The Role of Curcumin in Cancer Treatment
Abstract
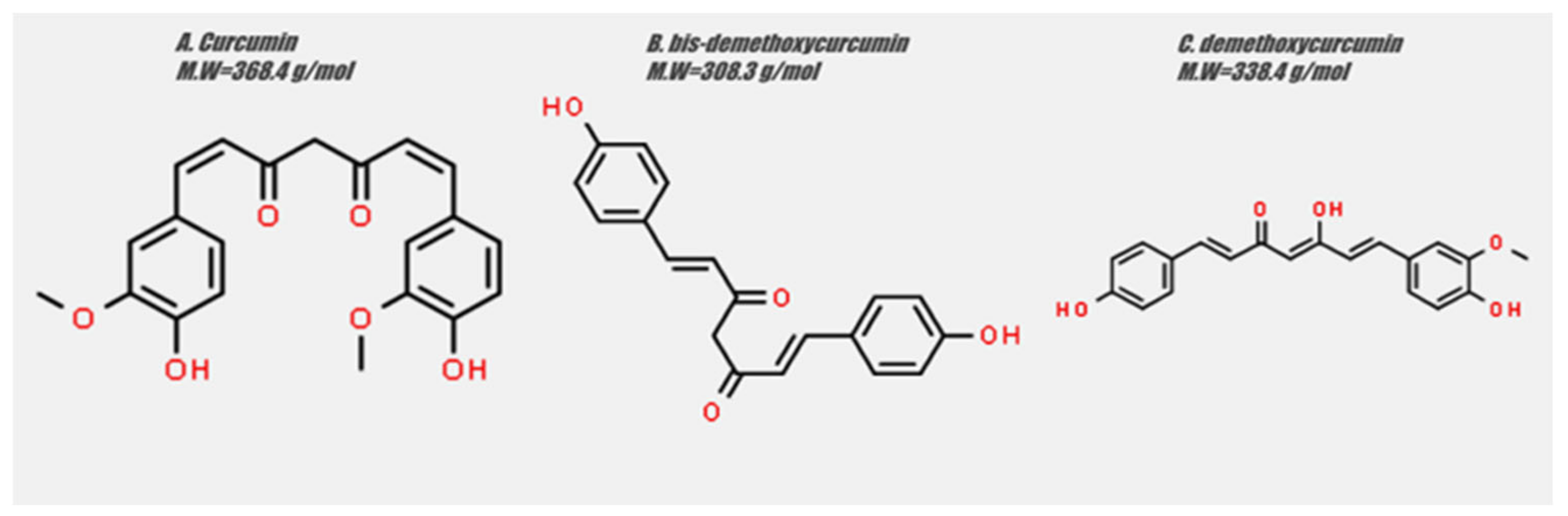
:1. Introduction
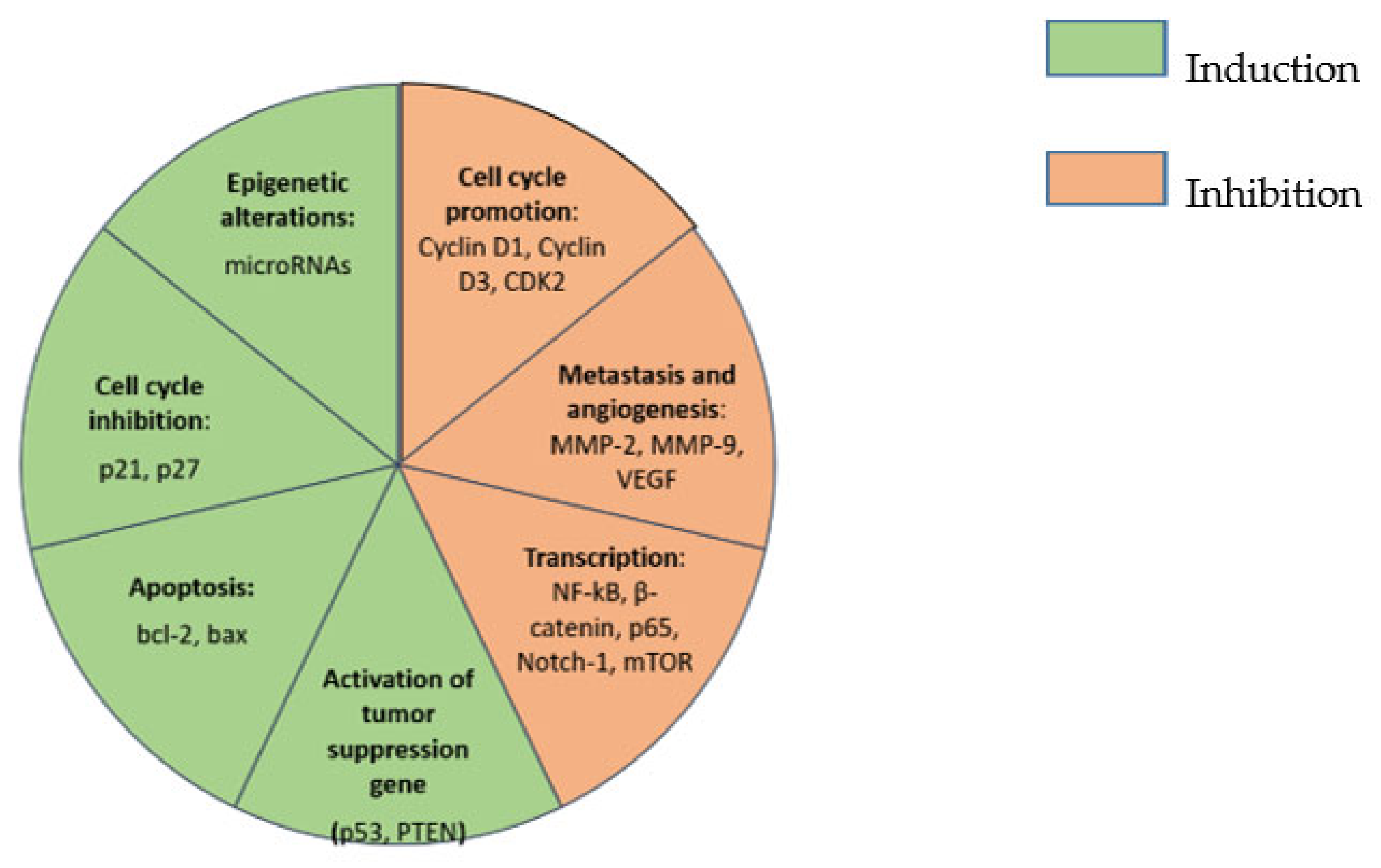
2. Immunomodulatory Effects of Curcumin
3. Lung Cancer
4. Breast Cancer
5. Prostate Cancer
6. Brain Tumors
7. Pancreatic Cancer
8. Gastric Cancer
9. Leukemia
10. Clinical Trials
11. Curcumin and Cancer-Associated Fibroblasts/Tumor-Associated Fibroblasts (CAFs/TAFs)
12. Potential Side Effects of Curcumin
13. Curcumin as Chemoprotective Agent in Cancer Chemotherapy
14. Curcumin Application: Limitations and Prospects
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Dutta, B. Study of secondary metabolite constituents and curcumin contents of six different species of genus curcuma. J. Med. Plants 2015, 3, 116–119. [Google Scholar]
- Kyritsis, A.; Bondy, M.; Levin, V. Modulation of Glioma Risk and Progression by Dietary Nutrients and Antiinflammatory Agents. Nutr. Cancer 2011, 63, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Heling’s, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action against Cancer; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Pentimalli, F.; Grelli, S.; Daniele, N.; Melino, G.; Amelio, I. Cell death pathologies: Targeting death pathways and the immune system for cancer therapy. Genes Immun. 2019, 20, 539–554. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrini, S.; Gorgoulis, V.; Halazonetis, T. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.I.A.; Jantan, I.; Haque, M.A. Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. Int. Immunopharmacol. 2017, 50, 291–304. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Nakamae, I.; Morimoto, T.; Shima, H.; Shionyu, M.; Fujiki, H.; Yoneda-Kato, N.; Yokoyama, T.; Kanaya, S.; Kakiuchi, K.; Shirai, T.; et al. Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules 2019, 24, 4067. [Google Scholar] [CrossRef] [Green Version]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-κB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri-Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ghildiyal, A.; Singh, S.; Siddiqui, S.; Kumari, P.; Arshad, M.; Mahdi, A. A Combinatorial effect of curcumin and tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL) in induction of apoptosis via inhibition of nuclear factor kappa activity and enhancement of caspase-3 activity in chronic myeloid cells: An in-vitro study. J. Cancer Res. Ther. 2018, 14, 1193–1200. [Google Scholar]
- Bessard, A.; Sole, V.; Bouchaud, G.; Quemener, A.; Jacques, Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor α fusion protein, in metastatic melanoma and colorectal cancer. Mol. Cancer Ther. 2009, 8, 2736–2745. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef]
- Das, L.; Vinayak, M. Curcumin Modulates Glycolytic Metabolism and Inflammatory Cytokines via Nrf 2 in Dalton’s Lymphoma Ascites Cells In Vivo. Anticancer Agents Med. Chem. 2018, 18, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. ‘’Spicing Up” of the Immune System by Curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Lantz, R.C.; Chena, G.J.; Solyomb, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 2005, 12, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Kim, K.H.; Lee, S.H.; Yoon, M.S.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Ahn, S.C.; Park, Y.C.; Park, Y.M. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J. Immunol. 2005, 174, 8116–8124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- South, E.H.; Exon, J.H.; Hendrix, K. Dietary curcumin enhances antibody response in rats. Immunopharmacol. Immunotoxicol. 1997, 19, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.B.; Yang, S.H.; Toth, B.; Kovalenko, A.; Wallach, D. Activation of the NLRP3 inflammasome by proteins that signal for necroptosis. Methods Enzymol. 