TGF-β Increases MFGE8 Production in Myeloid-Derived Suppressor Cells to Promote B16F10 Melanoma Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Cell Culture
2.2. FACS and Antibodies
2.3. Retroviral Overexpression
2.4. RNA Extraction and Quantitative RT-PCR
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Water-Soluble Tetrazolium Salts (WST) Assay
2.7. Migration and Invasion Assays
2.8. Microarray and Data Analysis
2.9. Statistical Analysis
3. Results
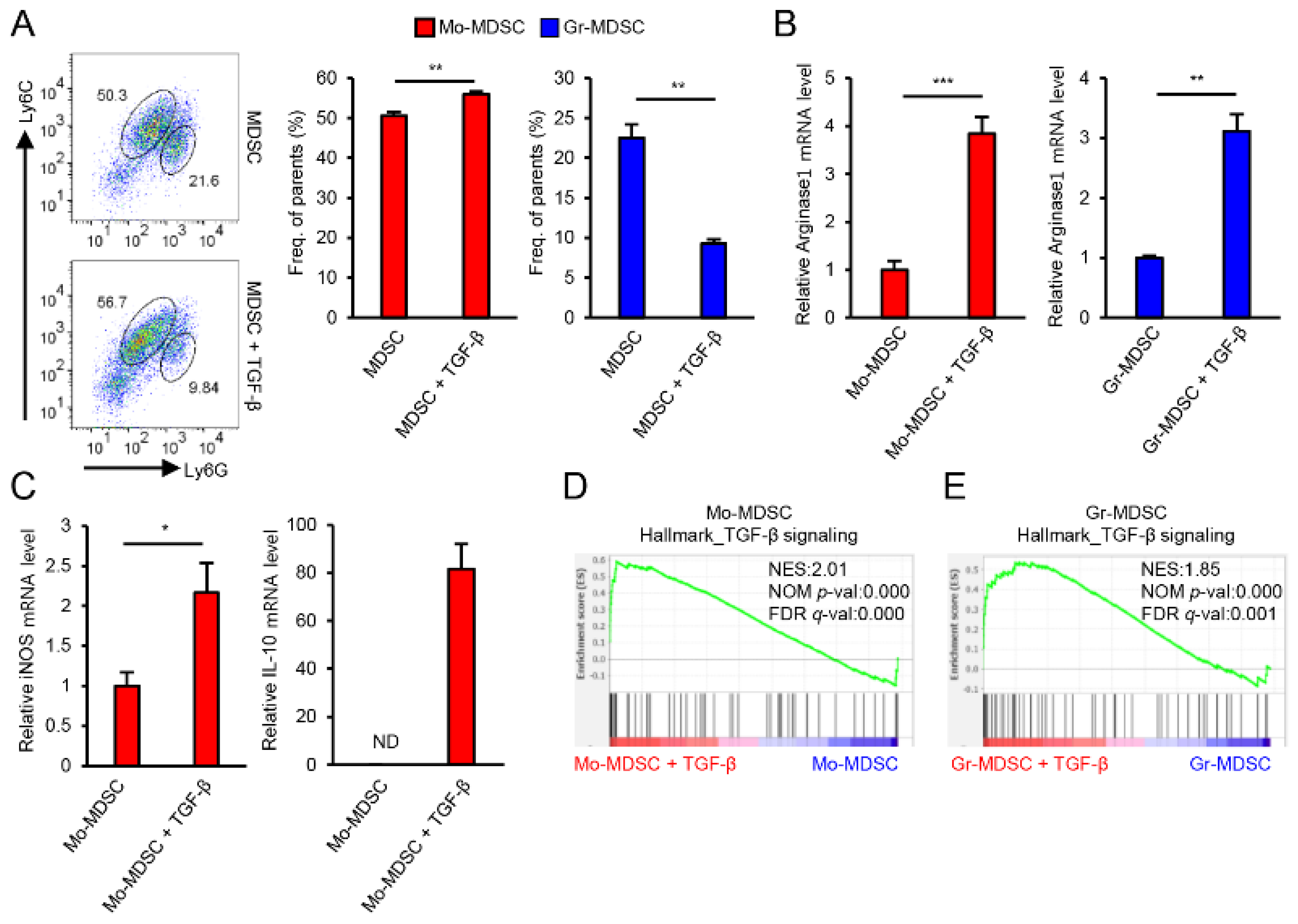
3.1. TGF-β Is an Important Regulator of MDSCs Immunosuppressive Function
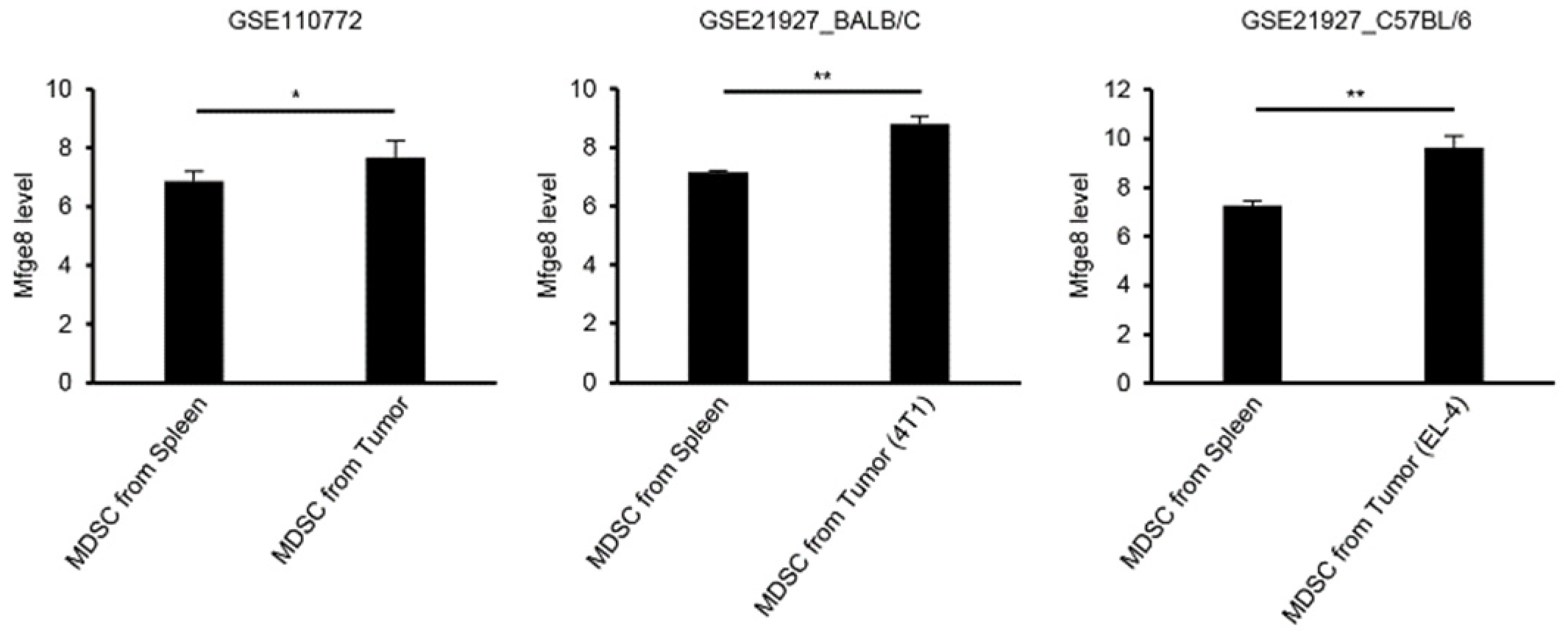
3.2. TGF-β Induces Gene Expression Changes including Pro-Metastatic Genes Such as Mfge8
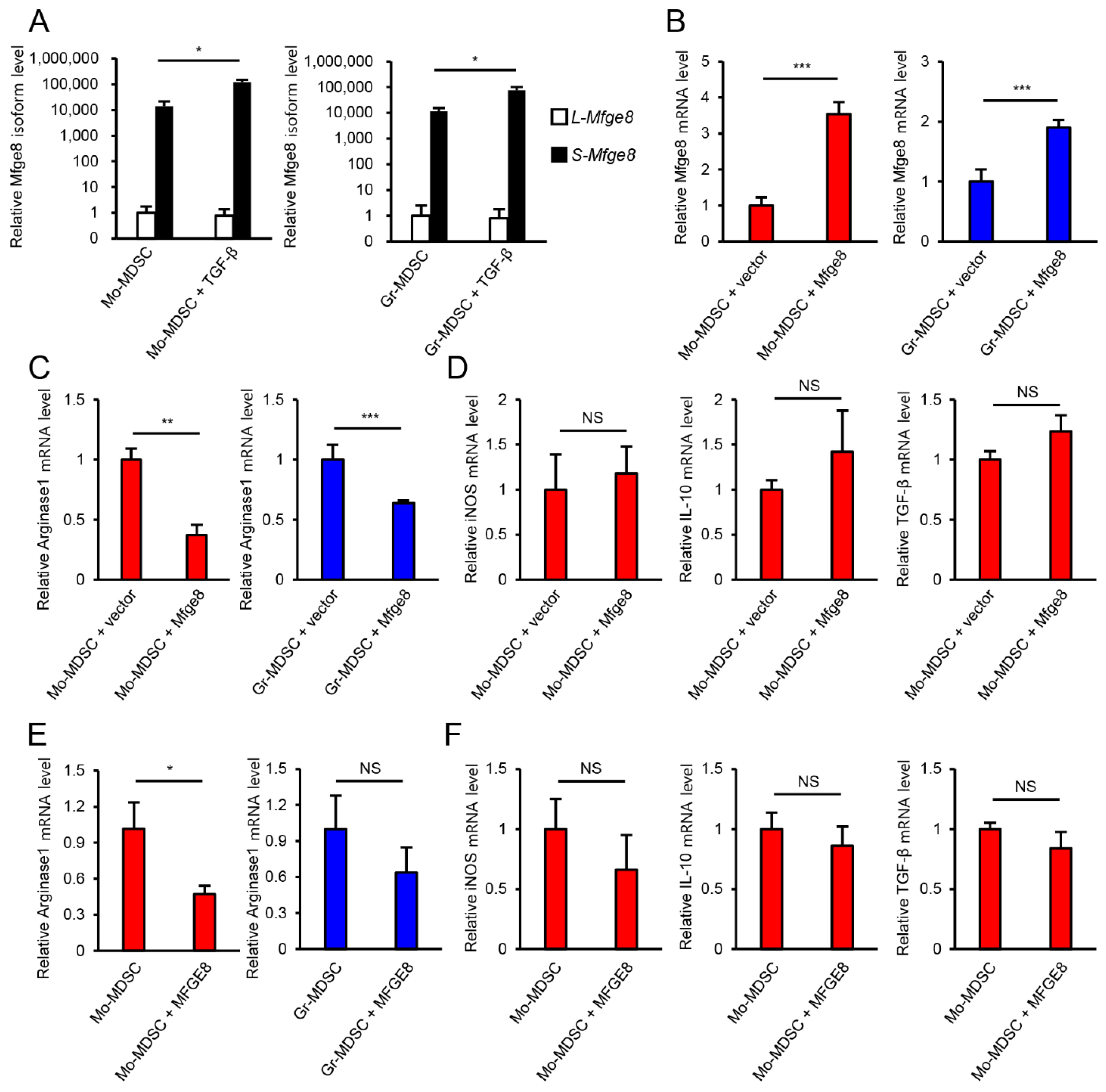
3.3. MFGE8 Does Not Promote the Immunosuppressive Function of MDSCs
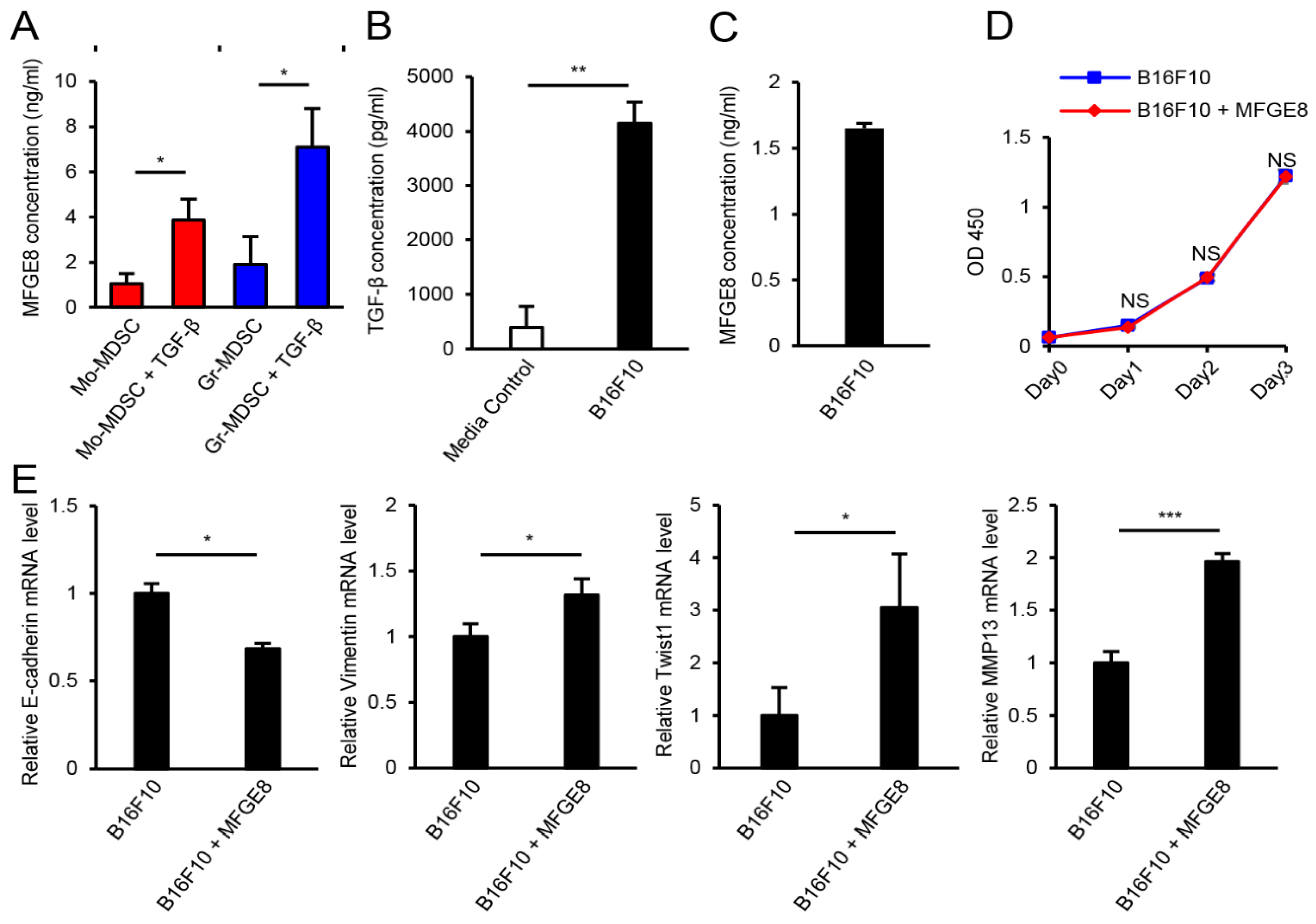
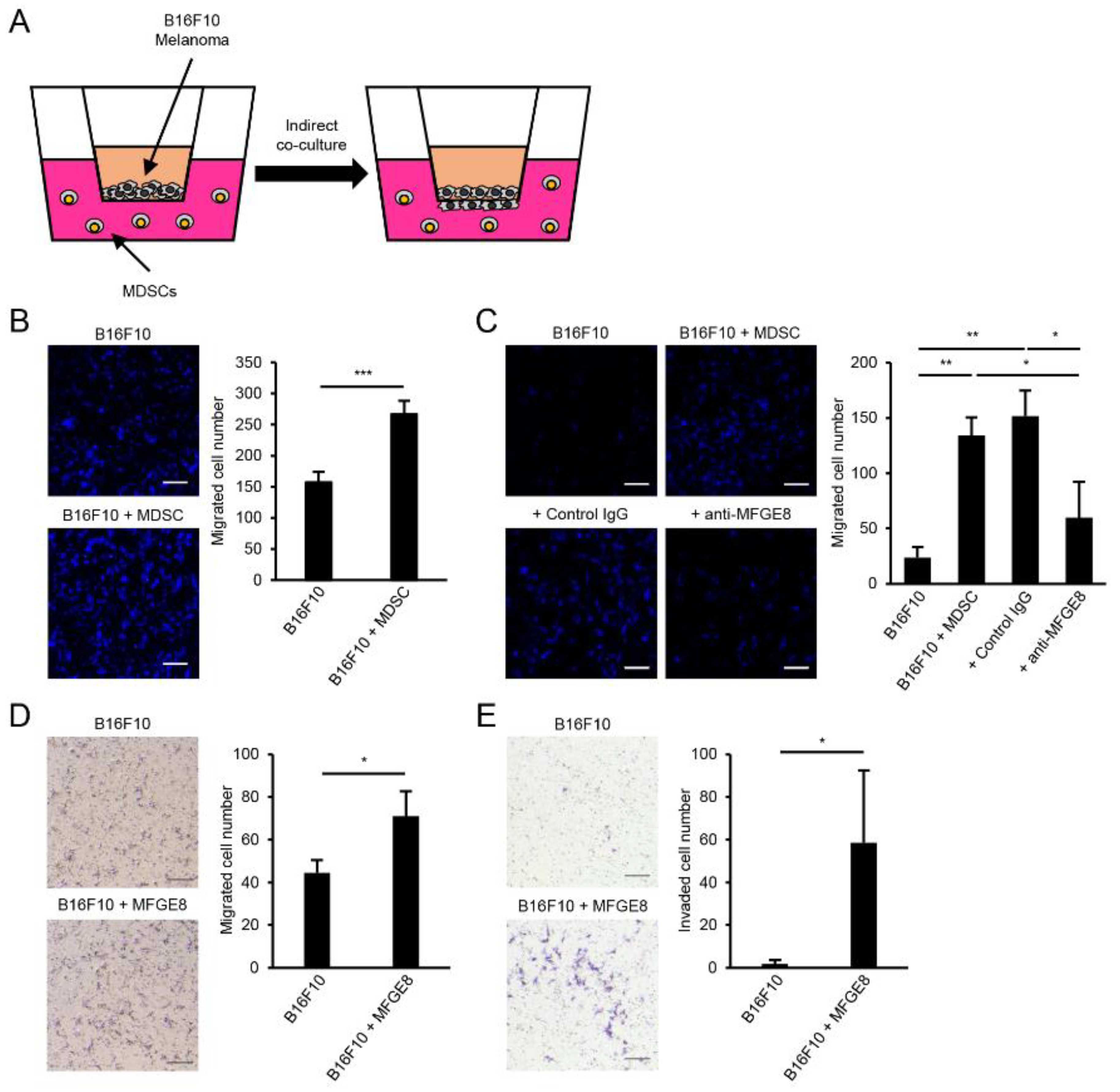
3.4. MFGE8 Induces Metastasis of B16F10 Melanoma
3.5. MDSCs Increase B16F10 Migration in an MFGE8-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene Name | Distance |
|---|---|
| Nt5e | 5.13 |
| Elovl3 | 4.97 |
| Mfge8 | 7.78 |
| Tshr | 6.51 |
| Gbp5 | 5.68 |
| Havcr2 | 3.46 |

References
- Bennett, J.A.; Rao, V.S.; Mitchell, M.S. Systemic Bacillus Calmette-Guerin (BCG) activates natural suppressor cells. Proc. Natl. Acad. Sci. USA 1978, 75, 5142–5144. [Google Scholar] [CrossRef]
- Dysthe, M.; Parihar, R. Myeloid-derived suppressor cells in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 117–140. [Google Scholar]
- Bosiljcic, M.; Cederberg, R.A.; Hamilton, M.J.; LePard, N.E.; Harbourne, B.T.; Collier, J.L.; Halvorsen, E.C.; Shi, R.; Franks, S.E.; Kim, A.Y.; et al. Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. 2019, 21, 103. [Google Scholar] [CrossRef]
