Myocardial Metabolic Response Predicts Chemotherapy Curative Potential on Hodgkin Lymphoma: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Image Analysis
2.3. Statistical Analysis
2.4. Study Approval
3. Results
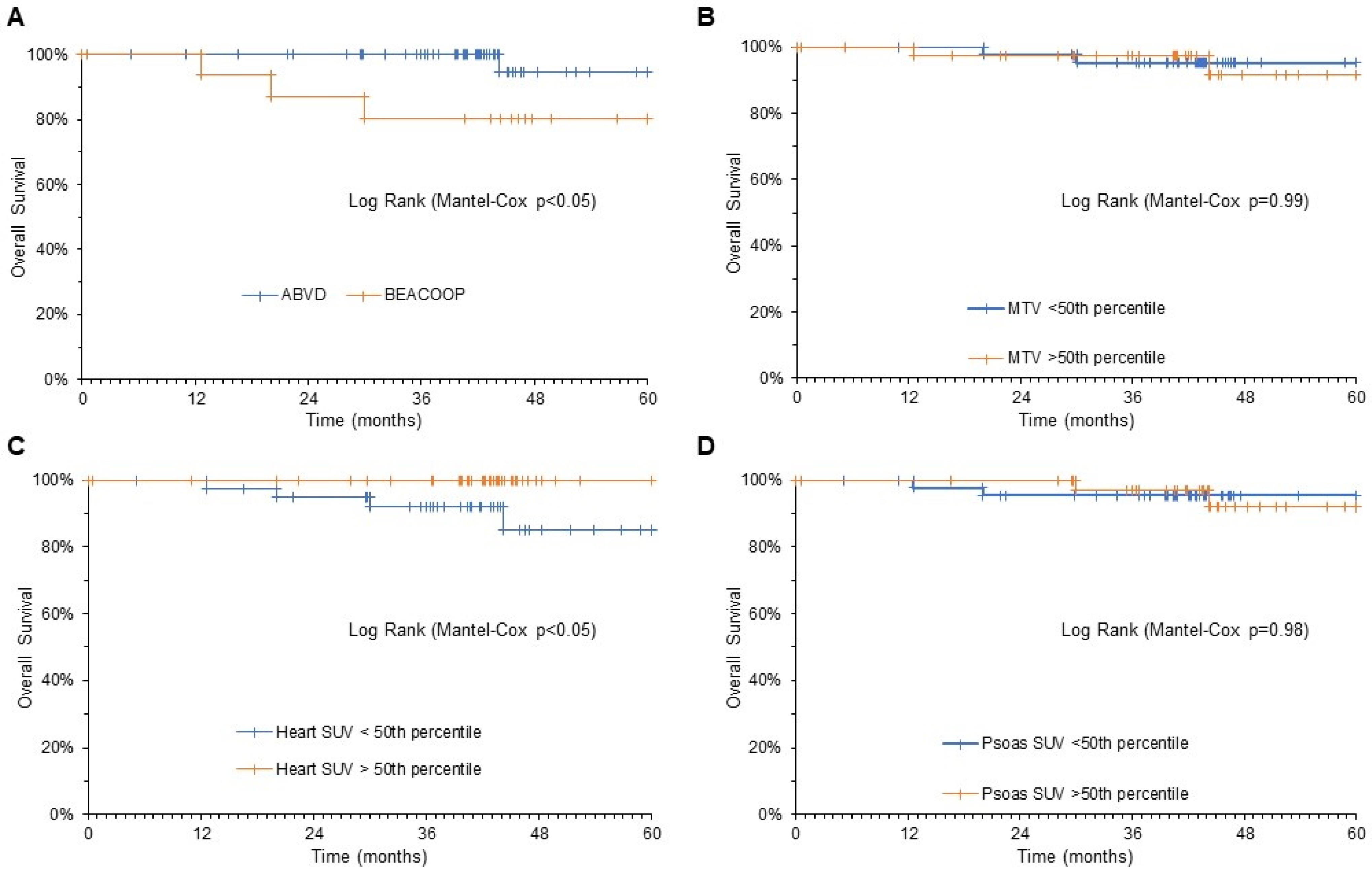
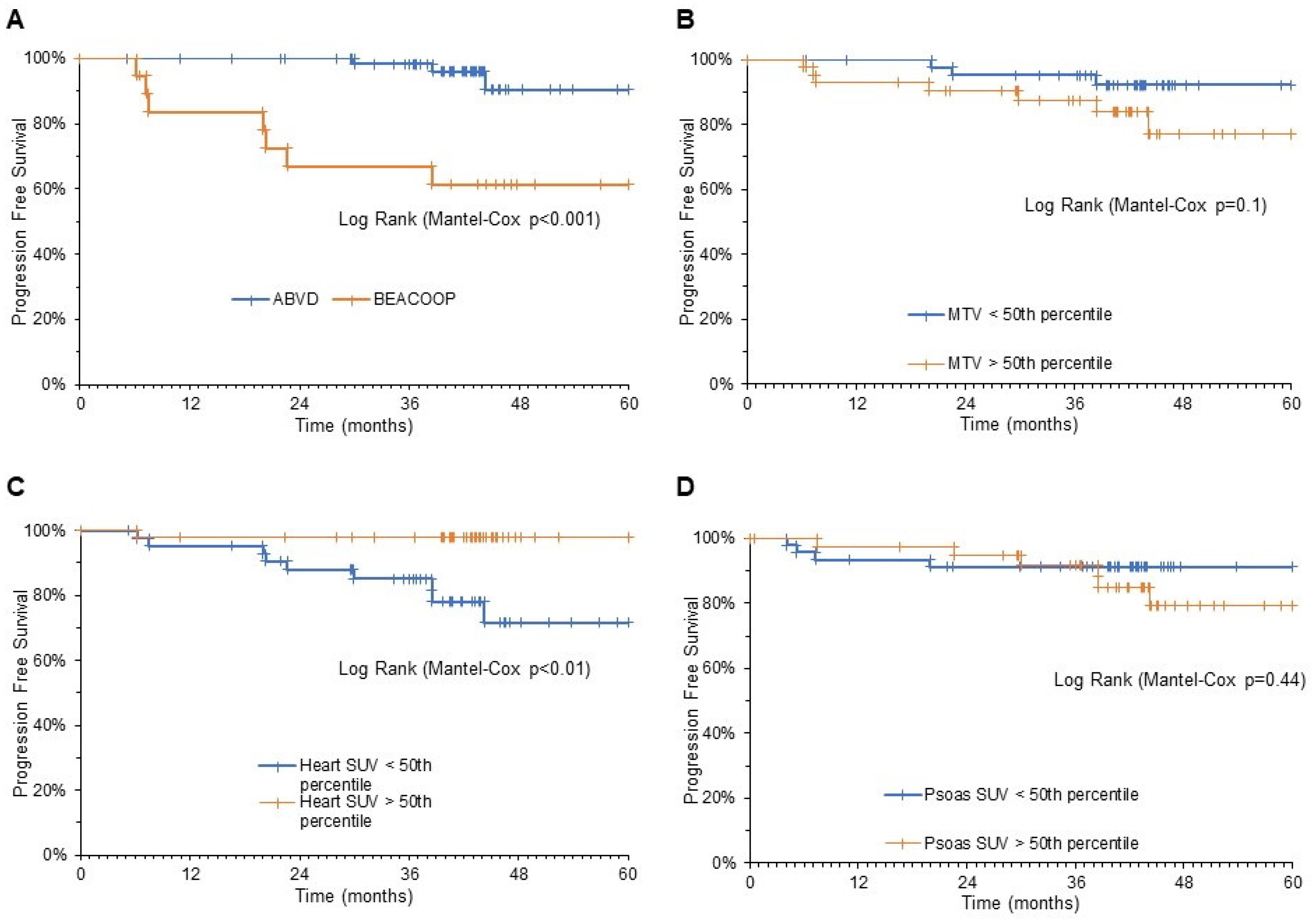
3.1. Clinical Characteristics and Follow-Up Data
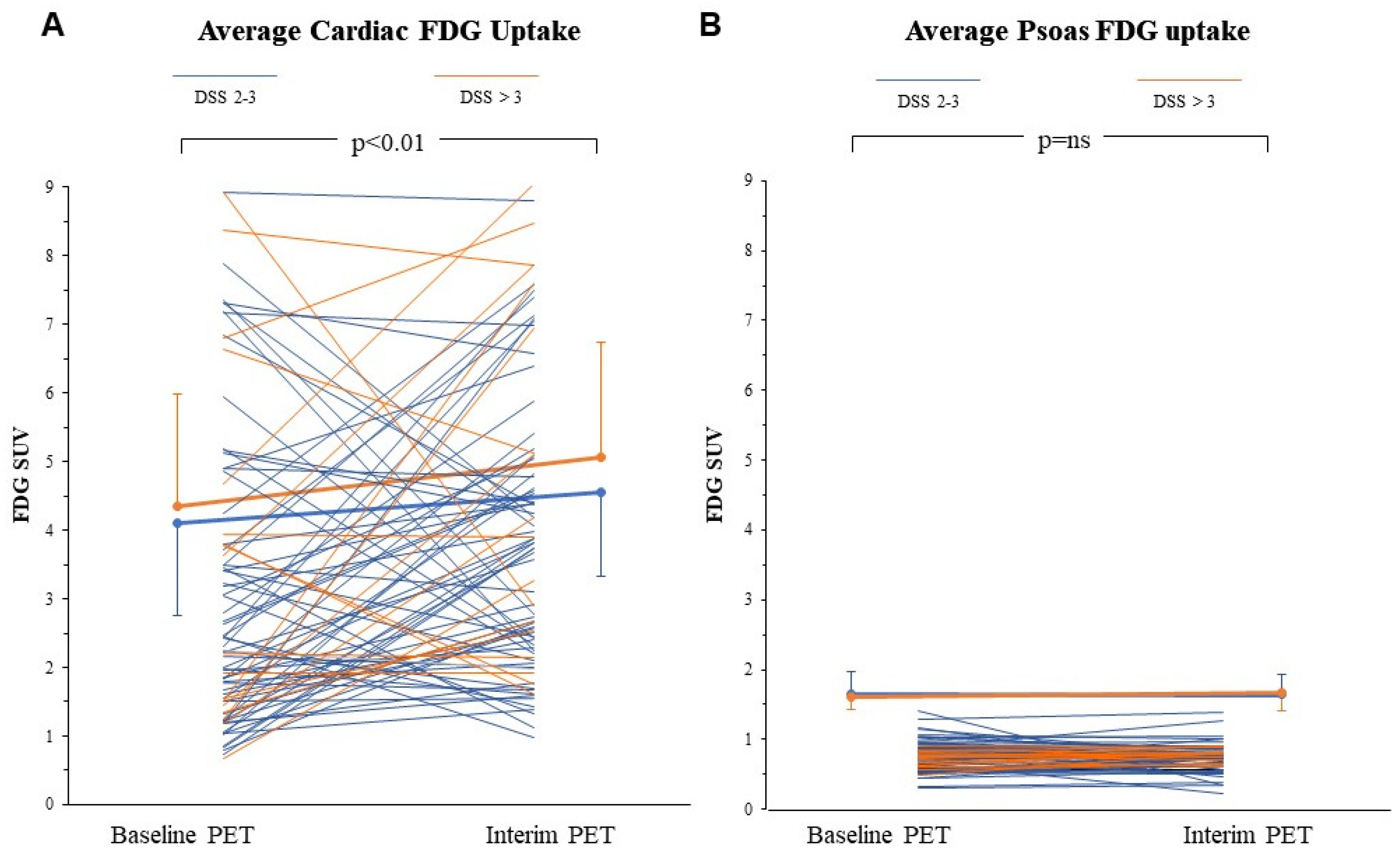
3.2. FDG Uptake Response to ABVD in the Heart and Skeletal Muscle
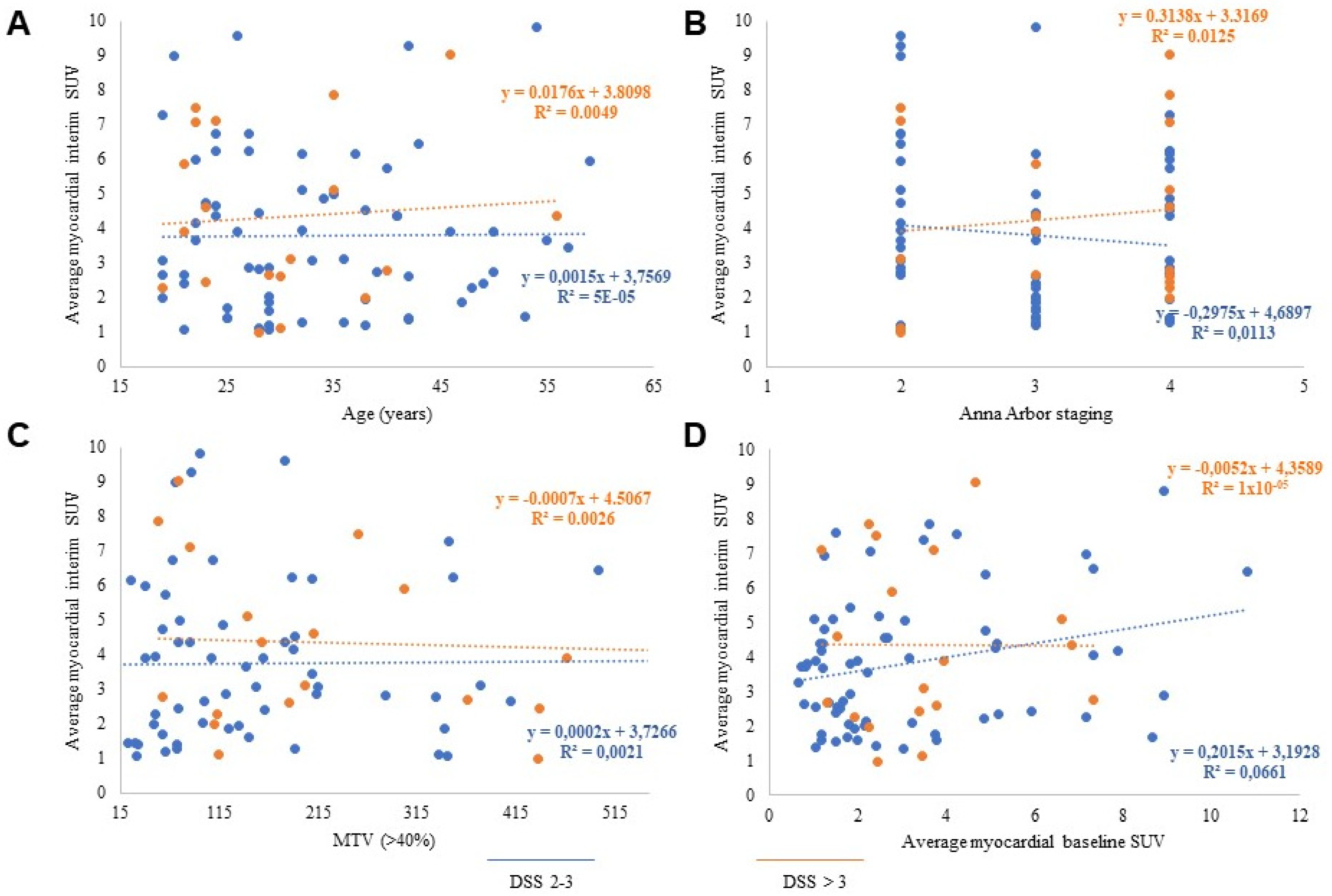
3.3. Predictive Power of Cardiac FDG Uptake
4. Discussion
4.1. Tumor Shutdown vs. Cardiac Lighting
4.2. Mechanisms Underlying Cardiac Lighting
4.3. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoppe, R.T.; Advani, R.H.; Ai, W.Z.; Ambinder, R.F.; Armand, P.; Bello, C.M.; Benitez, C.M.; Bierman, P.J.; Boughan, K.M.; Dabaja, B.; et al. Hodgkin Lymphoma, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 755–781. [Google Scholar] [CrossRef]
- Gallamini, A.; Tarella, C.; Viviani, S.; Rossi, A.; Patti, C.; Mulé, A.; Picardi, M.; Romano, A.; Cantonetti, M.; La Nasa, G.; et al. Early Chemotherapy Intensification With Escalated BEACOPP in Patients With Advanced-Stage Hodgkin Lymphoma With a Positive Interim Positron Emission Tomography/Computed Tomography Scan After Two ABVD Cycles: Long-Term Results of the GITIL/FIL HD 0607 Trial. J. Clin. Oncol. 2018, 36, 454–462. [Google Scholar] [CrossRef]