2014, 545, 67–81. [Google Scholar]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.M.; Thompson, J.K.; MacPherson, M.B.; Beuschel, S.L.; Westbom, C.M.; Sayan, M.; Shukla, A. Curcumin: A double hit on malignant mesothelioma. Cancer Prev. Res. 2014, 7, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Charlet, J.; Juncker, T.; Teiten, M.H.; Dicato, M.; Diederich, M. Effect of curcumin on nuclear factor κB signaling pathways in human chronic myelogenous K562 leukemia cells. Ann. N. Y. Acad. Sci. 2009, 1171, 436. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant Therapy with Bioavailability-Boosted Curcuminoids Suppresses Systemic Inflammation and Improves Quality of Life in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Bera, A.; Yoon, A.J.; Morselli, M.; Jeong, C.; Tosevska, A.; Dong, T.S.; Eklund, M.; Russ, E.; Nasser, H.; et al. A randomized, phase 1, placebo-controlled trial of APG-157 in oral cancer demonstrates systemic absorption and an inhibitory effect on cytokines and tumor-associated microbes. Cancer 2020, 8, 1668–1682. [Google Scholar] [CrossRef]
- Memon, H.; Patel, B. Immune checkpoint inhibitors in non-small cell lung cancer: A bird’s eye view. Life Sci. 2019, 233, 116713. [Google Scholar] [CrossRef]
- Wang, J.W.; Wang, X.; Wang, X.J.; Zheng, B.Z.; Wang, Y.; Liang, B. Curcumin inhibits the growth via Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7492–7499. [Google Scholar]
- Li, X.; Ma, S.; Yang, P.; Sun, B.; Zhang, Y.; Sun, Y.; Hao, M.; Mou, R.; Jia, Y. Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells. Oncol. Lett. 2018, 16, 6756–6762. [Google Scholar] [CrossRef]
- Wu, G.Q.; Chai, K.Q.; Zhu, X.M.; Jiang, H.; Wang, X.; Xue, Q.; Zheng, A.H.; Zhou, H.Y.; Chen, Y.; Chen, X.C.; et al. Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget 2016, 7, 26535–26550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Song, X.; Shang, M.; Zou, W.; Zhang, M.; Wei, H.; Shao, H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019, 15, 1243–1253. [Google Scholar] [CrossRef]
- Smagurauskaite, G.; Mahale, J.; Brown, K.; Thomas, A.L.; Howells, L.M. New paradigms to assess consequences of long-term, low-dose curcumin exposure in lung cancer cells. Molecules 2020, 25, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bland, A.R.; Bower, R.L.; Nimick, M.; Hawkins, B.C.; Rosengren, R.J.; Ashton, J.C. Cytotoxicity of curcumin derivatives in ALK positive non-small cell lung cancer. Eur. J. Pharmacol. 2019, 865, 172749. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Tian, Y.; Weiss, R.M.; Martin, D.T. Synergistic inhibition of GP130 and ERK signaling blocks chemoresistant bladder cancer cell growth. Cell. Signal. 2019, 63, 109381. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Shen, H.; Ran, R.; Xu, K.; Zhang, W.; Tong, X.; Feng, L. Curcumin co-treatment ameliorates resistance to gefitinib in drug-resistant NCI-H1975 lung cancer cells. J. Tradit. Chin. Med. 2017, 37, 355–360. [Google Scholar]
- Yoon, J.H.; Shin, J.W.; Pham, T.H.; Choi, Y.J.; Ryu, H.W.; Oh, S.R.; Oh, J.W.; Yoon, D.Y. Methyl lucidone induces apoptosis and G2/M phase arrest via the PI3K/Akt/NF-κB pathway in ovarian cancer cells. Pharm. Biol. 2020, 58, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Li, X.; Ho, C.T.; Lin, X.; Zhang, Y.; Li, B.; Chen, Z. Cocoa tea (Camellia ptilophylla) induces mitochondria-dependent apoptosis in HCT116 cells via ROS generation and PI3K/Akt signaling pathway. Food Res. Int. 2020, 129, 108854. [Google Scholar] [CrossRef]
- Zhou, G.Z.; Li, A.F.; Sun, Y.H.; Sun, G.C. A novel synthetic curcumin derivative MHMM-41 induces ROS-mediated apoptosis and migration blocking of human lung cancer cells A549. Biomed. Pharmacother. 2018, 103, 391–398. [Google Scholar] [CrossRef]
