Hypomethylating Agents (HMAs) as Salvage Therapy in Relapsed or Refractory AML: An Italian Multicentric Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
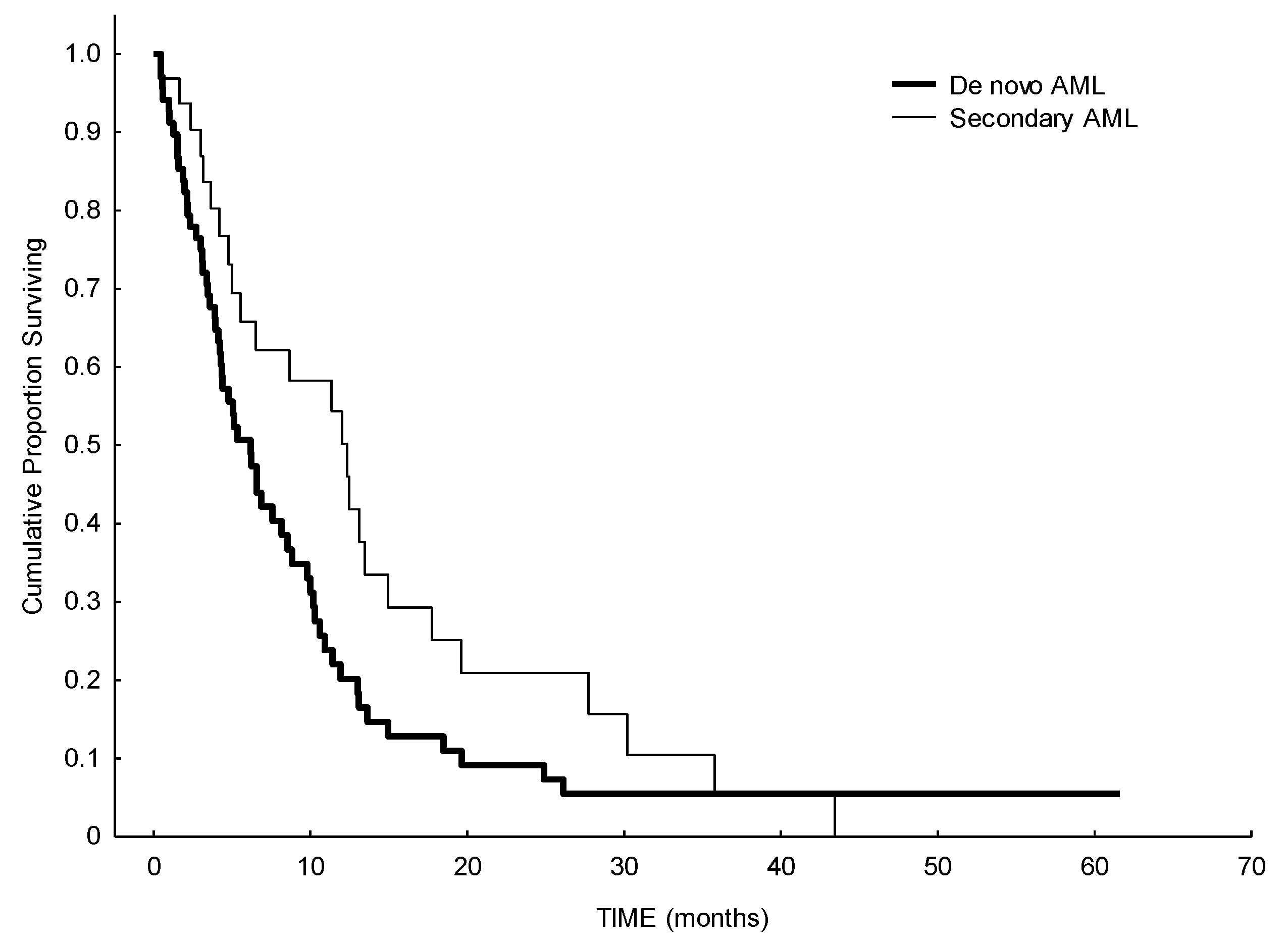
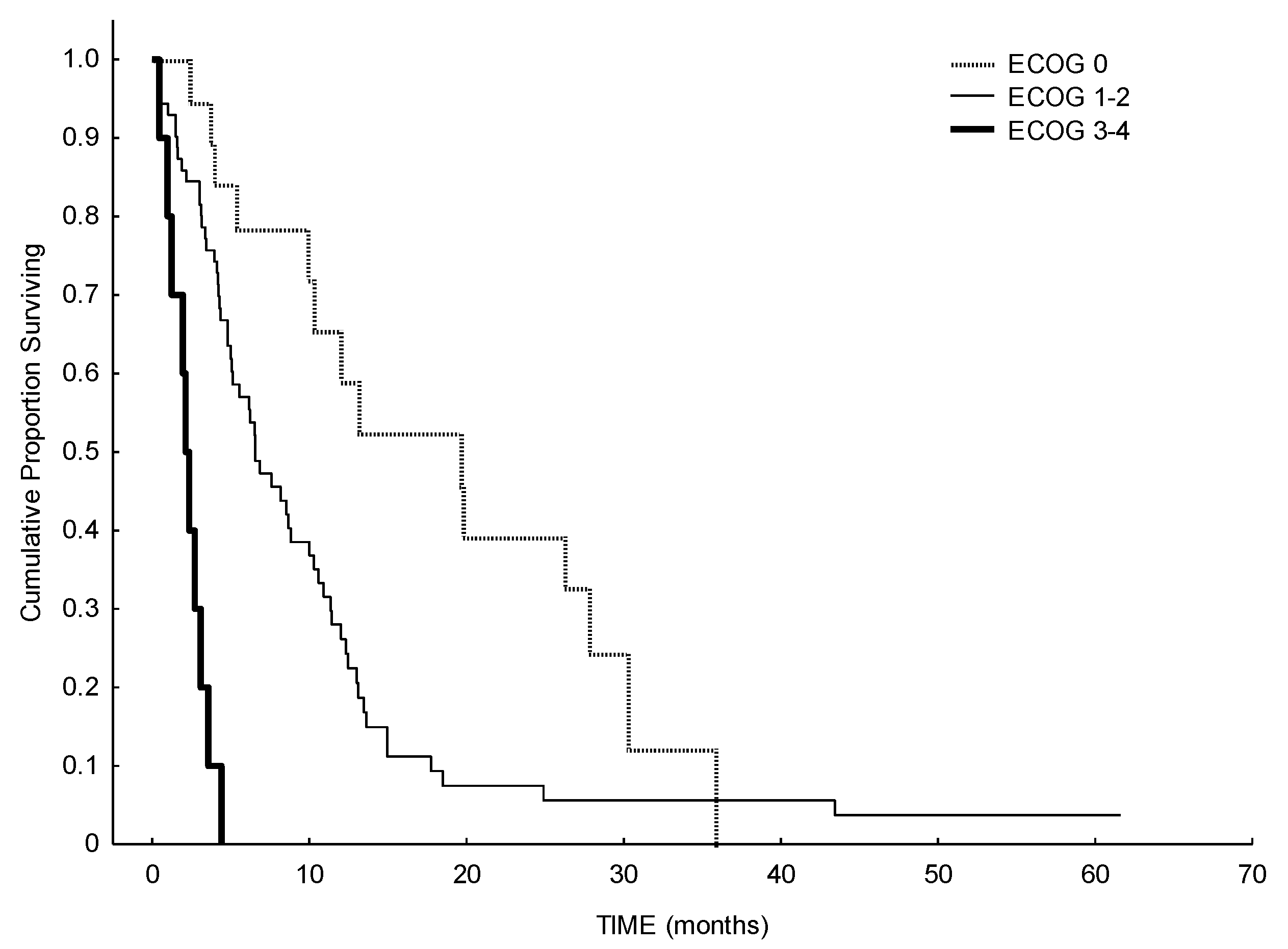
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ganzel, C.; Sun, Z.; Cripe, L.D.; Fernandez, H.F.; Douer, D.; Rowe, J.M.; Paietta, E.M.; Ketterling, R.; O’Connell, M.J.; Wiernik, P.H.; et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am. J. Hematol. 2018, 93, 1074–1081. [Google Scholar] [CrossRef] [Green Version]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, F.; Lessi, F.; Vitagliano, O.; Birkenghi, E.; Rossi, G. Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, B.C. Is there a standard of care for relapsed AML? Best Pract. Res. Clin. Haematol. 2018, 31, 384–386. [Google Scholar] [CrossRef]
- Thépot, S.; Itzykson, R.; Seegers, V.; Recher, C.; Raffoux, E.; Quesnel, B.; Delaunay, J.; Cluzeau, T.; Koka, A.M.; Stamatoullas, A.; et al. Azacitidine in untreated acute myeloid leukemia: A report on 149 patients. Am. J. Hematol. 2014, 89, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Maurillo, L.; Venditti, A.; Spagnoli, A.; Gaidano, G.; Ferrero, D.; Oliva, E.; Lunghi, M.; D’Arco, A.M.; Levis, A.; Pastore, D.; et al. Azacitidine for the treatment of patients with acute myeloid leukemia: Report of 82 patients enrolled in an Italian Compassionate Program. Cancer 2012, 118, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.P.; Chou, W.C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [Green Version]
