The Efficacy of Naïve versus Modified Mesenchymal Stem Cells in Improving Muscle Function in Duchenne Muscular Dystrophy: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
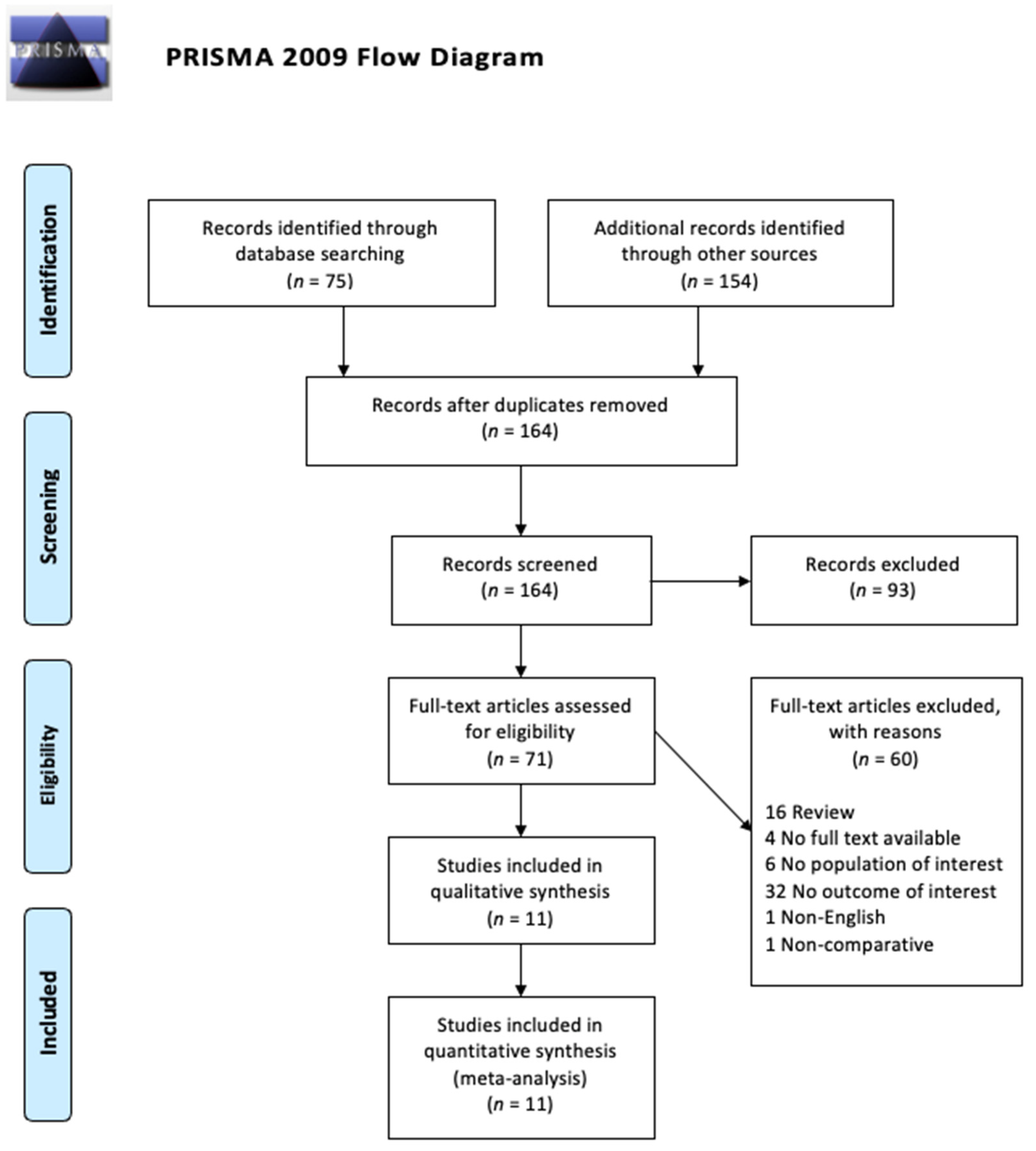
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Methodological Quality Assessment
3. Results
3.1. Study of Methodology Quality Assessment
3.2. Study of Characteristics
3.3. Cell Management and Injection
3.4. Measurement Instruments
3.5. Experimental Variables and Controls
3.6. Laboratory Effects and Proposed Mechanisms
3.6.1. MSCs Improved Life Expectancy and Limb Function
3.6.2. MSC Induced Myogenic Differentiation Is an Important Factor in Improving Outcomes
3.6.3. MSC Incorporation and Proliferation in Muscle Is Correlated with Improved Outcomes
3.6.4. Improved Exercise Resistance as a Major Mechanism of MSC’s Benefits
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laing, N.G.; Davis, M.R.; Bayley, K.; Fletcher, S.; Wilton, S.D. Molecular diagnosis of duchenne muscular dystrophy: Past, present and future in relation to implementing therapies. Clin. Biochem. Rev. 2011, 32, 129. [Google Scholar] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of dystrophin disrupts skeletal muscle signaling: Roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romitti, P.; Puzhankara, S.; Mathews, K.; Zamba, G.; Cunniff, C.; Andrews, J.; Matthews, D.; James, K.; Miller, L.; Druschel, C.; et al. Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years-four states, 2007. Morb. Mortal. Wkly. Rep. 2009, 58, 1119–1122. [Google Scholar]
- Naidoo, M.; Anthony, K. Dystrophin Dp71 and the neuropathophysiology of Duchenne muscular dystrophy. Mol. Neurobiol. 2020, 57, 1748–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landfeldt, E.; Lindgren, P.; Bell, C.F.; Schmitt, C.; Guglieri, M.; Straub, V.; Lochmüller, H.; Bushby, K. The burden of Duchenne muscular dystrophy: An international, cross-sectional study. Neurology 2014, 83, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.A.; Scutifero, M.; Cecio, M.R.; Torre, V.; De Luca, F.; Picillo, E.; et al. Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myol. 2012, 31, 121. [Google Scholar]
- BEYTÍA, M.D.; Vry, J.; Kirschner, J. Drug treatment of Duchenne muscular dystrophy: Available evidence and perspectives. Acta Myol. 2012, 31, 4. [Google Scholar]
- Sienkiewicz, D.; Kulak, W.; Okurowska-Zawada, B.; Paszko-Patej, G.; Kawnik, K. Duchenne muscular dystrophy: Current cell therapies. Ther. Adv. Neurol. Disord. 2015, 8, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Sanzarello, I.; Merlini, L.; Traina, F.; Rosa, M.A.; Faldini, C. Corticosteroid treatment impact on spinal deformity in Duchenne Muscular Dystrophy. Int. Sch. Res. Notices 2014, 2014, 965235. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybalka, E.; Timpani, C.A.; Debruin, D.A.; Bagaric, R.M.; Campelj, D.G.; Hayes, A. The failed clinical story of myostatin inhibitors against Duchenne muscular dystrophy: Exploring the biology behind the battle. Cells 2020, 9, 2657. [Google Scholar] [CrossRef]
- Klingler, W.; Jurkat-Rott, K.; Lehmann-Horn, F.; Schleip, R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012, 31, 184. [Google Scholar]
- Dzierlega, K.; Yokota, T. Optimization of antisense-mediated exon skipping for Duchenne muscular dystrophy. Gene Ther. 2020, 27, 407–416. [Google Scholar] [CrossRef]