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Buhl, T.; Jurlander, J.; Buus, S.; Keiding, S.; D’Amore, F.; Boesen, A.M.; et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006, 107, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Gallamini, A.; Hutchings, M.; Rigacci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; Di Raimondo, F.; D’Amore, F.; et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef] [Green Version]
- Borchmann, P.; Goergen, H.; Kobe, C.; Lohri, A.; Greil, R.; Eichenauer, D.A.; Zijlstra, J.M.; Markova, J.; Meissner, J.; Feuring-Buske, M.; et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017, 390, 2790–2802. [Google Scholar] [CrossRef]
- Heckmann, M.B.; Totakhel, B.; Finke, D.; Anker, M.S.; Müller-Tidow, C.; Haberkorn, U.; Katus, H.A.; Lehmann, L.H. Evidence for a cardiac metabolic switch in patients with Hodgkin’s lymphoma. ESC Heart Fail. 2019, 6, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Sarocchi, M.; Bauckneht, M.; Arboscello, E.; Capitanio, S.; Marini, C.; Morbelli, S.; Miglino, M.; Congiu, A.G.; Ghigliotti, G.; Balbi, M.; et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J. Transl. Med. 2018, 16, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauckneht, M.; Ferrarazzo, G.; Fiz, F.; Morbelli, S.; Sarocchi, M.; Pastorino, F.; Ghidella, A.; Pomposelli, E.; Miglino, M.; Ameri, P.; et al. Doxorubicin Effect on Myocardial Metabolism as a Prerequisite for Subsequent Development of Cardiac Toxicity: A Translational 18F-FDG PET/CT Observation. J. Nucl. Med. 2017, 58, 1638–1645. [Google Scholar] [CrossRef] [Green Version]
