; Brown, J.M.; Ali, K.; Vanhaesebroeck, B.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Btk-dependent Rac activation and actin rearrangement following FcepsilonRI aggregation promotes enhanced chemotactic responses of mast cells. J. Cell Sci. 2010, 123, 2576–2585. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Bruemmer, D. NR4A orphan nuclear receptors: Transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1535–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.F.; Cao, G.W. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J. Gastroenterol. 2012, 18, 6865–6873. [Google Scholar] [CrossRef]
- Maijenburg, M.W.; Gilissen, C.; Melief, S.M.; Kleijer, M.; Weijer, K.; Ten Brinke, A.; Roelofs, H.; Van Tiel, C.M.; Veltman, J.A.; De Vries, C.J.; et al. Nuclear receptors Nur77 and Nurr1 modulate mesenchymal stromal cell migration. Stem. Cells Dev. 2012, 21, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Mix, K.S.; McMahon, K.; McMorrow, J.P.; Walkenhorst, D.E.; Smyth, A.M.; Petrella, B.L.; Gogarty, M.; Fearon, U.; Veale, D.; Attur, M.G.; et al. Orphan nuclear receptor NR4A2 induces synoviocyte proliferation, invasion, and matrix metalloproteinase 13 transcription. Arthritis Rheum. 2012, 64, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hayashi, S.; Umezaki, M.; Yamamoto, T.; Kageyama-Yahara, N.; Kondo, T.; Kadowaki, M. Shikonin, a constituent of Lithospermum erythrorhizon exhibits anti-allergic effects by suppressing orphan nuclear receptor Nr4a family gene expression as a new prototype of calcineurin inhibitors in mast cells. Chem. Biol. Interact. 2014, 224, 117–127. [Google Scholar] [CrossRef]
- Song, C.H.; Joo, H.M.; Han, S.H.; Kim, J.I.; Nam, S.Y.; Kim, J.Y. Low-dose ionizing radiation attenuates mast cell migration through suppression of monocyte chemoattractant protein-1 (MCP-1) expression by Nr4a2. Int. J. Radiat. Biol. 2019, 95, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Isermann, P.; Lammerding, J. Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol. 2013, 23, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Rothballer, A.; Kutay, U. The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma 2013, 122, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, G.G.; Worman, H.J. Nuclear positioning. Cell 2013, 152, 1376–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisp, M.; Liu, Q.; Roux, K.; Rattner, J.B.; Shanahan, C.; Burke, B.; Stahl, P.D.; Hodzic, D. Coupling of the nucleus and cytoplasm: Role of the LINC complex. J. Cell Biol. 2006, 172, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Razafsky, D.; Hodzic, D. Bringing KASH under the SUN: The many faces of nucleo-cytoskeletal connections. J. Cell Biol. 2009, 186, 461–472. [Google Scholar] [CrossRef]
- Starr, D.A.; Fridolfsson, H.N. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 2010, 26, 421–444. [Google Scholar] [CrossRef] [Green Version]
- Bone, C.R.; Starr, D.A. Nuclear migration events throughout development. J. Cell Sci. 2016, 129, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Luxton, G.W.; Gundersen, G.G. Orientation and function of the nuclear-centrosomal axis during cell migration. Curr. Opin. Cell Biol. 2011, 23, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioka, Y.; Imaizumi, H.; Imada, J.; Katahira, J.; Matsuura, N.; Hieda, M. SUN1 splice variants, SUN1_888, SUN1_785, and predominant SUN1_916, variably function in directional cell migration. Nucleus 2016, 7, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, H.; Sato, K.; Nishihara, A.; Minami, K.; Koizumi, M.; Matsuura, N.; Hieda, M. X-ray-enhanced cancer cell migration requires the linker of nucleoskeleton and cytoskeleton complex. Cancer Sci. 2018, 109, 1158–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | Dose (Gy) | Time after Irradiation | Observed Effect on Actin CSK | Ref. |
|---|---|---|---|---|
| Mel270, BLM | 1–3 | 40 days | Increase in marginal actin filaments and decrease in internal ones | [66] |
| BALBc/3T3 SVT2 | 1, 2 | 24 h | Actin polymerization, increase actin filaments | [69,70] |
