Circulating MicroRNA-21 and MicroRNA-122 as Prognostic Biomarkers in Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. RNA Isolation and MiRNAs Analysis
2.3. HIF-1α Assay
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
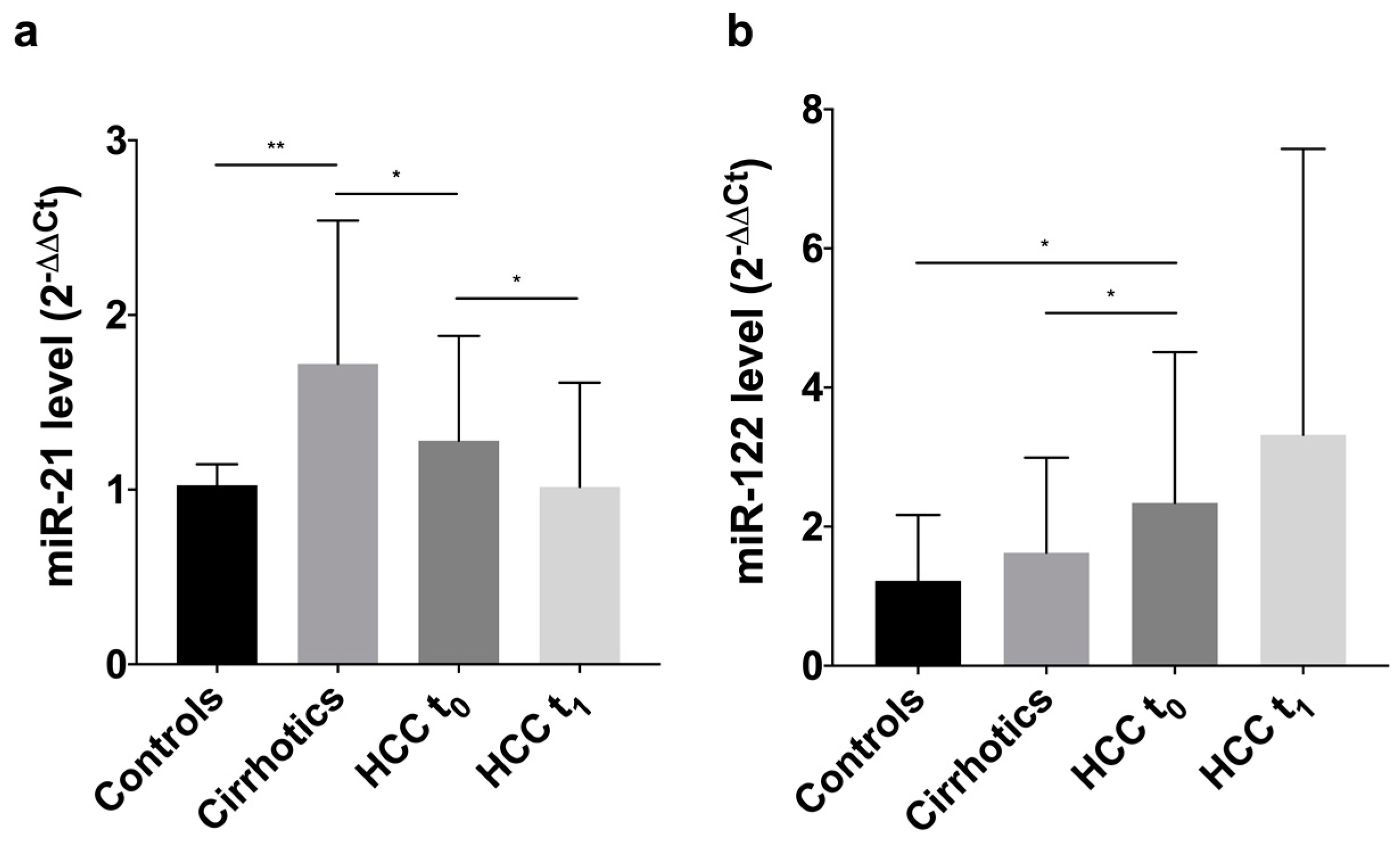
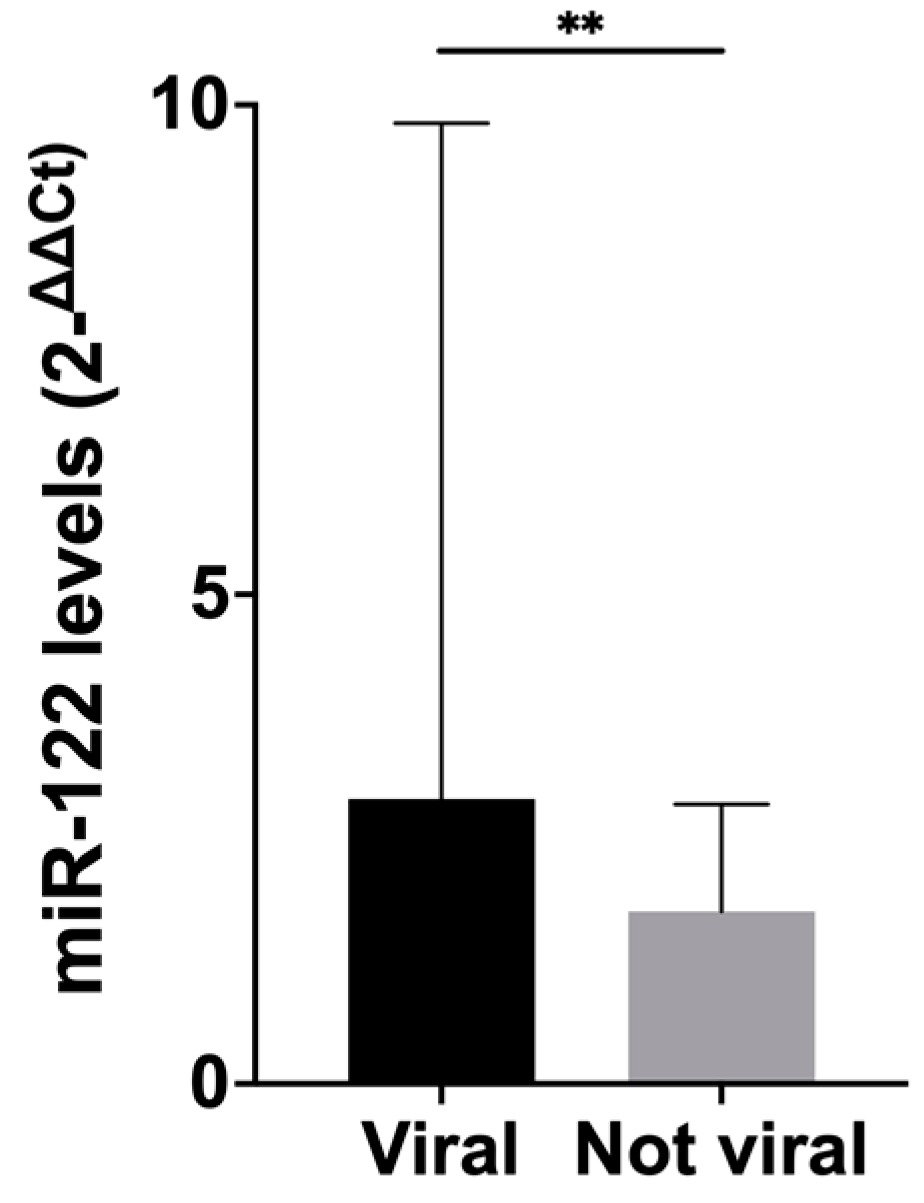
3.2. Levels of Circulating MiRNAs
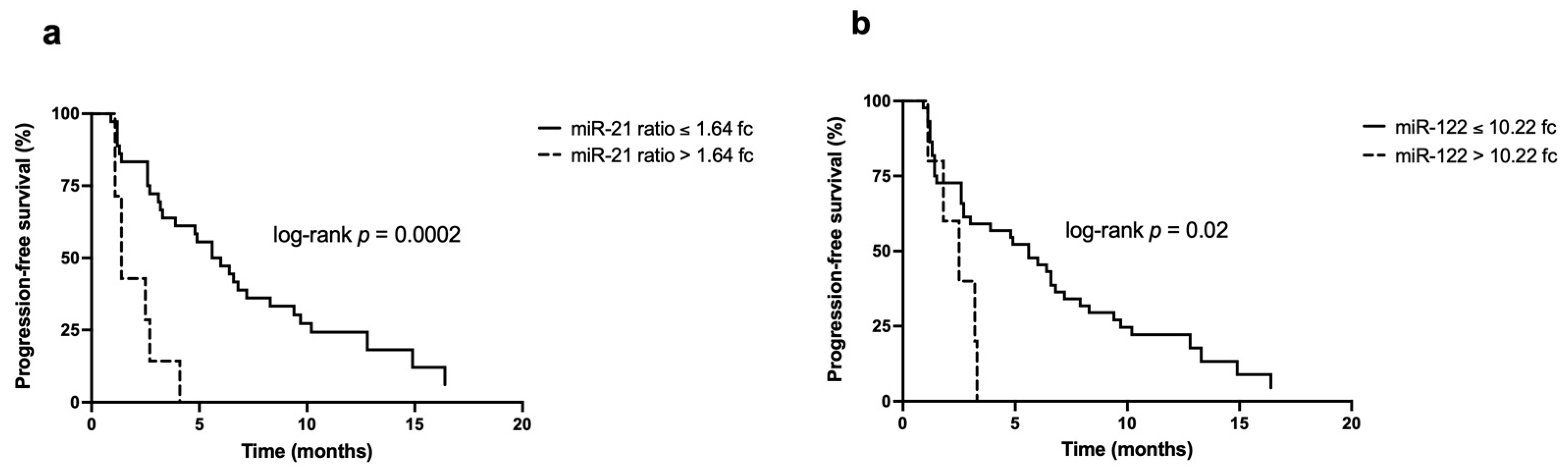
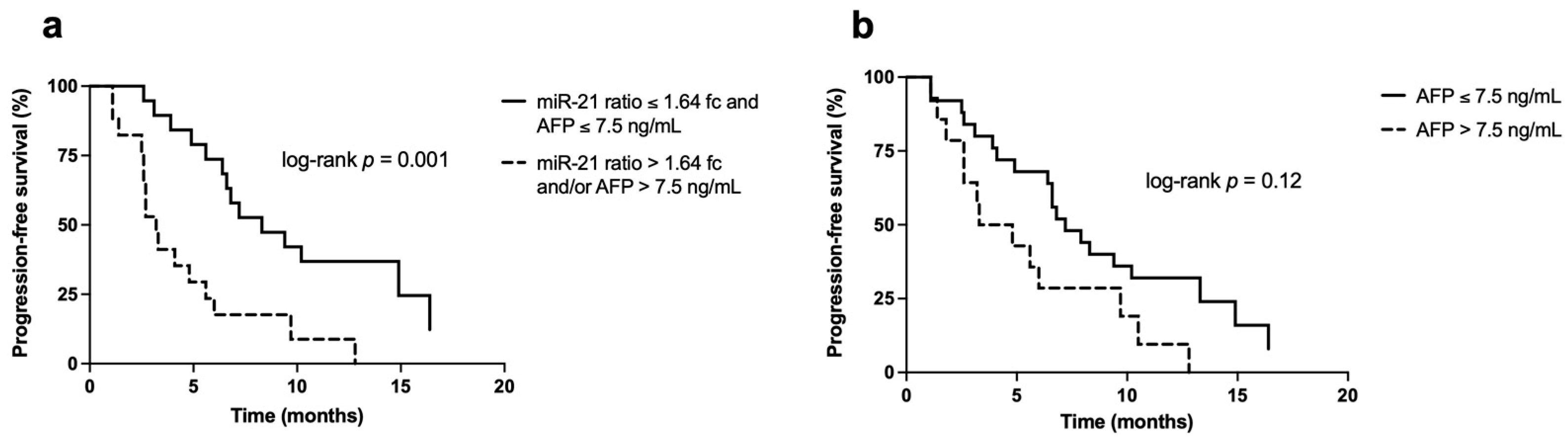
3.3. Survival Analysis
3.4. Univariate and Multivariate Analysis
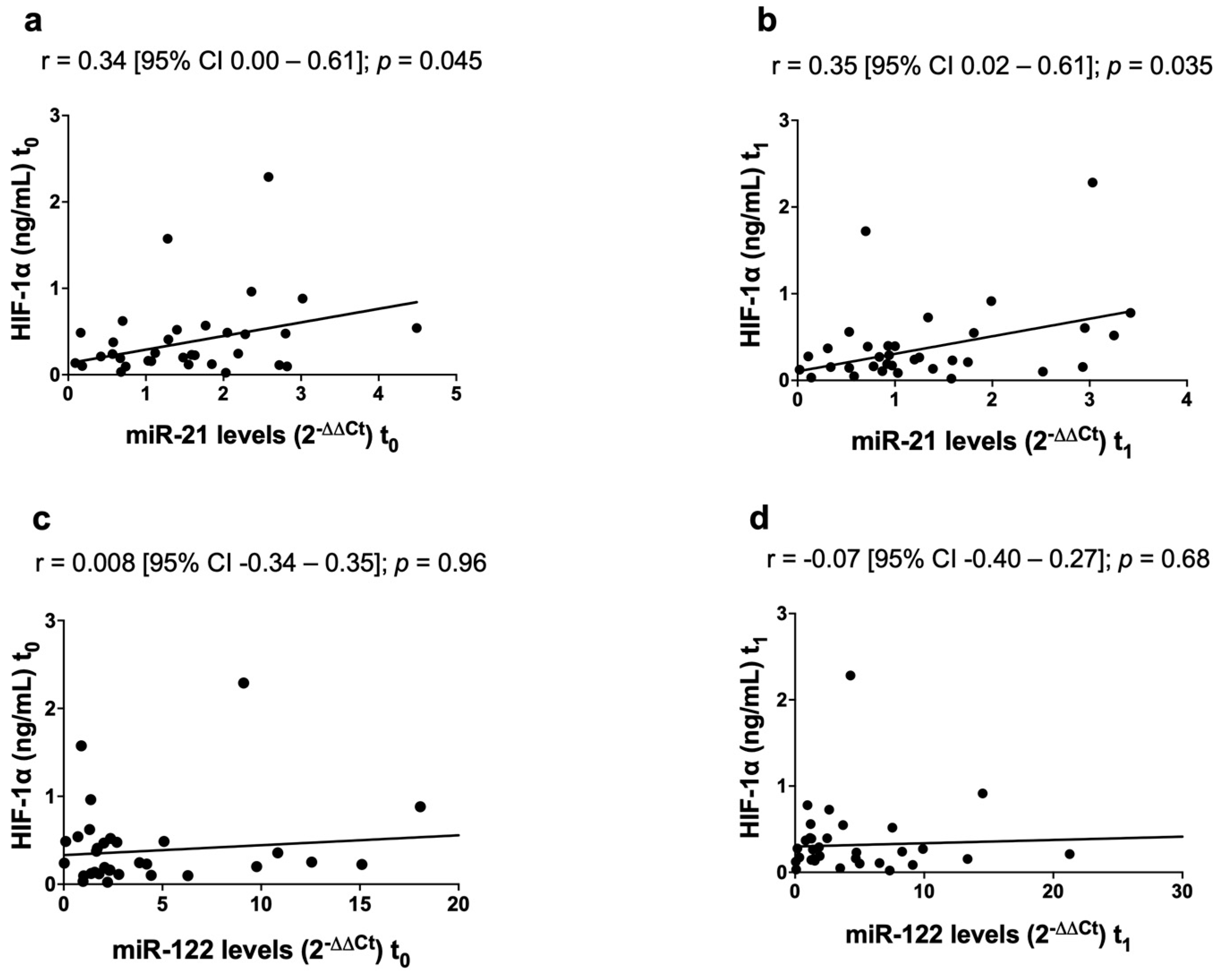
3.5. Correlation between MicroRNAs and HIF-1α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; van den Berg, A.; Huitema, S.; Gouw, A.S.H.; Molema, G.; de Jong, K.P. Correlation of microRNA-16, microRNA-21 and microRNA-101 expression with cyclooxygenase-2 expression and angiogenic factors in cirrhotic and noncirrhotic human hepatocellular carcinoma. PLoS ONE 2014, 9, e95826. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-S.; Yu, W.; Cui, H.; Wang, Y.-J.; Zhang, L.; Han, F.; Huang, T. Increased expression of miR-21 predicts poor prognosis in patients with hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 7234–7238. [Google Scholar] [PubMed]
- Yoon, J.S.; Kim, G.; Lee, Y.R.; Park, S.Y.; Tak, W.Y.; Kweon, Y.-O.; Park, J.G.; Lee, H.W.; Han, Y.S.; Ha, H.T.; et al. Clinical significance of microRNA-21 expression in disease progression of patients with hepatocellular carcinoma. Biomark. Med. 2018, 12, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Papaconstantinou, I.; Gazouli, M.; Lyberopoulou, A.; Polymeneas, G.; Voros, D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol. Carcinog. 2013, 52, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.; Banerjee, B.D.; Bharadwaj, M.; Husain, S.A.; Kar, P. Expression Analysis of MicroRNA-21 and MicroRNA-122 in Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2019, 9, 294–301. [Google Scholar] [CrossRef]