- Halaby, M.J.; Hezaveh, K.; Lamorte, S.; Ciudad, M.T.; Kloetgen, A.; MacLeod, B.L.; Guo, M.; Chakravarthy, A.; Medina, T.D.S.; Ugel, S.; et al. GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci. Immunol. 2019, 4, 8189. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): Impact on cancer and aging. J. Mol. Med. 2019, 97, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Kim, H.E.; Park, S.G. Insights into myeloid-derived suppressor cells in inflammatory diseases. Arch. Immunol. Ther. Exp. 2015, 63, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.-H.; Ye, M.B.; Lee, W.; Sim, K.-Y.; Kang, J.-A.; Kim, Y.-C.; et al. Myeloid-derived suppressor cells are controlled by regulatory T cells via TGF-β during murine colitis. Cell Rep. 2016, 17, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Marvel, D.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015, 125, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- Tesi, R.J. MDSC—The most important cell you have never heard of. Trends Pharmacol. Sci. 2019, 40, 4–7. [Google Scholar] [CrossRef]
- Tcyganov, E.; Mastio, J.; Chen, E.; Gabrilovich, D.I. Plasticity of myeloid-derived suppressor cells in cancer. Curr. Opin. Immunol. 2018, 51, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Watanabe, M. Myeloid-derived suppressor cells and therapeutic strategies in cancer. Mediat. Inflamm. 2015, 2015, 159269. [Google Scholar] [CrossRef] [PubMed]
- Trillo-Tinoco, J.; Sierra, R.A.; Mohamed, E.; Cao, Y.; Pulido, A.D.M.; Gilvary, D.L.; Anadon, C.M.; Costich, T.L.; Wei, S.; Flores, E.R.; et al. AMPK alpha-1 intrinsically regulates the function and differentiation of tumor myeloid-derived suppressor cells. Cancer Res. 2019, 79, 5034–5047. [Google Scholar] [CrossRef]