- Bauckneht, M.; Morbelli, S.; Fiz, F.; Ferrarazzo, G.; Piva, R.; Nieri, A.; Sarocchi, M.; Spallarossa, P.; Canepari, M.E.; Arboscello, E.; et al. A Score-Based Approach to 18F-FDG PET Images as a Tool to Describe Metabolic Predictors of Myocardial Doxorubicin Susceptibility. Diagnostics (Basel) 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Bauckneht, M.; Lai, R.; Miceli, A.; Schenone, D.; Cossu, V.; Donegani, M.I.; Raffa, S.; Borra, A.; Marra, S.; Campi, C.; et al. Spinal cord hypermetabolism extends to skeletal muscle in amyotrophic lateral sclerosis: A computational approach to [18F]-fluorodeoxyglucose PET/CT images. EJNMMI Res. 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Elstrom, R.L.; Tsai, D.E.; Vergilio, J.A.; Downs, L.H.; Alavi, A.; Schuster, S.J. Enhanced marrow [18F]fluorodeoxyglucose uptake related to myeloid hyperplasia in Hodgkin’s lymphoma can simulate lymphoma involvement in marrow. Clin. Lymphoma. 2004, 5, 62–64. [Google Scholar] [CrossRef]
- Lin, E.C. FDG PET/CT flip flop phenomenon in treated lymphoma of bone. Clin. Nucl. Med. 2006, 31, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Kazama, T.; Swanston, N.; Podoloff, D.A.; Macapinlac, H.A. Effect of colony-stimulating factor and conventional- or high-dose chemotherapy on FDG uptake in bone marrow. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Cerci, J.J.; Pracchia, L.F.; Linardi, C.C.; Pitella, F.A.; Delbeke, D.; Izaki, M.; Trindade, E.; Soares, J., Jr.; Buccheri, V.; Meneghetti, J.C. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J. Nucl. Med. 2010, 51, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Visser, L.; Roelofsen, H.; de Vries, M.; Diepstra, A.; van Imhoff, G.; van der Wal, T.; Luinge, M.; Alvarez-Llamas, G.; Vos, H.; et al. Proteomics analysis of Hodgkin lymphoma: Identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood 2008, 111, 2339–2346. [Google Scholar] [CrossRef]
- Gallamini, A. Positron emission tomography scanning: A new paradigm for the management of Hodgkin’s lymphoma. Haematologica 2010, 95, 1046–1048. [Google Scholar] [CrossRef]
- Bauckneht, M.; Pastorino, F.; Castellani, P.; Cossu, V.; Orengo, A.M.; Piccioli, P.; Emionite, L.; Capitanio, S.; Yosifov, N.; Bruno, S.; et al. Increased myocardial 18F-FDG uptake as a marker of Doxorubicin-induced oxidative stress. J. Nucl. Cardiol. 2020, 27, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khiati, S.; Dalla Rosa, I.; Sourbier, C.; Ma, X.; Rao, V.A.; Neckers, L.M.; Zhang, H.; Pommier, Y. Mitochondrial topoisomerase I (top1mt) is a novel limiting factor of doxorubicin cardiotoxicity. Clin. Cancer Res. 2014, 20, 4873–4881. [Google Scholar] [CrossRef] [Green Version]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Bauckneht, M.; Cossu, V.; Miceli, A.; Donegani, M.I.; Capitanio, S.; Morbelli, S.; Marini, C.; Sambuceti, G. FDG-PET Imaging of Doxorubicin-Induced Cardiotoxicity: A New Window on an Old Problem. Curr. Cardiovasc. Imaging Rep. 2019, 12, 41. [Google Scholar] [CrossRef]
- Wallace, K.B. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol. Toxicol. 2003, 93, 105–115. [Google Scholar] [CrossRef]