- Agudo, A.; Bonet, C.; Travier, N.; González, C.A.; Vineis, P.; Bueno-de-Mesquita, H.B.; Trichopoulos, D.; Boffetta, P.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J. Clin. Oncol. 2012, 30, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xie, W.; Wu, R.; Geng, H.; Zhao, L.; Xie, C.; Li, X.; Zhu, M.; Zhu, W.; Zhu, J.; et al. Inhibition of tobacco smoke-induced bladder MAPK activation and epithelial-mesenchymal transition in mice by curcumin. Int. J. Clin. Exp. Pathol. 2015, 8, 4503–4513. [Google Scholar]
- Liang, Z.; Wu, R.; Xie, W.; Zhu, M.; Xie, C.; Li, X.; Zhu, J.; Zhu, W.; Wu, J.; Geng, S.; et al. Curcumin reverses tobacco smoke-induced epithelial-mesenchymal transition by suppressing the MAPK pathway in the lungs of mice. Mol. Med. Rep. 2018, 17, 2019–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Zhu, J.; Yang, X.; Huang, C.; Zhou, L.; Meng, Z.; Li, X.; Zhong, C. TAp63α Is Involved in Tobacco Smoke-Induced Lung Cancer EMT and the Anti-Cancer Activity of Curcumin via miR-19 Transcriptional Suppression. Front. Cell Dev. Biol. 2021, 9, 645402. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Lheureux, S.; Denoyelle, C.; Ohashi, P.S.; De Bono, J.S.; Mottaghy, F.M. Molecularly targeted therapies in cancer: A guide for the nuclear medicine physician. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Januškevičienė, I.; Petrikaitė, V. Heterogeneity of breast cancer: The importance of interaction between different tumor cell popu lations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef]
- Yang, S.X.; Polley, E.; Lipkowitz, S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat. Rev. 2016, 45, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Steelman, L.S.; Martelli, A.M.; Cocco, L.; Libra, M.; Nicoletti, F.; Abrams, S.L.; McCubrey, J.A. The therapeutic potential of mTOR inhibitors in breast cancer. Br. J. Clin. Pharmacol. 2016, 82, 1189–1212. [Google Scholar] [CrossRef]
- Shan, H.; Yingchun, X.; Liwei, M.; Liming, H.; He, S. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar]
- Coker-Gurkan, A.; Celik, M.; Ugur, M.; Arisan, E.D.; Obakan-Yerlikaya, P.; Durdu, Z.B.; Palavan-Unsal, N. Curcumin inhibits autocrine growth hormone-mediated invasion and metastasis by targeting NF-κB signaling and polyamine metabolism in breast cancer cells. Amino Acids 2018, 50, 1045–1069. [Google Scholar] [CrossRef] [PubMed]
- Berrak, O.; Akkoc, Y.; Arisan, E.D.; Coker-Gurkan, A.; Obakan-Yerlikaya, P.; Palavan-Unsal, N. The inhibition of PI3K and NF-κB promoted curcumin-induced cell cycle arrest at G2/M via altering polyamine metabolism in Bcl-2 overexpressing MCF-7 breast cancer cells. Biomed. Pharm. 2016, 77, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Coker-Gurkan, A.; Bulut, D.; Genc, R.; Arisan, E.D.; Obakan-Yerlikaya, P.; Palavan-Unsal, N. Curcumin prevented human autocrine growth hormone (GH) signaling mediated NF-κB activation and miR-183-96-182 cluster stimulated epithelial mesenchymal transition in T47D breast cancer cells. Mol. Biol. Rep. 2019, 46, 355–369. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, L.; Jiang, X.; Wang, Y.; Wang, X.; Chen, B. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget 2018, 9, 11268–11278. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.M.; El-Kersh, D.M.; Ammar, R.A.; Adel, A.; Khalil, A.; Walid, H.; Eskander, K.; Hamdy, M.; Reda, N.; Mohsen, N.E.; et al. Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated multidrug resistance by curcumin and vitamin D3 increases sensitivity to paclitaxel in breast cancer. Chem. Biol. Interact. 2020, 315, 108865. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2010, 10, 20. [Google Scholar]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, D.; Ghosh, R. Chronic inflammatory mediators enhance prostate cancer development and progression. Biochem. Pharmacol. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and-2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, H.; Lu, Y.; Noguchi, T.; Muto, S.; Okada, H.; Kawato, S.; Horie, S. Modulation of AKR 1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018, 109, 1230–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavya, K.; Kumar, M.N.; Patil, R.H.; Hegde, S.M.; Kiran-Kumar, K.M.; Nagesh, R.; Babu, R.L.; Ramesh, G.T.; Chidananda-Sharma, S. Differential expression of AP-1 transcription factors in human prostate LNCaP and PC-3 cells: Role of Fra-1 in transition to CRPC status. Mol. Cell. Biochem. 2017, 433, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Wanli, Z.; Xudong, Z.; Guisong, Q.; Yuexian, G. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J. Biochem. Mol. Toxicol. 2018, 32, e22049. [Google Scholar]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer-I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000, 3, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Deeb, D.; Jiang, H.; Gao, X.; Hafner, M.S.; Wong, H.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol. Cancer Ther. 2004, 3, 803–812. [Google Scholar]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Dev. Ther. 2017, 11, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Nimick, M.; Cridge, A.G.; Hawkins, B.C.; Rosengren, R.J. Anticancer potential of novel curcumin analogs towards castrate-resistant prostate cancer. Int. J. Oncol. 2018, 52, 579–588. [Google Scholar] [CrossRef]
- Klinger, N.V.; Mittal, S. Therapeutic Potential of Curcumin for the Treatment of Brain Tumors. Oxid. Med. Cell. Longev. 2016, 2016, 9324085. [Google Scholar] [CrossRef]
- Weathers, S.P.; Gilbert, M.R. Advances in treating glioblastoma. F1000Prime Rep. 2014, 6, 46. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, W.; Liu, Y.; Chen, S.; Chin, S.; Qi, X.; Zhao, Y.; Liu, H.; Wang, J.; Mei, X.; et al. MicroRNA-378 enhances inhibitory effect of curcumin on glioblastoma. Oncotarget 2017, 8, 73938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, W.L.; Lin, H.Y.; Huang, C.Y.; Huang, B.R.; Lin, C.; Lu, D.Y.; Wei, K.C. Migration-prone glioma cells show curcumin resistance associated with enhanced expression of miR-21 and invasion/anti-apoptosis-related proteins. Oncotarget 2015, 6, 37770. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, W.; Long, L.; Zheng, B.; Ji, W.; Yang, N.; Zhang, Q.; Liang, Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012, 103, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Gersey, C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.C.; Wang, M.J.; Chiu, T.L. The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. Int. J. Mol. Med. 2010, 26, 217–224. [Google Scholar]
- Zhao, J.; Zhu, J.; Lv, X.; Xing, J.; Liu, S.; Chen, C.; Xu, Y. Curcumin potentiates the potent antitumor activity of ACNU against glioblastoma by suppressing the PI3K/AKT and NF-kappaB/COX-2 signaling pathways. OncoTargets Ther. 2017, 10, 5471–5482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotto-Filho, A.; Braganhol, E.; Klafke, K.; Figueiro, F.; Terra, S.R.; Paludo, F.J.; Morrone, M.; Bristot, I.J.; Battastini, A.M.; Forcelini, C.M.; et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015, 358, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojko, A.; Cierniak, A.; Adamczyk, A.; Ligeza, J. Modulatory effects of curcumin and tyrphostins (AG494 and AG1478) on growth regulation and viability of LN229 human brain cancer cells. Nutr. Cancer 2015, 67, 1170–1182. [Google Scholar] [CrossRef]