- Pu, C.; Huang, H.; Wang, Z.; Zou, W.; Lv, Y.; Zhou, Z.; Zhang, Q.; Qiao, L.; Wu, F.; Shao, S. Extracellular Vesicle-Associated mir-21 and mir-144 Are Markedly Elevated in Serum of Patients With Hepatocellular Carcinoma. Front. Physiol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Kim, S.S.; Cho, H.J.; Nam, J.S.; Kim, H.J.; Kang, D.R.; Won, J.H.; Kim, J.; Kim, J.K.; Lee, J.H.; Kim, B.H.; et al. Plasma MicroRNA-21, 26a, and 29a-3p as Predictive Markers for Treatment Response Following Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. J. Korean Med. Sci. 2018, 33, e6. [Google Scholar] [CrossRef]
- Suehiro, T.; Miyaaki, H.; Kanda, Y.; Shibata, H.; Honda, T.; Ozawa, E.; Miuma, S.; Taura, N.; Nakao, K. Serum exosomal microRNA-122 and microRNA-21 as predictive biomarkers in transarterial chemoembolization-treated hepatocellular carcinoma patients. Oncol. Lett. 2018, 16, 3267–3273. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Wang, L.; Wu, H.; Zhou, C.; Zhu, H.; Xu, N.; Xie, Y. Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival. PLoS ONE 2014, 9, e109347. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Jacquemin, E.; Munnich, A.; Lyonnet, S.; Henrion-Caude, A. miR-122, a paradigm for the role of microRNAs in the liver. J. Hepatol. 2008, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Nasser, M.W.; Wang, B.; Hsu, S.-H.; Datta, J.; Kutay, H.; Yadav, A.; Nuovo, G.; Kumar, P.; Ghoshal, K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J. Biol. Chem. 2009, 284, 32015–32027. [Google Scholar] [CrossRef]
- Xu, J.; Wu, C.; Che, X.; Wang, L.; Yu, D.; Zhang, T.; Huang, L.; Li, H.; Tan, W.; Wang, C.; et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol. Carcinog. 2011, 50, 136–142. [Google Scholar] [CrossRef]