- Sambuceti, G.; Cossu, V.; Bauckneht, M.; Morbelli, S.; Orengo, A.; Carta, S.; Ravera, S.; Bruno, S.; Marini, C. 18 F-fluoro-2-deoxy-d-glucose (FDG) uptake. What are we looking at? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef] [Green Version]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Biggi, A.; Gallamini, A.; Chauvie, S.; Hutchings, M.; Kostakoglu, L.; Gregianin, M.; Meignan, M.; Malkowski, B.; Hofman, M.S.; Barrington, S.F. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: Interpretation criteria and concordance rate among reviewers. J. Nucl. Med. 2013, 54, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambuceti, G.; Brignone, M.; Marini, C.; Massollo, M.; Fiz, F.; Morbelli, S.; Buschiazzo, A.; Campi, C.; Piva, R.; Massone, A.M.; et al. Estimating the whole bone-marrow asset in humans by a computational approach to integrated PET/CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1326–1338. [Google Scholar] [CrossRef]
- Fiz, F.; Marini, C.; Campi, C.; Massone, A.M.; Podestà, M.; Bottoni, G.; Piva, R.; Bongioanni, F.; Bacigalupo, A.; Piana, M.; et al. Allogeneic cell transplant expands bone marrow distribution by colonizing previously abandoned areas: An FDG PET/CT analysis. Blood 2015, 125, 4095–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Present Study | HD0607 Trial | ||
|---|---|---|---|
| Number | 86 | 782 | |
| Median age, years (range) | 29.5 (19–59) | 31 (14–60) | |
| ≥50 years | 8 (9%) | 10% | |
| Sex | |||
| Male | 41 (48%) | 49% | |
| Female | 45 (52%) | 51% | |
| WHO activity index | |||
| 0–1 | 77 (89%) | 90% | |
| >1 | 9 (11%) | 9% | |
| Ann Arbor Staging | |||
