Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study Protocol
2.2. Biochemical Analysis
2.3. Histological Analysis, Immunohistochemistry, and Immunofluorescent Staining
2.4. Western Blot Analysis
2.5. TdT-Mediated dUTP Nick End Labeling (TUNEL) Assay
2.6. Statistical Analysis
3. Results
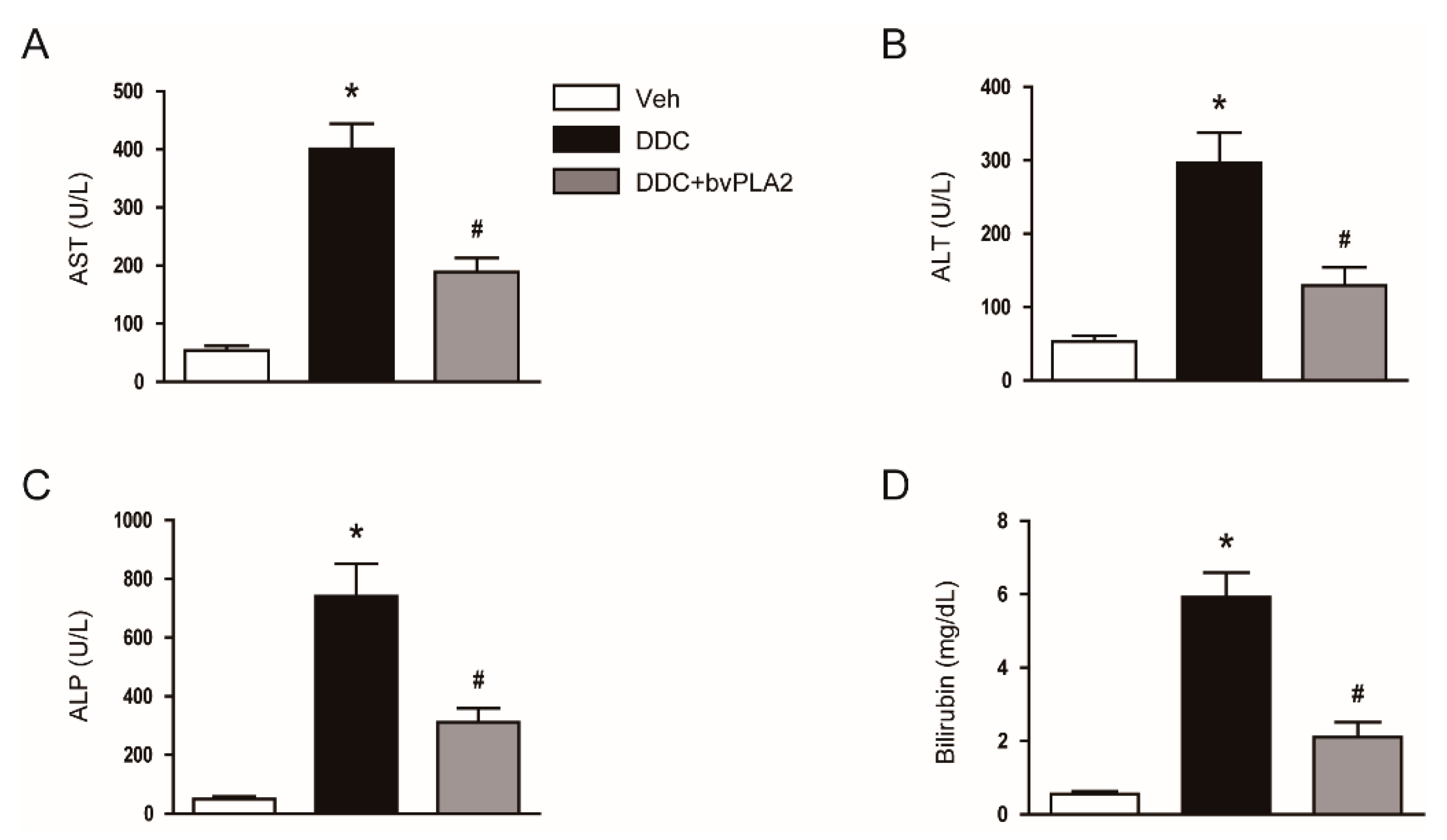
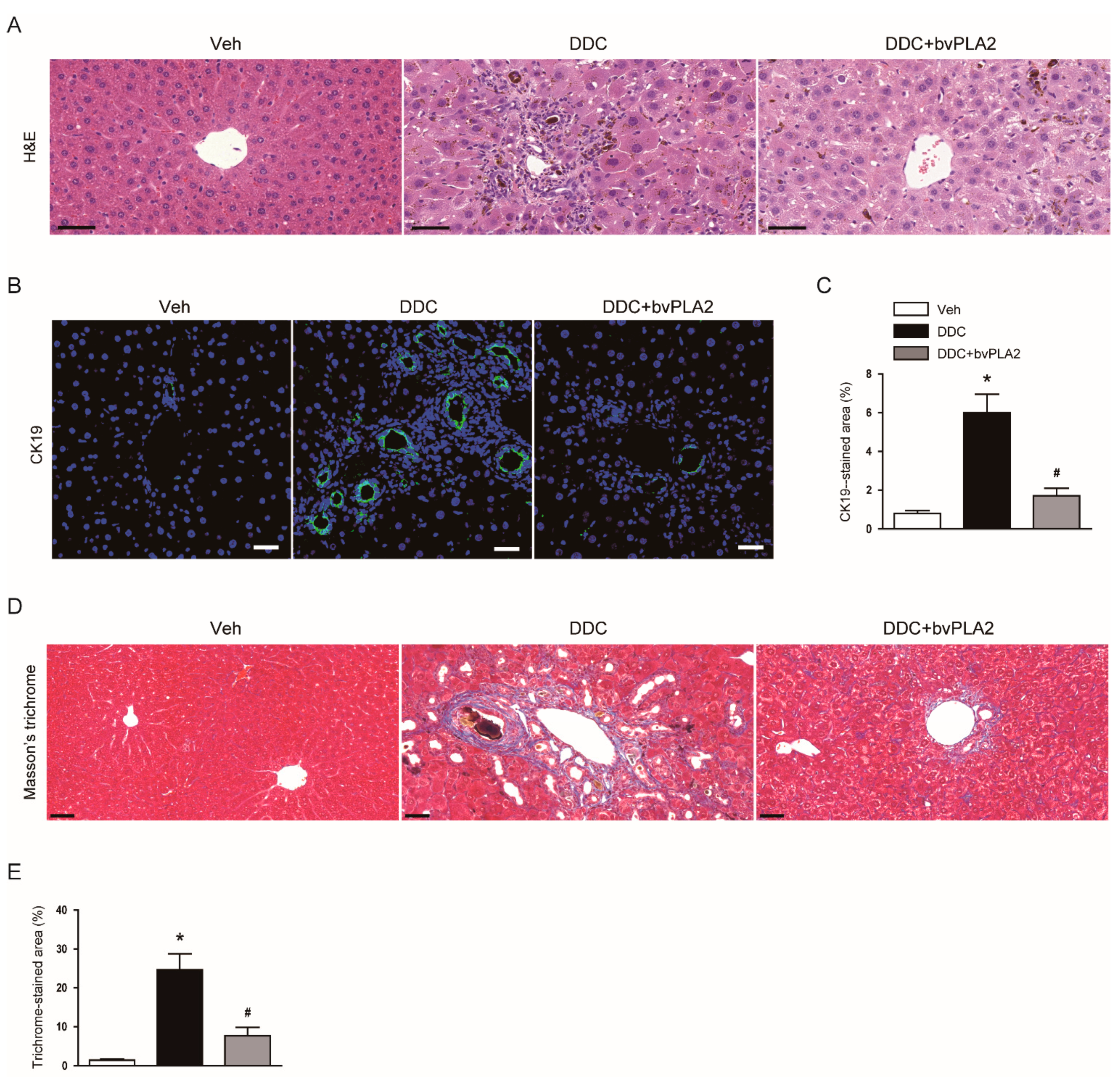
3.1. Administration of bvPLA2 Ameliorated Cholestatic Liver Injury and Fibrosis in DDC Diet-Fed Mice
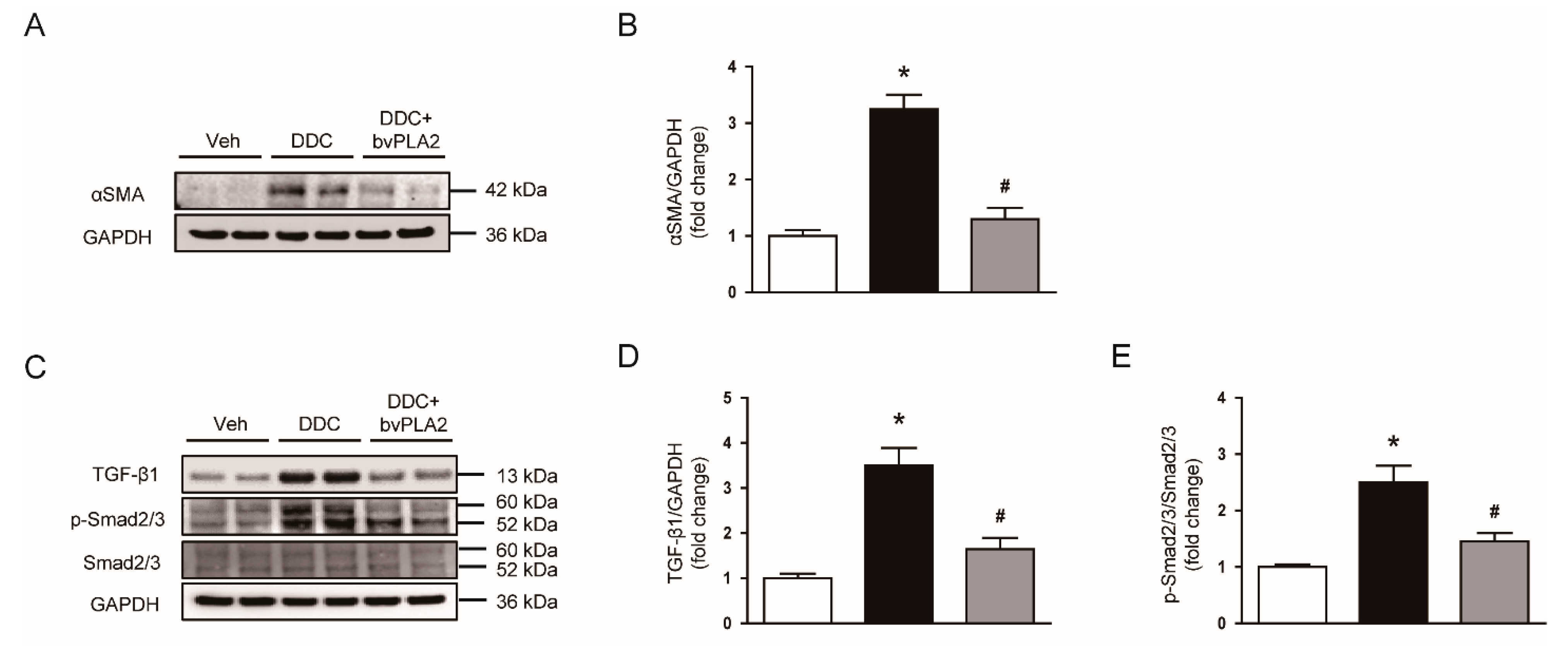
3.2. Administration of bvPLA2 Inhibited Expression of Extracellular Matrix Proteins, Myofibroblast Accumulation, and TGF-β Signaling Pathway in DDC Diet-Fed Mice
3.3. Administration of bvPLA2 Attenuated Apoptotic Cell Death in DDC Diet-Fed Mice
3.4. Administration of bvPLA2 Inhibited Cytokine Production in DDC Diet-Fed Mice
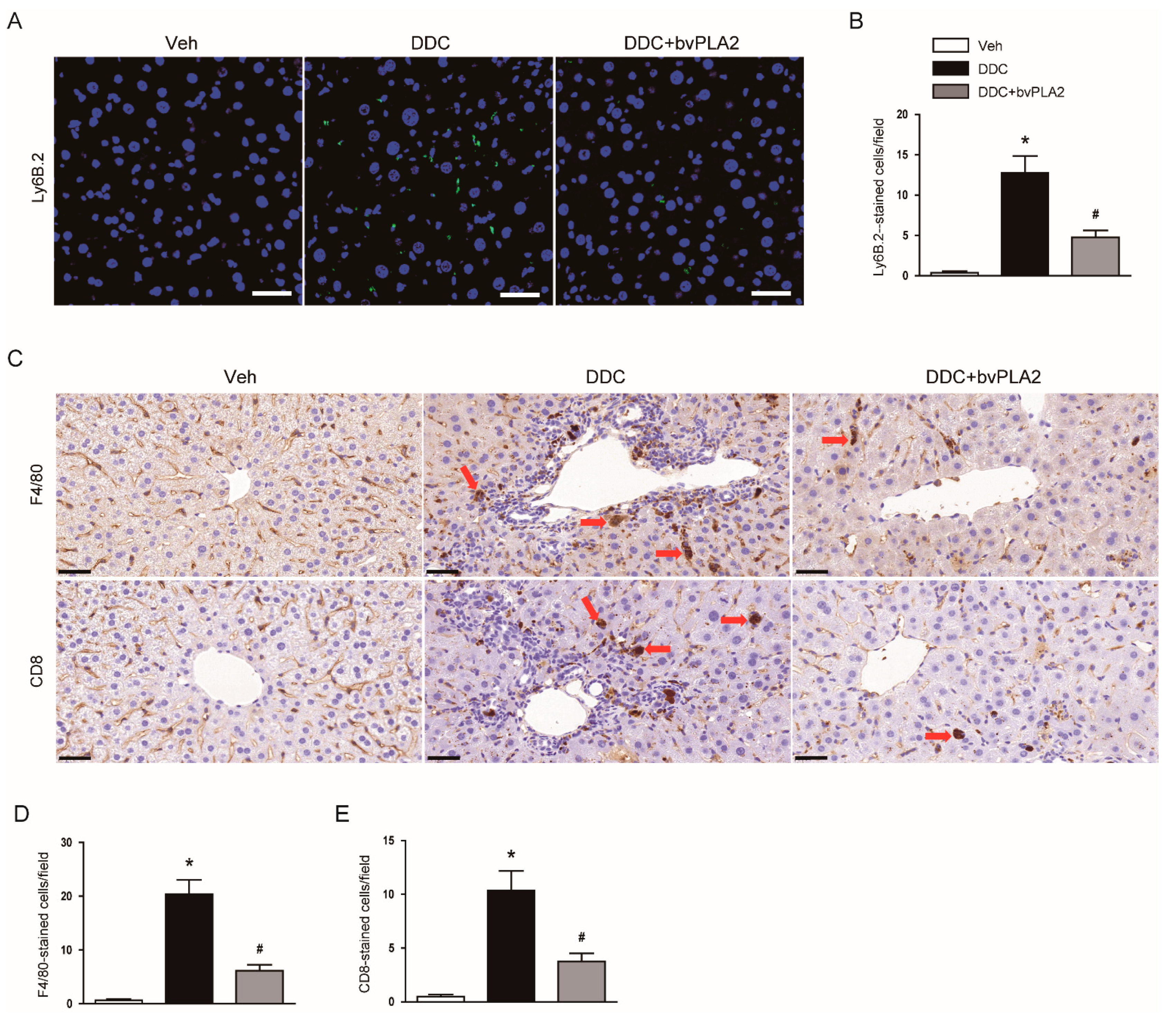
3.5. Administration of bvPLA2 Modulated Immune Cell Infiltration in DDC Diet-Fed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazaridis, K.N.; Strazzabosco, M.; Larusso, N.F. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology 2004, 127, 1565–1577. [Google Scholar] [CrossRef]
- Kriegermeier, A.; Green, R. Pediatric Cholestatic Liver Disease: Review of Bile Acid Metabolism and Discussion of Current and Emerging Therapies. Front. Med. (Lausanne) 2020, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Yokoda, R.T.; Rodriguez, E.A. Review: Pathogenesis of cholestatic liver diseases. World J. Hepatol. 