- Echevarría, L.; Aupy, P.; Goyenvalle, A. Exon-skipping advances for Duchenne muscular dystrophy. Hum. Mol. Genet. 2018, 27, R163–R172. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, O.; Yokota, T. Developing DMD therapeutics: A review of the effectiveness of small molecules, stop-codon readthrough, dystrophin gene replacement, and exon-skipping therapies. Expert Opin. Investig. Drugs 2021, 30, 167–176. [Google Scholar] [CrossRef]
- Maffioletti, S.M.; Noviello, M.; English, K.; Tedesco, F.S. Stem cell transplantation for muscular dystrophy: The challenge of immune response. BioMed Res. Int. 2014, 2014, 964010. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Dai, A.; Baspinar, O.; Yeşilyurt, A.; Sun, E.; Aydemir, Çİ.; Öztel, O.N.; Capkan, D.U.; Pinarli, F.; Agar, A.; Karaöz, E. Efficacy of stem cell therapy in ambulatory and nonambulatory children with Duchenne muscular dystrophy–Phase I–II. Degener. Neurol. Neuromuscul. Dis. 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemionow, M.; Malik, M.; Langa, P.; Cwykiel, J.; Brodowska, S.; Heydemann, A. Cardiac protection after systemic transplant of dystrophin expressing chimeric (DEC) cells to the mdx mouse model of Duchenne muscular dystrophy. Stem Cell Rev. Rep. 2019, 15, 827–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, R.Q.; He, J.; Zhang, Y.Y.; Xiong, F.; Ruan, G.P.; Zhu, X.Q.; Wang, Q.; Wang, J.X.; Zhu, G.X.; Zhao, J.; et al. Systemic delivery of human bone marrow embryonic-like stem cells improves motor function of severely affected dystrophin/utrophin–deficient mice. Cytotherapy 2014, 16, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Valadares, M.C.; Gomes, J.P.; Castello, G.; Assoni, A.; Pellati, M.; Bueno, C.; Corselli, M.; Silva, H.; Bartolini, P.; Vainzof, M.; et al. Human adipose tissue derived pericytes increase life span in Utrn tm1Ked Dmd mdx/J mice. Stem Cell Rev. Rep. 2014, 10, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Esper, G.V.; Pignatari, G.C.; Rodrigues, M.N.; Bertagnon, H.G.; Fernandes, I.R.; Nascimento, N.; Tabosa, A.M.; Beltrão-Braga, P.C.; Miglino, M.A. Aquapuncture using stem cell therapy to treat MdX mice. Evid. Based Complement. Altern. Med. 2015, 2015, 132706. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Szilagyi, E.; Cwykiel, J.; Domaszewska-Szostek, A.; Heydemann, A.; Garcia-Martinez, J.; Siemionow, K. Transplantation of Dystrophin Expressing Chimeric Human Cells of Myoblast/Mesenchymal Stem Cell Origin Improves Function in Duchenne Muscular Dystrophy Model. Stem Cells Dev. 2021, 30, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Nitahara-Kasahara, Y.; Kuraoka, M.; Oda, Y.; Hayashita-Kinoh, H.; Takeda, S.I.; Okada, T. Enhanced cell survival and therapeutic benefits of IL-10-expressing multipotent mesenchymal stromal cells for muscular dystrophy. Stem Cell Res. Ther. 2021, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Peng, F.; Xiong, F.; Shang, Y.; Zhao, C.; Li, W.; Zhang, C. Inhibition of myostatin promotes myogenic differentiation of rat bone marrow-derived mesenchymal stromal cells. Cytotherapy 2009, 11, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.Y.; Lei, Q.F.; Zhang, C.; Li, S.N. Improved motor function in dko mice by intravenous transplantation of bone marrow-derived mesenchymal stromal cells. Cytotherapy 2011, 13, 69–77. [Google Scholar] [CrossRef]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal models of Duchenne muscular dystrophy: From basic mechanisms to gene therapy. Dis. Models Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Deconinck, A.E.; Rafael, J.A.; Skinner, J.A.; Brown, S.C.; Potter, A.C.; Metzinger, L.; Watt, D.J.; Dickson, J.G.; Tinsley, J.M.; Davies, K.E. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 1997, 90, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Ruehle, M.A.; Stevens, H.Y.; Beedle, A.M.; Guldberg, R.E.; Call, J.A. Aggregate mesenchymal stem cell delivery ameliorates the regenerative niche for muscle repair. J. Tissue Eng. Regen. Med. 2018, 12, 1867–1876. [Google Scholar] [CrossRef]
- Rousseau, J.; Dumont, N.; Lebel, C.; Quenneville, S.P.; Côté, C.H.; Frenette, J.; Tremblay, J.P. Dystrophin expression following the transplantation of normal muscle precursor cells protects mdx muscle from contraction-induced damage. Cell Transplant. 2010, 19, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Łoboda, A.; Dulak, J. Muscle and cardiac therapeutic strategies for Duchenne muscular dystrophy: Past, present, and future. Pharmacol. Rep. 2020, 72, 1227–1263. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Full-text available | Full text not available |
| Written in English | Text not in English |
| Articles published in the last 20 years | No direct measurement of muscle function |
| Articles containing original data | Case reports, case series, reviews, letters, chapters |
| Studies related to mesenchymal stem cells and Duchenne muscular dystrophy |
| Study | MINORS Score |
|---|---|
| Dai A. et al. (2018) [19] | 13 of 16 |
| Siemionow M. et al. (2019) [20] | 22 of 24 |
| Pang R. et al. (2014) [21] | 22 of 24 |
| Valadares M. et al. (2014) [22] | 22 of 24 |
| Esper G. et al. (2015) [23] | 22 of 24 |
| Siemionow M. et al. (2021) [24] | 22 of 24 |
| Nitahara-Kashara et al. (2021) [25] | 22 of 24 |
| Geng J et al. (2009) [26] | 22 of 24 |
| Li Z. et al. (2011) [27] | 22 of 24 |
| Ruehle M. et al. (2018) [28] | 22 of 24 |
| Rousseau J. et al. (2010) [29] | 22 of 24 |
| Author | Journal | Year | Compartive/Non-Comparative | Cell Source | Subject Type; aGe at Treatment | Modified/Unmodified | Cell Harvesting and Processing | Injection Method | Number of Independent Measurements | Measurement Methodology * | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dai A. et al. (2018) [19] | Degenerative Neurological and Neuromuscular Disease | 2018 | Non-comparative | Human | Human; 7 to 14 years old | Unmodified | Umbilical cords were obtained from consenting patients delivering full-term infants by Caesarian section | 2 × 106 cells/kg/dose injections every 2 weeks for 4 months, alternating between systemic intra-arterial administration and local intramuscular injections | 5 | Electromyography; Spirometry; Echocardiography; Immunohistochemical analysis; Fluorescent in situ hybridization | [19] |
| Siemionow M. et al. (2019) [20] | Stem Cell Reviews and Reports | 2019 | Comparative | mdx mice; snj (wild type) mice | Mdx mice; 8 weeks old | Modified | Myoblasts were harvested from the hind limb muscles of both wild type and mdx mice. MSCs were harvested from the femur and tibia of mdx mice only. The fusion cells MBwt/MBmdx and MBwt/MSCmdx were created. Some MBwt, MBmdx, and MSCmdx were saved | 60 μL systemic-intraosseous saline injections to femoral bone | 3 | Histological and immunofluorescence of cardiac muscle cross sections; Echocardiography Assessment | [20] |