| II | 26 (30%) | 26% | |
| III | 30 (35%) | 32% | |
| IV | 30 (35%) | 32% | |
| International Prognostic Score (IPS) | |||
| 0–1 | 31 (37%) | 37% | |
| 2–3 | 46 (53%) | 51% | |
| >3 | 9 (10%) | 12% | |
| Large Nodal mass | |||
| <5 | 35 (41%) | 42% | |
| 5–7 | 14 (16%) | 18% | |
| 8–10 | 19 (22%) | 20% | |
| >10 | 18 (21%) | 20% | |
| B symptoms | 64 (75%) | 81% | |
| Variable | Strata | Num of Patients | Num of Events | % | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | ||||||
| Total | 86 | 10 | 12% | ||||||||
| Age | |||||||||||
| <median | 43 | 4 | 9% | 1 (ref) | |||||||
| >median | 43 | 6 | 14% | 1.32 | 0.37–4.70 | 0.67 | |||||
| Ann Arbor Staging | |||||||||||
| 2 | 28 | 3 | 11% | 1 (ref) | |||||||
| 3 | 28 | 1 | 4% | 0.38 | 0.04–3.71 | 0.41 | |||||
| 4 | 30 | 6 | 20% | 1.99 | 0.49–7.96 | 0.33 | |||||
| IPS Score | |||||||||||
| 1 | 26 | 3 | 12% | 1 (ref) | |||||||
| 2 | 27 | 2 | 7% | 2.74 | 0.53–14.22 | 0.23 | |||||
| 3 | 24 | 4 | 17% | 2.01 | 0.33–12.08 | 0.44 | |||||
| 4 | 9 | 1 | 11% | - | - | 0.98 | |||||
| MTV at baseline PET | |||||||||||
| <median | 43 | 3 | 7% | 1 (ref) | |||||||
| >median | 43 | 7 | 16% | 2.64 | 0.69–10.24 | 0.15 | |||||
| Deauville Score at Interim | |||||||||||
| 1–3 | 67 | 3 | 4% | 1 (ref) | 1 (ref) | ||||||
| 4–5 | 19 | 7 | 37% | 8.9 | 2.29–34.83 | 0.002 | 11 | 2.78–42.56 | 0.001 | ||
| Heart SUV at Interim | |||||||||||
| <median | 43 | 9 | 21% | 1 (ref) | 1 (ref) | ||||||
| >median | 43 | 1 | 2% | 0.1 | 0.01–0.80 | 0.03 | 0.08 | 0.01–0.63 | 0.017 | ||
| Skeletal muscle SUV at Interim | |||||||||||