- Oettle, H. Progress in the knowledge and treatment of advanced pancreatic cancer: From benchside to bedside. Cancer Treat. Rev. 2014, 40, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Masamune, A.; Suzuki, N.; Kikuta, K.; Satoh, M.; Satoh, K.; Shimosegawa, T. Curcumin blocks activation of pancreatic stellate cells. J. Cell. Biochem. 2006, 97, 1080–1093. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012, 72, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, C.; Xi, H.; Gao, Y.; Xu, D. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3 K/Akt pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Osterman, C.J.; Gonda, A.; Stiff, T.; Sigaran, U.; Valenzuela, M.M.A.; Bennit, H.R.; Moyron, R.B.; Khan, S.; Wall, N.R. Curcumin induces pancreatic adenocarcinoma cell death via reduction of the inhibitors of apoptosis. Pancreas 2016, 45, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, J.; Wan, L.; Zhou, X.; Wang, Z.; Wei, W. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol. Ther. 2015, 151, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.Z.; Ma, Y.; Ji, B.; Liu, Y.; Hwu, P.; Abbruzzese, J.L. Increased Cdc20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. J. Hematol. Oncol. 2012, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xue, Y.B.; Li, H.; Qiu, D.; Wang, Z.W.; Tan, S.S. Inhibition of Cell Survival by Curcumin Is Associated with Downregulation of Cell Division Cycle 20 (Cdc20) in Pancreatic Cancer Cells. Nutrients 2017, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Fassan, M.; Graham, D.Y. Epidemiology of gastric cancer. In Gastric Cancer; Springer: Cham, Switzerland, 2015; pp. 23–34. [Google Scholar]
- Yamashita, K.; Sakuramoto, S.; Watanabe, M. Genomic and epigenetic profiles of gastric cancer: Potential diagnostic and therapeutic applications. Surg. Today 2011, 41, 24–38. [Google Scholar] [CrossRef]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.; Cao, Y.; Han, G.; Zhang, Y.; You, Q.; Wang, Y.; Pan, Y. P53/microRNA-374b/AKT1 regulates colorectal cancer cell apoptosis in response to DNA damage. Int. J. Oncol. 2017, 50, 1785–1791. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Lian, N.; Zhang, F.; Chen, L.; Chen, Q.; Lu, C.; Bian, M.; Shao, J.; Wu, L.; Zheng, S. Activation of PPARgamma/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2017, 7, e2189. [Google Scholar] [CrossRef]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell. Physiol. 2017, 233, 4634–4642. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Liu, W.H.; Yuan, J.B.; Zhang, F.; Chang, J.X. Curcumin inhibits proliferation, migration and invasion of gastric cancer cells via Wnt3a/β-catenin/EMT signaling pathway. Zhongguo Zhong Yao Za Zhi 2019, 44, 3107–3115. [Google Scholar]
- Zand, A.; Imani, S.; Saadati, M.; Borna, H.; Ziaei, R.; Honari, H. Effect of age, gender and blood group on blood cancer types. Trauma Mon. 2010, 15, 111–114. [Google Scholar]
- Koohi, F.; Salehiniya, H.; Shamlou, R.; Eslami, S.; Ghojogh, Z.M.; Kor, Y.; Rafiemanesh, H. Leukemia in Iran: Epidemiology and morphology trends. Asian Pac. J. Cancer Prev. 2015, 16, 7759–7763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, S.; Raza, M.H.; Younas, M.; Naeem, F.; Adeeb, R.; Iqbal, J.; Anwar, P.; Sajid, U.; Manzoor, H.M. Molecular targets of curcumin and future therapeutic role in leukemia. J. Biosci. Med. 2018, 6, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2016, 91, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.X.; Xu, J.H.; Wu, G.H.; Chen, Y.Z. Inhibitory effect of curcumin on proliferation of K562 cells involves down-regulation of p210 (bcr/abl) initiated Ras signal transduction pathway. Acta Pharmacol. Sin. 2003, 24, 1155–1160. [Google Scholar]
- Mukherjee, A.; Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Curcumin boosts up the efficacy of imatinib mesylate in chronic myelogenic leukemia cell line K-562 by modulation of various markers. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 240–255. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Sundaram, C.; Reuter, S. Aggarwal BB: Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Pesakhov, S.; Nachliely, M.; Barvish, Z.; Aqaqe, N.; Schwartzman, B.; Voronov, E.; Sharoni, Y.; Studzinski, G.P.; Fiahman, D.; Danilenko, M. Cancer-selective cytotoxic Ca2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, J.; Xu, D.R.; Zheng, F.M.; Long, Z.J.; Huang, S.S.; Wu, X.; Zhou, W.H.; Hunag, R.W.; Liu, Q. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J. Transl. Med. 2011, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tima, S.; Ichikawa, H.; Ampasavate, C.; Okonogi, S.; Anuchapreeda, S. Inhibitory effect of turmeric curcuminoids on FLT3 expression and cell cycle arrest in the FLT3-overexpressing EoL-1 leukemic cell line. J. Nat. Prod. 2014, 77, 948–954. [Google Scholar] [CrossRef]