- Capietto, A.H.; Kim, S.; Sanford, D.E.; Linehan, D.C.; Hikida, M.; Kumosaki, T.; Novack, D.V.; Faccio, R. Down-regulation of PLCγ2-β-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. J. Exp. Med. 2013, 210, 2257–2271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Arvelo, F.; Sojo, F.; Cotte, C. Tumour progression and metastasis. Ecancermedicalscience 2016, 10, 617. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key criminals of tumor pre-metastatic niche formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Kujawski, M.; Kortylewski, M.; Lee, H.; Herrmann, A.; Kay, H.; Yu, H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Investig. 2008, 118, 3367–3377. [Google Scholar] [CrossRef]
- Tartour, E.; Pere, H.; Maillere, B.; Terme, M.; Merillon, N.; Taieb, J.; Sandoval, F.; Quintin-Colonna, F.; Lacerda, K.; Karadimou, A.; et al. Angiogenesis and immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011, 30, 83–95. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Syed, V. TGF-β signaling in cancer. J. Cell Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Ling, L.; van Dam, H.; Zhou, F.; Zhang, L. TGF-β signaling in cancer metastasis. Acta Biochim. Biophys. Sin. 2018, 50, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, L.; Leyman, S.; Nicolas, A.; Notebaert, S.; Dewulf, M.; Ngo, T.H.; Zuany-Amorim, C.; Amzallag, N.; Bernard-Pierrot, I.; Sastre-Garau, X.; et al. New blocking antibodies impede adhesion, migration and survival of ovarian cancer cells, highlighting MFGE8 as a potential therapeutic target of human ovarian carcinoma. PLoS ONE 2013, 8, e72708. [Google Scholar] [CrossRef]