- Jin, Y.; Wong, Y.S.; Goh, B.K.P.; Chan, C.Y.; Cheow, P.C.; Chow, P.K.H.; Lim, T.K.H.; Goh, G.B.B.; Krishnamoorthy, T.L.; Kumar, R.; et al. Circulating microRNAs as Potential Diagnostic and Prognostic Biomarkers in Hepatocellular Carcinoma. Sci. Rep. 2019, 9, 10464. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.-F.; Lai, L.; Jiang, B.-H. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS ONE 2011, 6, e19139. [Google Scholar] [CrossRef]
- Donnem, T.; Fenton, C.G.; Lonvik, K.; Berg, T.; Eklo, K.; Andersen, S.; Stenvold, H.; Al-Shibli, K.; Al-Saad, S.; Bremnes, R.M.; et al. MicroRNA signatures in tumor tissue related to angiogenesis in non-small cell lung cancer. PLoS ONE 2012, 7, e29671. [Google Scholar] [CrossRef]
- Sabry, D.; El-Deek, S.E.M.; Maher, M.; El-Baz, M.A.H.; El-Bader, H.M.; Amer, E.; Hassan, E.A.; Fathy, W.; El-Deek, H.E.M. Role of miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in colorectal carcinoma: Impact of HIF-1alpha-VEGF signaling pathway. Mol. Cell. Biochem. 2019, 454, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Csak, T.; Bala, S.; Lippai, D.; Satishchandran, A.; Catalano, D.; Kodys, K.; Szabo, G. microRNA-122 regulates hypoxia-inducible factor-1 and vimentin in hepatocytes and correlates with fibrosis in diet-induced steatohepatitis. Liver Int. 2015, 35, 532–541. [Google Scholar] [CrossRef]
- Ambade, A.; Satishchandran, A.; Szabo, G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and MIR-122-mediated HIF-1α activation. Sci. Rep. 2016, 6, 21340. [Google Scholar] [CrossRef]