- Zhu, G.H.; Dai, H.P.; Shen, Q.; Ji, O.; Zhang, Q.; Zhai, Y.L. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm. Biol. 2016, 4, 1303–1311. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2013, 88, 803–816. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.P.; Contesti, J.; Huergo-Zapico, L.; Lopez-Soto, A.; Fernández-Guizán, A.; Acebes-Huerta, A.; Gonzalez-Huerta, A.J.; Gonzalez, E.; Fernandez-Alvarez, C.; Gonzalez, S. Prognostic significance of CD8 and CD4 T cells in chronic lymphocytic leukemia. Leuk Lymphoma 2010, 51, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Kay, N.E.; Secreto, C.R.; Shanafelt, T.D. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin. Cancer Res. 2009, 15, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Chaitanya, G.; Alexander, J.S.; Babu, P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, D.; Singh, S.; Narayan, G. Curcumin induces apoptosis in Pre-B acute lymphoblastic leukemia cell lines Via PARP-1 cleavage. Asian Pac. J. Cancer Prev. 2016, 17, 3865–3869. [Google Scholar]
- Guo, Y.; Li, Y.; Shan, Q.; He, G.; Lin, J.; Gong, Y. Curcumin potentiates the anti-leukemia effects of imatinib by downregulation of the AKT/mTOR pathway and BCR/ABL gene expression in Ph+ acute lymphoblastic leukemia. Int. J. Biochem. Cell Biol. 2016, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadhan-Raj, S.V.; Weber, D.M.; Wang, M.; Giralt, S.A.; Thomas, S.K.; Alexanian, R.; Zhou, X.; Patel, P.; Bueso-Ramos, C.E.; Newman, R.; et al. Curcumin downregulates NF-kB and related genes in patients with multiple myeloma: Results of a phase I/II study. Blood 2007, 110, 1177. [Google Scholar] [CrossRef]
- Kalluri, R.; Michael, Z. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Marsh, T.; Pietras, K.; McAllister, S.S. Fibroblasts as architects ofcancer pathogenesis. Biochim. Biophys. Acta 2013, 1832, 1070–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.S.; Gond, Z.J.; Li, W.H.; Wang, X.; Ling, T.Y. Antifibrotic effect of curcumin in TGF-Β1-induced myofibroblastsfrom human Oral mucosa. Asian Pac. J. Cancer Prev. 2012, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, S.; Zhang, F.; Liu, G.; Wang, R.; London, S.D.; London, L. Curcumin modulates the inflammatory response and inhibitssubsequent fibrosis in a mouse model of viral-induced acute respira-tory distress syndrome. PLoS ONE 2013, 8, e57285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrayani, S.F.; Al-Khalaf, H.H.; Aboussekhra, A. Curcumintriggers P16-dependent senescence in active breast cancer-associatedfibroblasts and suppresses their paracrine procarcinogenic effects. Neoplasia 2013, 15, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Du, Q.; Zhang, Z.; Ma, J.; Han, L.; Wang, Y.; Yang, L.; Tao, N.; Qin, Z. Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch. Biochem. Biophys. 2020, 694, 108613. [Google Scholar] [CrossRef] [PubMed]
- Ba, P.; Xu, M.; Yu, M.; Li, L.; Duan, X.; Lv, S.; Fu, G.; Yang, J.; Yang, P.; Yang, C.; et al. Curcumin suppresses the proliferation and tumorigenicity of Cal27 by modulating cancer-associated fibroblasts of TSCC. Oral. Dis. 2020, 26, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Agency Response Letter GRAS Notice No. GRN 00460; CFSAN/Office of Food Additive Safety, 1011 U.S.; Food and Drug Administration: Silver Spring, MD, USA, 2013.