- Yamada, K.; Uchiyama, A.; Uehara, A.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S. MFG-E8 drives melanoma growth by stimulating mesenchymal stromal cell-induced angiogenesis and M2 polarization of tumor-associated macrophages. Cancer Res. 2016, 76, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Nakazaki, Y.; Carrasco, D.R.; Draganov, D.; Souders, N.; Johnson, M.; Mihm, M.C.; Dranoff, G. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008, 68, 8889–8898. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, L.; Sun, X.; Zhang, K.; Shen, H.; Tian, Y.; Sun, F.; Li, Y. MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumour Biol. 2017, 39, 1010428317707881. [Google Scholar] [CrossRef]
- Michalski, M.N.; Seydel, A.L.; Siismets, E.M.; Zweifler, L.E.; Koh, A.J.; Sinder, B.P.; Aguirre, J.I.; Atabai, K.; Roca, H.; McCauley, L.K. Inflammatory bone loss associated with MFG-E8 deficiency is rescued by teriparatide. FASEB J. 2018, 32, 3730–3741. [Google Scholar] [CrossRef]
- Aziz, M.M.; Ishihara, S.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kadowaki, Y.; Rumi, M.A.; Amano, Y.; Kinoshita, Y. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent αvβ3 integrin signaling. J. Immunol. 2009, 182, 7222–7232. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Yagita, H.; Yoshiyama, H.; Tahara, H. Putting the brakes on anticancer therapies: Suppression of innate immune pathways by tumor-associated myeloid cells. Trends Mol. Med. 2013, 19, 536–545. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Bierie, B.; Moses, H.L. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010, 21, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, R.; Ishihara, S.; Tada, Y.; Oka, A.; Sonoyama, H.; Fukuba, N.; Oshima, N.; Moriyama, I.; Yuki, T.; Kawashima, K.; et al. Role of milk fat globule-epidermal growth factor 8 in colonic inflammation and carcinogenesis. J. Gastroenterol. 2015, 50, 862–875. [Google Scholar] [CrossRef]