- Ju, C.; Wang, M.; Tak, E.; Kim, B.; Emontzpohl, C.; Yang, Y.; Yuan, X.; Kutay, H.; Liang, Y.; Hall, D.; et al. Hypoxia-inducible factor-1α-dependent induction of miR122 enhances hepatic ischemia tolerance. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Liu, K.; Min, X.-L.; Peng, J.; Yang, K.; Yang, L.; Zhang, X.-M. The Changes of HIF-1α and VEGF Expression After TACE in Patients With Hepatocellular Carcinoma. J. Clin. Med. Res. 2016, 8, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ducreux, M.; Lencioni, R.; Di Bisceglie, A.M.; Galle, P.R.; Dufour, J.F.; Greten, T.F.; Raymond, E.; Roskams, T.; De Baere, T.; et al. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.-W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Coulouarn, C.; Factor, V.M.; Andersen, J.B.; Durkin, M.E.; Thorgeirsson, S.S. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 2009, 28, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wang, Y.; Sai, W.; Zhang, L.; Miao, Y.; Cao, L.; Zhai, X.; Feng, X.; Yang, L. HBx-induced miR-21 suppresses cell apoptosis in hepatocellular carcinoma by targeting interleukin-12. Oncol. Rep. 2016, 36, 2305–2312. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Wang, S.; Wu, B.; Hao, J.; Fan, H.; Ju, Y.; Ding, Y.; Chen, L.; Chu, X.; et al. Hepatitis B virus mRNA-mediated miR-122 inhibition upregulates PTTG1-binding protein, which promotes hepatocellular carcinoma tumor growth and cell invasion. J. Virol. 2013, 87, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Nassirpour, R.; Mehta, P.P.; Yin, M.-J. miR-122 regulates tumorigenesis in hepatocellular carcinoma by targeting AKT3. PLoS ONE 2013, 8, e79655. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Hsu, P.W.-C.; Lai, T.-C.; Chau, G.-Y.; Lin, C.-W.; Chen, C.-M.; Lin, C.-D.; Liao, Y.-L.; Wang, J.-L.; Chau, Y.