| <median | 43 | 4 | 9% | 1 (ref) | |||||||
| >median | 43 | 6 | 14% | 1.72 | 0.48–6.1 | 0.41 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, C.; Bauckneht, M.; Borra, A.; Lai, R.; Donegani, M.I.; Miceli, A.; Campi, C.; Cossu, V.; Schenone, D.; Morbelli, S.; et al. Myocardial Metabolic Response Predicts Chemotherapy Curative Potential on Hodgkin Lymphoma: A Proof-of-Concept Study. Biomedicines 2021, 9, 971. https://doi.org/10.3390/biomedicines9080971
Marini C, Bauckneht M, Borra A, Lai R, Donegani MI, Miceli A, Campi C, Cossu V, Schenone D, Morbelli S, et al. Myocardial Metabolic Response Predicts Chemotherapy Curative Potential on Hodgkin Lymphoma: A Proof-of-Concept Study. Biomedicines. 2021; 9(8):971. https://doi.org/10.3390/biomedicines9080971
Chicago/Turabian StyleMarini, Cecilia, Matteo Bauckneht, Anna Borra, Rita Lai, Maria Isabella Donegani, Alberto Miceli, Cristina Campi, Vanessa Cossu, Daniela Schenone, Silvia Morbelli, and et al. 2021. "Myocardial Metabolic Response Predicts Chemotherapy Curative Potential on Hodgkin Lymphoma: A Proof-of-Concept Study" Biomedicines 9, no. 8: 971. https://doi.org/10.3390/biomedicines9080971
APA StyleMarini, C., Bauckneht, M., Borra, A., Lai, R., Donegani, M. I., Miceli, A., Campi, C., Cossu, V., Schenone, D., Morbelli, S., Chauvie, S., Piana, M., Gallamini, A., & Sambuceti, G. (2021). Myocardial Metabolic Response Predicts Chemotherapy Curative Potential on Hodgkin Lymphoma: A Proof-of-Concept Study. Biomedicines, 9(8), 971. https://doi.org/10.3390/biomedicines9080971