- Zhou, P.; Zhi, X.; Zhou, T.; Chen, S.; Li, X.; Wang, L.; Yin, L.; Shao, Z.; Ou, Z. Overexpression of Ecto-5′-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol. Ther. 2007, 6, 426–431. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Zhou, X.; Zhou, T.; Ma, D.; Chen, S.; Zhi, X.; Yin, L.; Shao, Z.; Ou, Z.; Zhou, P. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J. Cancer Res. Clin. Oncol. 2007, 134, 365–372. [Google Scholar] [CrossRef]
- Westerberg, R.; Tvrdik, P.; Undén, A.-B.; Månsson, J.-E.; Norlén, L.; Jakobsson, A.; Holleran, W.H.; Elias, P.M.; Asadi, A.; Flodby, P.; et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J. Biol. Chem. 2004, 279, 5621–5629. [Google Scholar] [CrossRef]
- Otani, A.; Ishihara, S.; Aziz, M.M.; Oshima, N.; Mishima, Y.; Moriyama, I.; Yuki, T.; Amano, Y.; Ansary, M.M.; Kinoshita, Y. Intrarectal administration of milk fat globule epidermal growth factor-8 protein ameliorates murine experimental colitis. Int. J. Mol. Med. 2012, 29, 349–356. [Google Scholar]
- Mishiro, T.; Kusunoki, R.; Otani, A.; Ansary, M.U.; Tongu, M.; Harashima, N.; Yamada, T.; Sato, S.; Amano, Y.; Itoh, K.; et al. Butyric acid attenuates intestinal inflammation in murine DSS-induced colitis model via milk fat globule-EGF factor 8. Lab. Investig. 2013, 93, 834–843. [Google Scholar] [CrossRef]
- Godoy, P.; Cadenas, C.; Hellwig, B.; Marchan, R.; Stewart, J.D.; Reif, R.; Lohr, M.; Gehrmann, M.; Rahnenführer, J.; Schmidt, M.; et al. Interferon-inducible guanylate binding protein (GBP2) is associated with better prognosis in breast cancer and indicates an efficient T cell response. Breast Cancer 2012, 21, 491–499. [Google Scholar] [CrossRef]
- Yu, S.; Yu, X.; Sun, L.; Zheng, Y.; Chen, L.; Xu, H.; Jin, J.; Lan, Q.; Chen, C.C.; Li, M. GBP2 enhances glioblastoma invasion through Stat3/fibronectin pathway. Oncogene 2020, 39, 5042–5055. [Google Scholar] [CrossRef]