- Filì, C.; Candoni, A.; Zannier, M.E.; Olivieri, J.; Imbergamo, S.; Caizzi, M.; Nadali, G.; di Bona, E.; Ermacora, A.; Gottardi, M.; et al. Efficacy and toxicity of Decitabine in patients with acute myeloid leukemia (AML): A multicenter real-world experience. Leuk. Res. 2019, 76, 33–38. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Itzykson, R.; Thépot, S.; Berthon, C.; Delaunay, J.; Bouscary, D.; Cluzeau, T.; Turlure, P.; Prébet, T.; Dartigeas, C.; Marolleau, J.P.; et al. Azacitidine for the treatment of relapsed and refractory AML in older patients. Leuk. Res. 2015, 39, 124–130. [Google Scholar] [CrossRef]
- Craddock, C.; Labopin, M.; Robin, M.; Finke, J.; Chevallier, P.; Yakoub-Agha, I.; Bourhis, J.H.; Sengelov, H.; Blaise, D.; Luft, T.; et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica 2016, 101, 879–883. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, E.K.; Feldman, E.J.; Christos, P.J.; Rohan, S.D.; Lagassa, C.B.; Ippoliti, C.; Scandura, J.M.; Carlson, K.; Roboz, G.J. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk. Lymphoma 2013, 54, 2003–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, M.; DeVeaux, M.; Montesinos, P.; Itzykson, R.; Ritchie, E.K.; Sekeres, M.A.; Barnard, J.D.; Podoltsev, N.A.; Brunner, A.M.; Komrokji, R.S.; et al. Hypomethylating agents in relapsed and refractory AML: Outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, F. Is complete remission key in elderly patients with AML? Lancet Haematol. 2016, 3, e212–e213. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Schuh, A.C. Response Kinetics and Clinical Benefits of Nonintensive AML Therapies in the Absence of Morphologic Response. Clin Lymphoma Myeloma Leuk. 2020, 20, e66–e75. [Google Scholar] [CrossRef] [Green Version]
- Ivanoff, S.; Gruson, B.; Chantepie, S.P.; Lemasle, E.; Merlusca, L.; Harrivel, V.; Charbonnier, A.; Votte, P.; Royer, B.; Marolleau, J.P. 5-Azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am. J. Hematol. 2013, 88, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Podoltsev, N.A.; Stahl, M.; Zeidan, A.M.; Gore, S.D. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev 2017, 31, 43–62. [Google Scholar] [CrossRef]