| Pang R. et al. (2014) [21] | Cytotherapy | 2014 | Comparative | Human | Mdx mice; 6 weeks old | Unmodified | To induce myogenic differentiation, cells were cultured in myogenic differentiation medium (Lonza, Basel, Switzerland) for 10 days. | Two million ELSCs or MSCs in 500 μL of saline were injected into each mouse through the tail vein. For control, 500 μL of saline was injected through the tail vein. | 9 | Analysis of motor function via traction, rotating rod, and running wheel tests; CK activity via NADPH fluorescence kit followed by spectrophotometry; Immunohistochemistry; Western blotting; RT-PCR; Histological analysis | [21] |
| Valadares M. et al. (2014) [22] | Stem Cell Reviews and Reports | 2014 | Comparative | Human | Mdx mice; 7 weeks old | Unmodified | Four tissue specimens from a 46-year-old healthy female donor, namely: adipose tissue, muscle, fallopian tubes and endometrium were obtained from total hysterectomy procedures. All pericyte populations were confirmed to have at least 75 % purity after sorting. | Injected intraperitonially with 1 million viable cells (or vehicle), once a week, for a total of 8 weeks, without any immunosuppression. These seven groups were comprised by animals receiving either: vehicle, fibroblasts, myoblasts, pericytes (endometrium), pericytes (fallopian tubes), pericytes (adipose tissue) and pericytes (muscle). | 9 | Analysis of motor function via ambulation test, grip test, and rotarod; Myogenic differentiation; qPCR; PCR; Western blot; Immunohistochemistry; Histological analysis | [22] |
| Esper G. et al. (2015) [23] | Evidence-Based Complementary and Alternative Medicine | 2015 | Comparative | Human | Mdx mice; 4 to 6 weeks old | Modified | MSCs derived from deciduous teeth were obtained using a modified Miura’s protocol with collagenase type I for pulp digestion | 1 × 104 cells suspended in 20 microliters saline injected every three weeks, totaling three injections via acupoint at Bladder 47, 49, and 52. | 3 | Analysis of motor function via wire test; CK-NAC; Histological analysis | [23] |
| Siemionow M. et al. (2021) [24] | Stem Cells and Development | 2021 | Comparative | Human | Mdx mice; 6 to 8 weeks old | Modified | Allogenic human myoblasts and normal human bone marrow-derived MSCs underwent fusion exvivo using polyethylene. Cells presenting double PKH26/PKH67 staining were sorted through fluoresence-activated cell sorting and used for in vitro and invivo analysis | 0.25 × 106 fused cells or 0.5 × 106 of unfused cells were suspended in 60 microliters of Dulbecco’s phosphate-buffered saline. This volume was delivered into the left gastrocnemius of each mouse through six injections | 6 | Analysis of motor function via wire hanging and grip strength test, in situ muscle force test, and ex vivo muscle force test; Histological analysis; Immunofluoresence analysis | [24] |
| Nitahara-Kashara et al. (2021) [25] | Stem Cell Research & Therapy | 2021 | Comparative | Sprague-Dawley rats, Human, Beagle dogs | NOD/SCID mice, Beagle dogs, Canine X-linked muscular dystrophy model (CXMDj); 3 to 52 months old | Modified | MSCs isolated from Sprague-Dalwey rat bone marrow were transduced with a luciferase-expressing retroviral vector. Canine CD271 + MSCs were also transduced with a luciferase-expressing retroviral vector as well as an enhnaced green fluorescent protein (eGFP) or MyoD-expressing adenoviral vector. MSCs or human dental pulp stem cells (hDPSCs) were transduced with adeno-associated virus (AAV)/eGFP or control AAV1/IL-10 vectors | 5.0–10.0 × 106 luciferase-expressing rat MSCs were injected intramuscularly into the right or lift hindlimb muscle of NOD/SCID mice pretreated with cardiotoxin 1 day before treatment. 