- Cao, L.; Ji, Y.; Zeng, L.; Liu, Q.; Zhang, Z.; Guo, S.; Guo, X.; Tong, Y.; Zhao, X.; Li, C.-M.; et al. P200 family protein IFI204 negatively regulates type I interferon responses by targeting IRF7 in nucleus. PLoS Pathog. 2019, 15, e1008079. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Sun, Z.; Zhang, X.; Lu, L.; Wang, Y.; Zhang, M. S100A9 and ORM1 serve as predictors of therapeutic response and prognostic factors in advanced extranodal NK/T cell lymphoma patients treated with pegaspargase/gemcitabine. Sci. Rep. 2016, 6, 23695. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Men, Q.; Su, X.; Chen, W.; Zou, L.; Li, Q.; Song, M.; Ouyang, D.; Chen, Y.; Li, Z.; et al. Downregulated expression of TSHR is associated with distant metastasis in thyroid cancer. Oncol. Lett. 2017, 14, 7506–7512. [Google Scholar] [CrossRef] [PubMed]
- Tyrkalska, S.; Candel, S.; Angosto, D.; Gómez-Abellán, V.; Martín-Sánchez, F.; García-Moreno, D.; Zapata-Pérez, R.; Sánchez-Ferrer, A.; Sepulcre, M.P.; Pelegrin, P.; et al. Neutrophils mediate Salmonella typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nat. Commun. 2016, 7, 12077. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.R.; Wellington, D.A.; Kumar, P.; Kassa, H.; Booth, C.J.; Cresswell, P.; MacMicking, J.D. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 2012, 336, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cao, Z.; Wang, L.; Wan, Y.; Peng, N.; Wang, Q.; Chen, X.; Zhou, Y.; Zhu, Y. Inducible GBP5 mediates the antiviral response via interferon-related pathways during influenza A virus infection. J. Innate Immun. 2017, 9, 419–435. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, Y.; Yin, K.; Ma, J.; Tian, J.; Zhang, Y.; Mao, L.; Xu, H.; Wang, S. LncRNA AK036396 inhibits maturation and accelerates immunosuppression of polymorphonuclear myeloid-derived suppressor cells by enhancing the stability of ficolin B. Cancer Immunol. Res. 2020, 8, 565–577. [Google Scholar] [CrossRef]
- Sharad, S.; Sztupinszki, Z.; Chen, Y.; Kuo, C.; Ravindranath, L.; Szallasi, Z.; Petrovics, G.; Sreenath, T.L.; Dobi, A.; Rosner, I.L.; et al. Analysis of PMEPA1 isoforms (a and b) as selective inhibitors of androgen and TGF-β signaling reveals distinct biological and prognostic features in prostate cancer. Cancers 2019, 11, 1995. [Google Scholar] [CrossRef]
- Hafler, D.A.; Kuchroo, V. TIMs: Central regulators of immune responses. J. Exp. Med. 2008, 205, 2699–2701. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5+ myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. 2017, 78, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Al Khamees, B.; Jia, D.; Li, L.; Couture, J.F.; Figeys, D.; Jinushi, M.; Wang, L. MFG-E8 is critical for embryonic stem cell-mediated t cell immunomodulation. Stem Cell Rep. 2015, 5, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cerdeira, C.; Carnero Gregorio, M.; Lopez-Barcenas, A.; Sanchez-Blanco, E.; Sanchez-Blanco, B.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Guzman, R.A. Advances in immunotherapy for melanoma: A comprehensive review. Mediat. Inflamm. 2017, 2017, 3264217. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Ware, O.; Bosenberg, M. Genetics of metastasis: Melanoma and other cancers. Clin. Exp. Metastasis 2018, 35, 379–391. [Google Scholar] [CrossRef]

| Group | Gene Name | Function |
|---|---|---|
| Up | Nt5e | Promote tumor migration [37,38] |
| Elovl3 | Fatty acid chain elongation [39] | |