-P.; et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 2009, 49, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.S.; Li, J.S. High expression of miR-21 is not a predictor of poor prognosis in all patients with hepatocellular carcinoma. Mol. Clin. Oncol. 2018, 8, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Jiang, W.; Li, Q.; Lan, Y. The clinical significance of microRNA-122 in predicting the prognosis of patients with hepatocellular carcinoma: A meta-analysis validated by the Cancer Genome Atlas dataset. Medicine 2019, 98, e14810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.X.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Long, X.R.; Zhang, Y.J.; Zhang, M.Y.; Chen, K.; Zheng, X.F.S.; Wang, H.Y. Identification of an 88-microRNA signature in whole blood for diagnosis of hepatocellular carcinoma and other chronic liver diseases. Aging 2017, 9, 1565–1584. [Google Scholar] [CrossRef][Green Version]
- Guo, X.; Lv, X.; Lv, X.; Ma, Y.; Chen, L.; Chen, Y. Circulating miR-21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget 2017, 8, 44050–44058. [Google Scholar] [CrossRef]
- Qi, P.; Cheng, S.; Wang, H.; Li, N.; Chen, Y.; Gao, C. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS ONE 2011, 6, e28486. [Google Scholar] [CrossRef]
- Koberle, V.; Kronenberger, B.; Pleli, T.; Trojan, J.; Imelmann, E.; Peveling-Oberhag, J.; Welker, M.-W.; Elhendawy, M.; Zeuzem, S.; Piiper, A.; et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur. J. Cancer 2013, 49, 3442–3449. [Google Scholar] [CrossRef]
- Bihrer, V.; Friedrich-Rust, M.; Kronenberger, B.; Forestier, N.; Haupenthal, J.; Shi, Y.; Peveling-Oberhag, J.; Radeke, H.H.; Sarrazin, C.; Herrmann, E.; et al. Serum miR-122 as a biomarker of necroinflammation in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 2011, 106, 1663–1669. [Google Scholar] [CrossRef]