- Palmieri, R.; Paterno, G.; de Bellis, E.; Mercante, L.; Buzzatti, E.; Esposito, F.; del Principe, M.I.; Maurillo, L.; Buccisano, F.; Venditti, A. Therapeutic Choice in Older Patients with Acute Myeloid Leukemia: A Matter of Fitness. Cancers 2020, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Klepin, H.D.; Estey, E.; Kadia, T. More Versus Less Therapy for Older Adults with Acute Myeloid Leukemia: New Perspectives on an Old Debate. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 421–432. [Google Scholar] [CrossRef]
- Goodyear, O.C.; Dennis, M.; Jilani, N.Y.; Loke, J.; Siddique, S.; Ryan, G.; Nunnick, J.; Khanum, R.; Raghavan, M.; Cook, M.; et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012, 119, 3361–3369. [Google Scholar] [CrossRef]
- Schroeder, T.; Rautenberg, C.; Haas, R.; Germing, U.; Kobbe, G. Hypomethylating agents for treatment and prevention of relapse after allogeneic blood stem cell transplantation. Int. J. Hematol. 2018, 107, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaut, D.; Burkenroad, A.; Duong, T.; Feammelli, J.; Sasine, J.; Schiller, G. Venetoclax combination therapy in relapsed/refractory acute myeloid leukemia: A single institution experience. Leuk. Res. 2020, 90, 106314. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | N (%) | |
|---|---|---|
| Sex | M F | 68 (68%) 32 (32%) |
| WHO 2016 | AML with recurrent genetic abnormalities AML/MRC Therapy Related AML AML, NOS not available | 5 (5%) 27 (27%) 2 (2%) 61 (61%) 5 (5%) |
| ELN2017 Risk score | Favorable Intermediate Adverse not available | 10 (10%) 50 (0%) 33 (33%) 7 (7%) |
| Genetic mutations | FLT3 NPM1 CEBPA | 7 (7%) 10 (10%) 2 2(2%) |
| Type of AML | Secondary AML De novo AML Relapsed AML Refractory AML not available | 32 (32%) 68 (68%) |