| HUVEC | 2–8 | n.a. | Remodelling of the actin CSK | [72] |
| LN229 U87 | 2 | 20, 40 h | Activation of small GTPases Rac1K, increase in G-actin, decrease in F-actin | [77] |
| Cortical neurons | 2, 4 | 24 h | Decomposition and rearrangement of the F-actin | [79] |
| Calu-3 16HBE14o- | 2–10 | 4 h | Increase in F-actin depolymerization | [88] |
| MC3T3-E1 | 0.5, 5 | 5 days | Decrease in F-actin expression, expression of RhoA, ROCK1, and p-cofilin due to actin depolymerization | [89] |
| Murine exorbital lacrimal gland cells | 0.036 | 4, 8 h | Actin depolymerization, increase in the cellular area (the outcomes were reversible after 24 h) | [92] |
| TSCC | 0–4 | 24 h | Disorganization of the F-actin | [93,94] |
| Cell Line | Dose (Gy) | Time after Irradiation | Observed Effect on Cell Adhesion | Ref. |
|---|---|---|---|---|
| MDA-MB-231 | 10 | 24 h | Increase in the connection between cells and FN | [99] |
| U-87 MG U-373 MG MDA-MB-231 | 0, 2, 4, 8 | 24, 48 and 72 h | Increased cell adhesion due to the activity of FAK and Src | [109] |
| HMEC-1 | 15 | 15 min | Increase cell adhesion due to FAs formation through the activation of RhoA/ROCK signalling pathways | [114,115] |
| BALBc/3T3 SVT2 | 1, 2, 4, 8 | 24, 72 h | Increased adhesion | [69,116,117,118] |
| MCF10A | 2, 10 | 24 h | The decreased adhesion resulted in inverse proportionality with the delivered dose. (The effects were reversible after 72 h) | [119] |
| MDA-MB-231 | 2, 10 | 24, 72 h | Decrease adhesion with lower dose on the softer substrate, the opposite phenomenon was observed on the stiffer substrate |
| Cell Line | Dose (Gy) | Time after Irradiation | Observed Effect on Cell Migration | Ref. |
|---|---|---|---|---|
| BALBc/3T3, SVT2 | 4, 8 | 24, 72 h | Reduced speed and motility. (The effects were reversible after 72 h for BALBc/3T3) | [71] |
| BALBc/3T3, SVT2 | 1, 2 | 6, 24 h | Reduced speed and motility | [117] |
| MCF10A | 2, 10 | 24, 72 h | After 24 h cells showed an increased motility with 2 Gy; 72 h after treatment cells showed a reduced motility | [119] |
| MDA-MB-231 | 2, 10 | 24, 72 h | After 24 h cells showed an increase in the migration velocity (this effect was reversible after 72 h) | |
| TSCC (Tca-8113) | 0–4 | 24 h | Increase in cell migration in a dose-dependent manner | [93] |
| U251, U87 | 0–10 | 24 h | Increase in cell migration due to the expression of MMP-2 and MMP-9 enzymes | [137,149] |
| IOMM-Lee, CH-157-MN | 7 | 24 h | Increase in cell motility due to the overexpression of α3β1 integrin | [152] |
| NIH-3T3 | 1–8 | 21 days | Increase in cell migration due to the expression of 𝛼5β3 integrin | [153] |
| HMEC-1 | 15 | 15 min | Decrease in cell motility | [115] |
| RBL-2H3 | 0.01, 0.05, 0.1, 0.5 | N.A. | Decrease in cell migration through the suppression of the MCP-1 | [174] |
| MDA-MB-231 | 0.5 | 24, 48 h | The expression of SUN1 and SUN2 proteins was necessary for the radiation-induced migration of cells | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Verde, G.; Artiola, V.; Panzetta, V.; Pugliese, M.; Netti, P.A.; Fusco, S. Cytoskeleton Response to Ionizing Radiation: A Brief Review on Adhesion and Migration Effects. Biomedicines 2021, 9, 1102. https://doi.org/10.3390/biomedicines9091102
La Verde G, Artiola V, Panzetta V, Pugliese M, Netti PA, Fusco S. Cytoskeleton Response to Ionizing Radiation: A Brief Review on Adhesion and Migration Effects. Biomedicines. 2021; 9(9):1102. https://doi.org/10.3390/biomedicines9091102
Chicago/Turabian StyleLa Verde, Giuseppe, Valeria Artiola, Valeria Panzetta, Mariagabriella Pugliese, Paolo A. Netti, and Sabato Fusco. 2021. "Cytoskeleton Response to Ionizing Radiation: A Brief Review on Adhesion and Migration Effects" Biomedicines 9, no. 9: 1102. https://doi.org/10.3390/biomedicines9091102
APA StyleLa Verde, G., Artiola, V., Panzetta, V., Pugliese, M., Netti, P. A., & Fusco, S. (2021). Cytoskeleton Response to Ionizing Radiation: A Brief Review on Adhesion and Migration Effects. Biomedicines, 9(9), 1102. https://doi.org/10.3390/biomedicines9091102