1.0 × 107 of both eGFP-MSCs and IL-10-MSCs were injected into the right and left hindlimb, respectively. AAV1/IL-10 transduced Luc-CD271 + MSCs (2.4–2.7 × 107 cells/2 mL) were injected into the muscles of healthy beagles on days 0 and 50 without immunosupressants. The tibialis anterior muscles were pretreated by injecting 10 nmol/kg of cardiotoxin. hDPSCs or AAV1/IL-10 transduced hDPSCs (4.0 × 106 cells/mL/kg body weight at a rate of 1 mL/min) was adminstered intravenously into CXMDj using nine injections at two week intervals. | 13 | Analysis of motor function via 15-m running time and counts of spontaneous locomotor activity; Creatinine kinase; Alanine aminotransferase; Aspartate aminotransferase; blood urea nitrogen; Histological analysis; Immunohistochemical analysis; ELISA; Luciferase reporter assays; Biodistribution of MSCs; Cytokine/cytokine array; MRI | [25] |
| Geng J et al. (2009) [26] | Cytotherapy | 2009 | Comparative | Sprague-Dawley rats | Mdx mice; 7 to 9 weeks old | Modified | Femur and tibia bone marrow mesenchymal stem cells were incubated in complete medium containing 10 μM 5-AzaC for 24 h to induce differentiation, and incubated with or without 10–100 μg/mL polyclonal anti-myostatin Ab in differentiation medium | 1.2 × 107 MSC of the mixture were infused per mouse through the tail vein. One day before transplantation, mdx mice in the Ab transplantation group were injected intraperitoneally with anti-myostatin antibody (6 mg/kg/week). | 5 | Immunofluorescence analysis; RT-PCR; Motor function via Rota-rod; CK-NAC; Western blot | [26] |
| Li Z. et al. (2011) [27] | Cytotherapy | 2011 | Comparative | Sprague-Dawley rats | Mdx mice; 6 weeks old | Unmodified | Bone marrow MSCs were extracted from the femurs and tibias of male rats. | Cell density was adjusted to 1 × 107/mL. A volume of 0.5 mL MSC suspended in PBS was administered via tail vein injection into each experimental dko mouse, and matched controls were administered equivalent injections of PBS. | 5 | Analysis of motor function via traction, rotating rod, and running wheel tests; Histological analysis; Detection of grafted cells | [27] |
| Ruehle M. et al. (2018) [28] | Journal of Tissue Engineering and Regenerative Medicine | 2018 | Comparative | Wild type C57BL/6 mice | Mdx mice; 12 weeks old | Modified | Mesenchymal stem cells were lentivirally GFP-labelled to confirm multipotency and proliferative capacity. Some cells were formed into aggregates by centrifuging and incubating overnight to form spheroidal aggregates | Mice received local 100 μL injections containing MSC aggregates, MSC single cells, or saline. 5 × 105 MSCs were in each injection except for saline | 4 | Peak isometric torque via servomotor and Pt-Ir needle electrodes; Histological analysis; Cell immunomodulatory factor quantification | [28] |
| Rousseau J. et al. (2010) [29] | Cell Transplantation | 2010 | Comparative | Normal C57BL/10 J and C57BL/10 J mdx/mdx mice | Mdx mice; 12 month old | Unmodified | Muscles dissected from arms and legs were cut into small fragments and digested with collagenase and dispase. After 48 hrs in culture, Muscle precursor cells were frozen in nitrogen until transplantation. | A total of 1.5 million cells were injected in several sites throughout each of the left and right extensor digitorum longus | 2 | Analysis of motor function via electrode stimulation in organ bath; Immunohistochemistry | [29] |