2020, 12, 423–435. [Google Scholar] [CrossRef]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kim, K.H. Clinical Effectiveness and Adverse Events of Bee Venom Therapy: A Systematic Review of Randomized Controlled Trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.S.; Yoon, H.; Kang, G.H.; Hwang, D.S.; Kim, S.K.; Chung, H.S.; et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, G.; Sohn, S.-H.; Park, S.; Jung, K.-H.; Lee, J.M.; Yang, J.; Cho, J.; Bae, H. Regulatory T Cells Contribute to the Inhibition of Radiation-Induced Acute Lung Inflammation via Bee Venom Phospholipase A2 in Mice. Toxins 2016, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-H.; Lee, S.; Choi, D.B.; Shin, D.; Kim, J.; Yang, H.; Bae, H. Bee Venom Phospholipase A2 Ameliorates Atherosclerosis by Modulating Regulatory T Cells. Toxins 2020, 12, 609. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.-W.; Kim, H.; Chung, D.K. Bee Venom Alleviates Atopic Dermatitis Symptoms through the Upregulation of Decay-Accelerating Factor (DAF/CD55). Toxins 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Baek, H.; Shin, D.; Lee, G.; Park, S.; Lee, S.; Choi, D.; Kim, W.; Bae, H. Protective Effects of Intratracheally-Administered Bee Venom Phospholipase A2 on Ovalbumin-Induced Allergic Asthma in Mice. Toxins 2016, 8, 269. [Google Scholar] [CrossRef]
- Kim, H.; Keum, D.J.; Kwak, J.W.; Chung, H.-S.; Bae, H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef]