- Waidmann, O.; Bihrer, V.; Pleli, T.; Farnik, H.; Berger, A.; Zeuzem, S.; Kronenberger, B.; Piiper, A. Serum microRNA-122 levels in different groups of patients with chronic hepatitis B virus infection. J. Viral Hepat. 2012, 19, e58–e65. [Google Scholar] [CrossRef]
- Qiu, L.; Fan, H.; Jin, W.; Zhao, B.; Wang, Y.; Ju, Y.; Chen, L.; Chen, Y.; Duan, Z.; Meng, S. miR-122-induced down-regulation of HO-1 negatively affects miR-122-mediated suppression of HBV. Biochem. Biophys. Res. Commun. 2010, 398, 771–777. [Google Scholar] [CrossRef]
- Spaniel, C.; Honda, M.; Selitsky, S.R.; Yamane, D.; Shimakami, T.; Kaneko, S.; Lanford, R.E.; Lemon, S.M. microRNA-122 abundance in hepatocellular carcinoma and non-tumor liver tissue from Japanese patients with persistent HCV versus HBV infection. PLoS ONE 2013, 8, e76867. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, X.; Dai, C.; Shang, C. High serum microRNA-122 level is independently associated with higher overall survival rate in hepatocellular carcinoma patients. Tumour Biol. 2015, 36, 4773–4776. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, J.K.; Nam, J.S.; Wang, H.J.; Lee, J.H.; Kim, B.W.; Kim, S.S.; Noh, C.K.; Shin, S.J.; Lee, K.M.; et al. High circulating microRNA-122 expression is a poor prognostic marker in patients with hepatitis B virus-related hepatocellular carcinoma who undergo radiofrequency ablation. Clin. Biochem. 2015, 48, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Nam, J.S.; Cho, H.J.; Won, J.H.; Kim, J.W.; Ji, J.-H.; Yang, M.J.; Park, J.H.; Noh, C.-K.; Shin, S.J.; et al. Plasma micoRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2017, 32, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S. Progression-free survival: Starting point or endpoint in advanced HCC trial design? J. Hepatol. 2019, 70, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