| Study | Variables | Controls | Modified/Unmodified | Effects on Muscle | Conclusions |
|---|---|---|---|---|---|
| Siemionow M. et al. (2019) [20] | MBwt + MBmdx | Wild type with no treatment | Modified | Increased ejection fraction and fractional shortening in MBwt/MBmdx and MBwt/MSCmdx compared to saline injected mdx controls | Fused cells are more efficacious in countering the effects of systolic dysfunction due to better engraftment potential and lower levels of rejection. |
| MBwt + MSCmdx | Mdx mice with saline injection only | Increased ejection fraction and fractional shortening in MBwt/MBmdx and MBwt/MSCmdx compared to not-fused cells | MSCs are effective at maintaining cardiac function | ||
| MBwt/MBmdx | MBwt/MBmdx and MBwt/MSCmdx mice had ejection values 10% and 20% higher, respectively, compared to vehicle injected controls | ||||
| MBwt/MSCmdx | |||||
| Dai A. et al. (2018) [19] | Human wharton jelly-derived mesenchymal stem cells | None | Unmodified | Increased FVC and FEV1 for all patients | Positive effect for vital functions |
| Increased or equal ejection fraction for 8 out of 9 patients | Questionable benefits in terms motility despite drop in CK | ||||
| No statistically significant difference between pre and post measure of muscle strength | |||||
| Significant difference between pre and posttreatment EMG measurement of amplitude in right and left suralis | |||||
| Ruehle M. et al. (2018) [28] | Wild-type mice with myotoxic injury injected with single cell MSCs | Wild-type mice with myotoxic injury with saline only injections | Modified | No difference in peak isometric torque of the ankle plantar flexor muscles prior to treatment | Single cell MSCs are not effective at improving muscle function |
| Wild-type mice with myotoxic injury injected with aggregated MSC | Mdx mice with no myotoxic injury with saline only injections | Aggregate treated mice had significantly greater torque than saline for both types of mice | Aggregated MSCs are effective at improving muscle function | ||
| Mdx mice with no myotoxic injury injected with single MSCs | Aggregate MSCs had greater peak isometric torque compared to single cell for both types of mice | Aggregated MSCs may have avoided rapid clearance from tissue and remained within muscle longer than single cells, as aggregates could be identified for up to 7 days in the cardiotoxin-injured wild-type mice and for up to 3 days in the dystrophic tissue, whereas single cells could not be reliably identified by histology at any time point | |||
| Mdx mice with no myotoxic injury injected with aggregated MSCs | Single cell MSCs had the same peak isometric torque as saline | Wild-type mice with myotoxic injury and mdx mice do not have a significant impact on the efficacy of stem cells | |||
| Geng J et al. (2009) [26] | Rat bone marrow mesenchymal stem cells (transplant) | No transplant | Modified | No difference between the no transplant and transplant group | MSCs alone are ineffective at improving muscle function |
| Rat bone marrow mesenchymal stem cells + myostatin antibody (Ab transplant) | Ab transplant group greatly improved motor function compared to no transplant and transplant | Inhibition of myostatin improves the MSC ability to improve muscle function | |||