- Pose, E.; Sancho-Bru, P.; Coll, M. 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine Diet: A Rodent Model in Cholestasis Research. Methods Mol. Biol. 2019, 1981, 249–257. [Google Scholar]
- Gijbels, E.; Pieters, A.; De Muynck, K.; Vinken, M.; Devisscher, L. Rodent models of cholestatic liver disease: A practical guide for translational research. Liver Int. 2021, 41, 656–682. [Google Scholar] [CrossRef]
- Kim, J.-Y.; An, H.-J.; Kim, W.-H.; Park, Y.-Y.; Park, K.D.; Park, K.-K. Apamin suppresses biliary fibrosis and activation of hepatic stellate cells. Int. J. Mol. Med. 2017, 39, 1188–1194. [Google Scholar] [CrossRef]
- Best, J.; Verhulst, S.; Syn, W.K.; Lagaisse, K.; van Hul, N.; Heindryckx, F.; Sowa, J.P.; Peeters, L.; Van Vlierberghe, H.; Leclercq, I.A.; et al. Macrophage Depletion Attenuates Extracellular Matrix Deposition and Ductular Reaction in a Mouse Model of Chronic Cholangiopathies. PLoS ONE 2016, 11, e0162286. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, J.-H.; Kim, K.; Leem, J.; Park, K.-K. Melatonin Inhibits Transforming Growth Factor-β1-Induced Epithelial–Mesenchymal Transition in AML12 Hepatocytes. Biology 2019, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, J.-H.; Jeon, E.J.; Leem, J.; Park, K.