- Lamb, S.R.; Wilkinson, S.M. Contact allergy to tetrahydrocurcumin. Contact Dermat. 2003, 48, 227. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Nouzari, S.M.H.; Jalalian, H.R.; Sahebkar, A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebo-controlled trial. J. Funct. Foods 2014, 6, 615–622. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Ehtejab, G. A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J. Cancer Sci. Ther. 2013, 5, 320–324. [Google Scholar]
- Borsari, M.; Ferrari, E.; Grandi, R.; Saladini, M. Curcuminoids as potential new iron-chelating agents: Spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorg. Chim. Acta 2002, 328, 61–68. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J.T.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sun, W.; Huang, C.K.; Wang, L.; Xia, M.M.; Cui, X.; Hu, G.X.; Wang, Z.S. Inhibitory effects of curcumin on activity of cytochrome P450 2C9 enzyme in human and 2C11 in rat liver microsomes. Drug Dev. Ind. Pharm. 2015, 41, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Volak, L.P.; Ghirmai, S.; Cashman, J.R.; Court, M.H. Curcuminoids inhibit multiple human cytochromes P450 (CYP), UDP-glucuronosyltransferase (UGT), and sulfotransferase (SULT) enzymes, while piperine is a relatively selective CYP3A4 inhibitor. Drug Metab. Dispos. 2008, 36, 1594–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.C.; Zhao, L.X.; Lou, H.X. Curcumin alters the pharmacokinetics of warfarin and clopidogrel in Wistar rats but has no effect on anticoagulation or antiplatelet aggregation. Planta Med. 2013, 79, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, B.H.; Prakash, N.; Jayakumar, K. Modification of pharmacokinetics of norfloxacin following oral administration of curcumin in rabbits. J. Vet. Sci. 2009, 10, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Sulakhiya, K.; Barua, C.C.; Mundhe, N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol. Cell. Biochem. 2017, 431, 113–122. [Google Scholar] [CrossRef]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A controlled study of a lecithinized delivery system of curcumin (Meriva(R)) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Prev. Biomark. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiborr, C.; Eckert, G.P.; Rimbach, G.; Frank, J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 2010, 397, 1917–1925. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Lu, M.; Meng, Z.; Chen, X.; Jing, J.; Li, J.; Yao, W.; Zhu, H.; Fu, T. Treatment of metastatic lung cancer via inhalation administration of curcumin composite particles based on mesoporous silica. Eur. J. Pharm. Sci. 2019, 134, 246–255. [Google Scholar]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.Y.; Priyadarsini, K.I. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic. Biol. Med. 2005, 6, 811–822. [Google Scholar] [CrossRef]
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of Curcumin by Complexation with Divalent Cations in Glycerol/Water System. Bioinorg. Chem. Appl. 2010, 2010, 292760. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Wu, Z.; Xie, Q.T.; Cheng, J.S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Cell Signaling Pathways | Type of Effect | Used Model | Tested Dosage | References |
|---|---|---|---|---|---|
| Lung cancer | Wnt/β-catenin | Downregulation/inhibition | Human cell line A549 | 60 μM | [35] |
| VEGF | Downregulation/inhibition | Nude mice | 100 mg/kg | [36] | |
| NF-κB | Downregulation/inhibition | Nude mice | 100 mg/kg | [36] | |
| NOTCH 1 | Downregulation/inhibition | Human lung cancer cell lines | 6 μΜ | [37] | |
| ERK ½ | Downregulation/inhibition | Human NCI-H1975 line | 10 ng/mL | [41] | |
| Breast cancer | Akt/mTOR | Downregulation/inhibition | Human breast cell lines | 10 or 30 μΜ | [55] |
| NF-κB | Downregulation/inhibition | Human breast cell lines | 20 or 25 μΜ | [56] | |
| Autocrine GH | Downregulation/inhibition | T47D human breast cells | 20 μΜ | [58] | |
| Bcl-2 and Bcl- xL | Downregulation/inhibition | T47D human breast cells | 20 μΜ | [58] | |
| MDR-1 | Downregulation/inhibition | MCF-7 breast cancer cell line | 1.3 μΜ | [60] | |