| Variable | Cirrhotics (n = 28) | HCC (n = 54) | p† | |
|---|---|---|---|---|
| Males—n (%) | 20 (71.4) | 44 (81.5) | 0.40 | |
| Age (years) | 63.5 (49.3–72.0) | 67.0 (61.8–76.0) | 0.13 | |
| Cirrhosis—n (%) | 28 (100) | 48 (88.9) | 0.09 | |
| Etiology—n (%) | HBV | 3 (10.7) | 6 (11.1) | 0.07 |
| HCV | 4 (14.3) | 20 (37.1) | ||
| Alcohol | 16 (57.1) | 16 (29.6) | ||
| Other | 5 (17.9) | 12 (22.2) | ||
| CRPH—n (%) | 26 (92.9) | 33 (60.4) | 0.002 | |
| Child-Pugh A—n (%) | 13 (46.4) | 47 (87.0) | 0.0002 | |
| MELD score | 13 (10–19) | 8 (7–11) | <0.0001 | |
| Bilirubin (μmol/L) | 23.5 (13.4–68.6) | 15.0 (10.0–20.0) | 0.006 | |
| Albumin (g/dL) | 3.5 (2.9–4.0) | 4.0 (3.5–4.3) | 0.007 | |
| INR | 1.32 (1.15–1.60) | 1.12 (1.09–1.21) | 0.0003 | |
| Number of nodules | 2 (1–4) | |||
| Diameter (cm) | 2.2 (1.8–3.6) | |||
| MVI and/or EHS—n (%) | 4 (7.5) | |||
| BCLC stage—n (%) | 0/A | 30 (55.6) | ||
| B/C | 24 (44.4) | |||
| Previous treatments—n (%) | LR/ABL | 13 (24.0) | ||
| TACE | 9 (16.7) | |||
| ABL/LR + TACE | 21 (38.9) | |||
| No | 11 (20.4) | |||
| Radiological response | CR | 24 (44.5%) | ||
| PR | 16 (29.6%) | |||
| SD | 4 (7.4%) | |||
| PD | 10 (18.5%) | |||
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | aHR (95% CI) | p | ||
| AFP (ng/mL) | ≤7.5 | Ref | - | Ref | - |
| >7.5 | 2.52 (1.37–4.66) | 0.003 | 4.31 (1.66–11.20) | 0.003 | |
| miR-21 ratio (2−ΔΔCt) | ≤1.64 | Ref | - | Ref | - |
| >1.64 | 4.95 (1.93–12.65) | 0.001 | 8.61 (2.03–36.47) | 0.003 | |
| miR-122 (2−ΔΔCt) | ≤10.22 | Ref | - | Ref | - |
| >10.22 | 2.98 (1.10–8.09) | 0.03 | 2.11 (0.46–9.78) | 0.3 | |
| Radiological response | CR/PR | Ref | - | Ref | - |
| SD/PD | 6.37 (2.91–13.95) | <0.0001 | 10.44 (2.74–39.79) | 0.001 | |
| Number of nodules | ≤3 | Ref | - | Ref | - |