-K. Melatonin Prevents Transforming Growth Factor-β1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms. Antioxidants 2020, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Leem, J.; Jeon, E.J. Protective Effects of Melatonin Against Aristolochic Acid-Induced Nephropathy in Mice. Biomolecules 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Gwon, M.-G.; An, H.-J.; Kim, J.-Y.; Kim, W.-H.; Gu, H.; Kim, H.-J.; Leem, J.; Jung, H.J.; Park, K.-K. Anti-fibrotic effects of synthetic TGF-β1 and Smad oligodeoxynucleotide on kidney fibrosis in vivo and in vitro through inhibition of both epithelial dedifferentiation and endothelial-mesenchymal transitions. FASEB J. 2020, 34, 333–349. [Google Scholar] [CrossRef]
- Rana, J.; Biswas, M. Regulatory T cell therapy: Current and future design perspectives. Cell. Immunol. 2020, 356, 104193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Okabe, H.; Popovic, B.; Preziosi, M.E.; Pradhan-Sundd, T.; Poddar, M.; Singh, S.; Bell, A.; England, S.G.; Nagarajan, S.; et al. Loss of Wnt Secretion by Macrophages Promotes Hepatobiliary Injury after Administration of 3,5-Diethoxycarbonyl-1, 4-Dihydrocollidine Diet. Am. J. Pathol. 2019, 189, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, M.; Sun, X.; Qin, J.; Yu, C.; Wen, Y.; Zhang, Q.; Gu, J.; Xia, Q.; Kong, X. DJ-1 deficiency attenuates expansion of liver progenitor cells through modulating the inflammatory and fibrogenic niches. Cell Death Dis. 2016, 7, e2257. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.; Lee, C.; Cheng, C.; Chou, H.C.; Yang, H.; Bae, H. Targeting of adipose tissue macrophages by bee venom phospholipase A2 attenuates high-fat diet-induced obesity. Int. J. Obes. (Lond.) 2021, 45, 1656–1667. [Google Scholar] [CrossRef]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.S.; Onori, P.; Wise, C.; Yang, F.; Marzioni, M.; Alvaro, D.; Franchitto, A.; Mancinelli, R.; Alpini, G.; Munshi, M.K.; et al. Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig. Liver Dis. 2010, 42, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.L.; Feng, D.C.; Radaeva, S.; Kong, X.N.; Wang, L.; Liu, Y.; Li, Q.; Shen, H.; Gao, Y.P.; Müllenbach, R.; et al. IFN-γ inhibits liver progenitor cell proliferation in HBV-infected patients and in 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet-fed mice. J. Hepatol. 2013, 59, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.-J.; Briz, O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009, 14, 1677–1689. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, K.-H.; Kim, S.-J.; Lee, W.-R.; Lee, K.-G.; Park, K.-K. Bee venom protects hepatocytes from tumor necrosis factor-alpha and actinomycin D. Arch. Pharm. Res. 2010, 33, 215–223. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, K.-W.; Choi, S.-M.; Yang, E.J. Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells. Toxins 2015, 7, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Park, S.-Y.; Ku, S.J.; Ryu, K.; Kim, Y.; Bae, H.; Lee, Y.-S. Bee Venom Phospholipase A2 Induces Regulatory T Cell Populations by Suppressing Apoptotic Signaling Pathway. Toxins 2020, 12, 198. [Google Scholar] [CrossRef]