| FEN1 | Downregulation/inhibition | MCF-7 breast cancer cell line | 0–50 μmol/L | [59] | |
| Brain cancer | STAT3 | Downregulation/inhibition | Human GBM stem cells | 25 μΜ | [82] |
| IAP | Downregulation/inhibition | Human GBM stem cells | 25 μΜ | [82] | |
| MAPK | Upregulation/activation | Human GBM stem cells | 25 μΜ | [82] | |
| Pancreatic cancer | Platelet-derived growth factor | Downregulation/inhibition | Rat pancreatic stellate cells | 25 μΜ | [90] |
| PI3 K/Akt | Downregulation/inhibition | Panc-1 human pancreatic cells | 20 μΜ | [93] | |
| IAP | Downregulation/inhibition | PANC-1 human cells | 10/50/100μΜ | [94] | |
| Cdc20 | Downregulation/inhibition | Patu8988 and Panc-1 human cell lines | 10 or 20 μΜ | [97] | |
| Gastric cancer | PI3K | Downregulation/inhibition | Human SGC-7901 and BGC-823 cells | 10/20/40 μΜ | [104] |
| Wnt3 a/β-catenin/EMT | Downregulation/inhibition | Human gastric cell lines | 20 μΜ | [106] | |
| BCL-2 | Downregulation/inhibition | Human gastric cell lines | 20 μΜ | [106] | |
| Leukemia | |||||
| -CML | MAPK | Downregulation/inhibition | Human K562 cell line | 5 or 10 mg/L | [111] |
| Hsp90 | Downregulation/inhibition | Human K562 cell line | 30 μΜ | [112] | |
| p210 BCR-ABL | Downregulation/inhibition | Human K562 cell line | 5 or 10 mg/L | [111] | |
| AML | Bcl-2 | Downregulation/inhibition | Primary human CD34+ AML cells | 0–80 μΜ | [116] |
| MAPK | Downregulation/inhibition | Human SHI-1 cells | 6.25–25 μM | [117] | |
| MMP | Downregulation/inhibition | Human SHI-1 cells | 6.25–25 μM | [117] | |
| CLL | NF-κB | Downregulation/inhibition | Human CLL B cells | 10–12.5 μΜ | [120] |
| STAT3 | Downregulation/inhibition | Human CLL B cells | 10–12.5 μΜ | [120] | |
| AKT | Downregulation/inhibition | Human CLL B cells | 10–12.5 μΜ | [120] | |
| XIAP | Downregulation/inhibition | Human CLL B cells | 10–12.5 μΜ | [120] | |
| Mcl-1 | Downregulation/inhibition | Human CLL B cells | 10–12.5 μΜ | [120] | |
| ALL | AKT/mTOR | Downregulation/inhibition | Human ALL cell lines | 0–40 μΜ | [123] |
| ABL/STAT5 | Downregulation/inhibition | Human ALL cell lines | 0–40 μΜ | [123] | |
| BCR/ABL | Downregulation/inhibition | Human ALL cell lines | 0–40 μΜ | [123] |
| Cancer | Drug | Title | NCT | Phase | Estimated/Actual Completion Date |
|---|---|---|---|---|---|
| Breast | Curcumin | A „Window Trial” on Curcumin for Invasive Breast Cancer Primary Tumors | NCT03980509 | 1 | 30 December 2022 |
| Curcumin ® (CUC-01) and Paclitaxel | ‘’Curcumin” in Combination with Chemotherapy in Advanced Breast Cancer | NCT03072992 | 2 | 30 June 2019 | |
| Prostate | Curcumin | Trial of Curcumin to Prevent Progression of Low-risk Prostate Cancer Under Active Surveillance | NCT03769766 | 3 | November 2026 |
| Curcumin and radiation | Nanocurcumin for Prostate Cancer Patients Undergoing Radiotherapy (RT) | NCT02724618 | 2 | April 2022 | |
| Colorectal | Avastin/FOLFIRI and curcumin | Avastin/FOLFIRI in Combination with Curcumin in Colorectal Cancer Patients with Unresectable Metastasis | NCT02439385 | 2 | 1 August 2019 |
| Cervical | Curcumin | Curcumin in Advanced Cervical Cancer | NCT04294836 | 2 | 31 December 2023 |
| Chronic Lymphocytic Leukemia (CLL), Small Lymphocytic Lymphoma (SLL) | Curcumin and cholecalciferol | Curcumin and Cholecalciferol in Treating Patients With Previously Untreated Stage 0–II Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma | NCT02100423 | 2 | 13 December 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. https://doi.org/10.3390/biomedicines9091086
Zoi V, Galani V, Lianos GD, Voulgaris S, Kyritsis AP, Alexiou GA. The Role of Curcumin in Cancer Treatment. Biomedicines. 2021; 9(9):1086. https://doi.org/10.3390/biomedicines9091086
Chicago/Turabian StyleZoi, Vasiliki, Vasiliki Galani, Georgios D. Lianos, Spyridon Voulgaris, Athanasios P. Kyritsis, and George A. Alexiou. 2021. "The Role of Curcumin in Cancer Treatment" Biomedicines 9, no. 9: 1086. https://doi.org/10.3390/biomedicines9091086
APA StyleZoi, V., Galani, V., Lianos, G. D., Voulgaris, S., Kyritsis, A. P., & Alexiou, G. A. (2021). The Role of Curcumin in Cancer Treatment. Biomedicines, 9(9), 1086. https://doi.org/10.3390/biomedicines9091086