| Li Z. et al. (2011) [27] | MSC transplantation | PBS injection only wild type | Unmodified | Until 15 weeks after transplantation, no abnormalities were observed in either the diet or behavior of transplanted mice | MSCs provide significant improvements in motor function and lifespan, although it is still significantly worse than normal mice |
| PBS injection only dko mice | The median lifespan of normal control mice was significantly higher than both other groups, but the median lifespan of the transplantation group (35 wks) was significantly higher than that of the control mice (22 wks) | ||||
| All motor function tests showed significant improvement in experimental mice compared to control groups. Normal control mice performed significantly better than experimental mice, though | |||||
| Pang R. et al. (2014) [21] | ELSC injection | Saline injection only dko mice | Unmodified | ELSC transplanted mice had significantly improved motor function in all tests compared with dko mice transplanted with MSCs or saline | ELSCs were superior to MSCs by every metric, even though MSCs were already significantly better than saline only injections. The increased myogenic differentiation of ELSCs may be responsible for this discrepancy |
| MSC injection | |||||
| Valadares M. et al. (2014) [22] | Fibroblast injection | Vehicle (HBSS) injection only in dko mice | Unmodified | Endometrial-derived pericytes showed significant effects related to the age of the onset of injections. The younger the dko mice started being treated, the better the survival. This observation was not seen in any other treated groups | All cells were ineffective at engrafting and providing significant benefits to muscle function |
| Myoblast injection | Only adipose derived pericytes increased life expectancy in mdx mice | ||||
| Endometrial pericyte injection | Despite improved survival in adipose pericyte injected mice, none of the physical tests revealed differences between the groups | ||||
| Fallopian tube pericyte injection | No human cells were found in any analyzed tissues and no difference in the HE stained sections of the gastrocnemius muscle | ||||
| Adipose pericyte injection | No in vitro myogenic potential in pericytes derived from any tissue source was found | ||||
| Muscle pericyte injection | |||||
| Esper G. et al. (2015) [23] | False aquapuncture with SHEDs | No aquapuncture | Modified | Strength improvement in mice with SHED/true acupoints and only slight improvement with saline/true acupoints and SHED/false acupoints compared to controls | Acupuncture and MSCs each have a beneficial effect in muscle force that can complement each other |
| True aquapuncture with saline | Although creatine kinase decreased in all treatments, only SHED/true acupoint was able to improve force | ||||
| True aquapuncture with SHEDs | |||||
| Rousseau J. et al. (2010) [29] | Mdx mice transplanted with 10 J MPCs | No treatment mdx mice | Unmodified | There was no significant force difference observed between the different groups, even those injected with cardiotoxin | Replacing dystrophin can increase resistance to exercise and contraction-induced injury but not sufficiently enoigh to increase the strength of the muscle |
| Mdx mice transplanted with mdx MPCs | Mdx mice injected with cardiotoxin only | Mdx mice with 10 J MPCs transplanted increased muscle protection against eccentric contractions | |||