| 54 (54%) 44 (44%) 2 (2%) | ||
| N° of previous lines of therapy before HMA | 1 2 ≥3 | 60 (60%) 29 (29%) 11 (11%) |
| HMA | Azacytidine Decitabine | 80 (80%) 20 (20%) |
| AlloSCT before HMA | YES NO | 20 (20%) 80 (80%) |
| Response to HMA | Response (CR, PR or CRi) Stable disease (SD) No response | 24 (24%) 26 (26%) 50 (50%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lessi, F.; Laurino, M.; Papayannidis, C.; Vitagliano, O.; Grimaldi, F.; Lazzarotto, D.; Gottardi, M.; Crisà, E.; Riva, M.; Reda, G.; et al. Hypomethylating Agents (HMAs) as Salvage Therapy in Relapsed or Refractory AML: An Italian Multicentric Retrospective Study. Biomedicines 2021, 9, 972. https://doi.org/10.3390/biomedicines9080972
Lessi F, Laurino M, Papayannidis C, Vitagliano O, Grimaldi F, Lazzarotto D, Gottardi M, Crisà E, Riva M, Reda G, et al. Hypomethylating Agents (HMAs) as Salvage Therapy in Relapsed or Refractory AML: An Italian Multicentric Retrospective Study. Biomedicines. 2021; 9(8):972. https://doi.org/10.3390/biomedicines9080972
Chicago/Turabian StyleLessi, Federica, Marica Laurino, Cristina Papayannidis, Orsola Vitagliano, Francesco Grimaldi, Davide Lazzarotto, Michele Gottardi, Elena Crisà, Marta Riva, Gianluigi Reda, and et al. 2021. "Hypomethylating Agents (HMAs) as Salvage Therapy in Relapsed or Refractory AML: An Italian Multicentric Retrospective Study" Biomedicines 9, no. 8: 972. https://doi.org/10.3390/biomedicines9080972
APA StyleLessi, F., Laurino, M., Papayannidis, C., Vitagliano, O., Grimaldi, F., Lazzarotto, D., Gottardi, M., Crisà, E., Riva, M., Reda, G., Ermani, M., Semenzato, G., Trentin, L., & Ferrara, F. (2021). Hypomethylating Agents (HMAs) as Salvage Therapy in Relapsed or Refractory AML: An Italian Multicentric Retrospective Study. Biomedicines, 9(8), 972. https://doi.org/10.3390/biomedicines9080972