- Ikeno, Y.; Ohara, D.; Takeuchi, Y.; Watanabe, H.; Kondoh, G.; Taura, K.; Uemoto, S.; Hirota, K. Foxp3+ Regulatory T Cells Inhibit CCl4-Induced Liver Inflammation and Fibrosis by Regulating Tissue Cellular Immunity. Front. Immunol. 2020, 11, 584048. [Google Scholar] [CrossRef]
- Roh, Y.S.; Park, S.; Lim, C.W.; Kim, B. Depletion of Foxp3+ Regulatory T Cells Promotes Profibrogenic Milieu of Cholestasis-Induced Liver Injury. Dig. Dis. Sci. 2015, 60, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Taketomi, Y.; Murakami, M. Metabolic regulation by secreted phospholipase A 2. Inflamm. Regen. 2016, 36, 7. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Jang, H.-J.; Leem, J.; Kim, G.-M. Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice. Biomedicines 2021, 9, 992. https://doi.org/10.3390/biomedicines9080992
Kim J-Y, Jang H-J, Leem J, Kim G-M. Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice. Biomedicines. 2021; 9(8):992. https://doi.org/10.3390/biomedicines9080992
Chicago/Turabian StyleKim, Jung-Yeon, Hyo-Jeong Jang, Jaechan Leem, and Gyun-Moo Kim. 2021. "Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice" Biomedicines 9, no. 8: 992. https://doi.org/10.3390/biomedicines9080992
APA StyleKim, J.-Y., Jang, H.-J., Leem, J., & Kim, G.-M. (2021). Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice. Biomedicines, 9(8), 992. https://doi.org/10.3390/biomedicines9080992