| Siemionow M. et al. (2021) [24] | MB/MSC fused cells | DPBS injection only | Modified | MB/MSC fused cells significantly improved in vivo muscle force and reduced fibrosis compared with vehicle-injected controls and non fused MB and MSC cells | Fused cells were more effective at improving muscle function than unfused cells |
| Not-fused MB and MSC | |||||
| Nitahara-Kashara et al. (2021) [25] | Mice transplanted with IL-10 MSCs | Mice transplanted with GFP-MSCs | Modified | Muscle function was not measured specifically in mice, but IL-10 expressing MSCs had significantly more cell survival and were more effective at enhancing post-transplantation retention. Il-10-Luc-MSCs were retained for more than 67 days after transplantation | Higher retention in early stages exerts a significant effect on long-term engraftment |
| Beagles transplanted with cardiotoxin and IL-10-Luc-CD271 + MSCs | Beagles transplanted with cardiotoxin and MyoD-Luc-CD271 + MSCs | Lucisferase activity, which correlates to the number of MSCs, tended to be higher in IL-10-Luc-CD271 + MSCs. IL-10 levels in IL-10-Luc-CD271+ MSC treated tibialis anterior muscles increased, while those in MYoD-Luc-CD271 +MSC-treated muscles did not. | IL-10 expressing CD271+ MSCs could survive long term and engraft after intramuscular regeneration | ||
| CXMDj transplanted with AAV1/IL-10-transduced hDPSCs | CXMDj transplanted with hDPSCs | Significantly higher torque was found in IL-10-hDPSC-treated CXMDj than in control CXMDj, which had similar values to hDPSC-treated CXMDj. hDPSC and IL-10-hDPSC-treated CXMDj maintained a 15-m running speed and were active at 3 to 12 months of age. Only IL-10-hDPSC was effective at reducing CK levels and lactic acid before and after exercise | Only IL-10-hDPSCs exert a protective effect against dystrophic damage caused by exercise | ||
| IL-10-hDPSCs were only detected in the TA muscles and not in other organs. Dystrophin expression was undetectable in the muscle tissues of hDPSC-treated CXMDj | |||||
| Il-10-hDPSCs decreased inflammation and necrotic/edematous lesions and increased the weight and area of muscles. hDPSCs significantly limited the infiltration of nuclei, indicating a midler phenotype |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, O.Y.-J.; Chen, Y.-F.; Xu, H.-T.; Lee, C.-W. The Efficacy of Naïve versus Modified Mesenchymal Stem Cells in Improving Muscle Function in Duchenne Muscular Dystrophy: A Systematic Review. Biomedicines 2021, 9, 1097. https://doi.org/10.3390/biomedicines9091097
Shen OY-J, Chen Y-F, Xu H-T, Lee C-W. The Efficacy of Naïve versus Modified Mesenchymal Stem Cells in Improving Muscle Function in Duchenne Muscular Dystrophy: A Systematic Review. Biomedicines. 2021; 9(9):1097. https://doi.org/10.3390/biomedicines9091097
Chicago/Turabian StyleShen, Oscar Yuan-Jie, Yi-Fan Chen, Hong-Tao Xu, and Chien-Wei Lee. 2021. "The Efficacy of Naïve versus Modified Mesenchymal Stem Cells in Improving Muscle Function in Duchenne Muscular Dystrophy: A Systematic Review" Biomedicines 9, no. 9: 1097. https://doi.org/10.3390/biomedicines9091097
APA StyleShen, O. Y.-J., Chen, Y.-F., Xu, H.-T., & Lee, C.-W. (2021). The Efficacy of Naïve versus Modified Mesenchymal Stem Cells in Improving Muscle Function in Duchenne Muscular Dystrophy: A Systematic Review. Biomedicines, 9(9), 1097. https://doi.org/10.3390/biomedicines9091097