| Mfge8 | Anti-inflammatory [40,41], Tumor metastasis [27,30] | |
| Down | Gbp2 * | Better prognosis in breast cancer [42], Glioblastoma invasion [43] |
| Ifi204 * | Negatively regulate type 1 interferon response [44] | |
| Gm12250 * | Unknown | |
| Gr-Up | Orm1* | Therapeutic response predictor in NK/T lymphoma [45] |
| Tshr | Inhibit metastasis of thyroid cancer [46] | |
| Awat1 * | Unknown | |
| Gr-Down | Gbp4 * | Inflammasome activation [47] |
| Gbp5 | Inflammasome assembly [48], Stimulation of NF-κB signaling pathway [49] | |
| Prss34 * | Unknown | |
| Mo-Up | Fcnb * | Maintain immunosuppressive function of Gr-MDSCs [50] |
| Pmepa1 * | Prostate cancer metastasis regulator [51] | |
| Havcr2 | T cell exhaustion and tolerance [52] | |
| Mo-Down | Gm4951 * | Unknown |
| Gbp8 * | Unknown | |
| Iigp1 * | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Yang, T.; Lee, W.; Park, S.-G. TGF-β Increases MFGE8 Production in Myeloid-Derived Suppressor Cells to Promote B16F10 Melanoma Metastasis. Biomedicines 2021, 9, 896. https://doi.org/10.3390/biomedicines9080896
Lim H, Yang T, Lee W, Park S-G. TGF-β Increases MFGE8 Production in Myeloid-Derived Suppressor Cells to Promote B16F10 Melanoma Metastasis. Biomedicines. 2021; 9(8):896. https://doi.org/10.3390/biomedicines9080896
Chicago/Turabian StyleLim, Heejin, Taewoo Yang, Wongeun Lee, and Sung-Gyoo Park. 2021. "TGF-β Increases MFGE8 Production in Myeloid-Derived Suppressor Cells to Promote B16F10 Melanoma Metastasis" Biomedicines 9, no. 8: 896. https://doi.org/10.3390/biomedicines9080896
APA StyleLim, H., Yang, T., Lee, W., & Park, S.-G. (2021). TGF-β Increases MFGE8 Production in Myeloid-Derived Suppressor Cells to Promote B16F10 Melanoma Metastasis. Biomedicines, 9(8), 896. https://doi.org/10.3390/biomedicines9080896