| >3 | 2.30 (1.24–4.25) | 0.008 | 0.51 (0.12–2.16) | 0.4 | |
| Diameter (cm) | ≤5 | Ref | - | Ref | - |
| >5 | 2.23 (0.97–5.16) | 0.06 | 1.78 (0.47–6.78) | 0.4 | |
| CRPH | No | Ref | - | Ref | - |
| Yes | 1.71 (0.92–3.17) | 0.09 | 0.56 (0.23–1.35) | 0.2 | |
| BCLC stage | 0/A | Ref | - | Ref | - |
| B/C | 2.54 (1.36–4.74) | 0.003 | 3.50 (0.77–15.96) | 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelizzaro, F.; Cardin, R.; Sartori, A.; Imondi, A.; Penzo, B.; Aliberti, C.; Ponzoni, A.; Vitale, A.; Cillo, U.; Farinati, F. Circulating MicroRNA-21 and MicroRNA-122 as Prognostic Biomarkers in Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization. Biomedicines 2021, 9, 890. https://doi.org/10.3390/biomedicines9080890
Pelizzaro F, Cardin R, Sartori A, Imondi A, Penzo B, Aliberti C, Ponzoni A, Vitale A, Cillo U, Farinati F. Circulating MicroRNA-21 and MicroRNA-122 as Prognostic Biomarkers in Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization. Biomedicines. 2021; 9(8):890. https://doi.org/10.3390/biomedicines9080890
Chicago/Turabian StylePelizzaro, Filippo, Romilda Cardin, Anna Sartori, Angela Imondi, Barbara Penzo, Camillo Aliberti, Alberto Ponzoni, Alessandro Vitale, Umberto Cillo, and Fabio Farinati. 2021. "Circulating MicroRNA-21 and MicroRNA-122 as Prognostic Biomarkers in Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization" Biomedicines 9, no. 8: 890. https://doi.org/10.3390/biomedicines9080890
APA StylePelizzaro, F., Cardin, R., Sartori, A., Imondi, A., Penzo, B., Aliberti, C., Ponzoni, A., Vitale, A., Cillo, U., & Farinati, F. (2021). Circulating MicroRNA-21 and MicroRNA-122 as Prognostic Biomarkers in Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization. Biomedicines, 9(8), 890. https://doi.org/10.3390/biomedicines9080890








