Colorectal Cancer, Liver Metastases and Biotherapies
Abstract
1. Introduction
2. Biology of the Metastatic Colorectal Cancer
3. Current Management of Liver Metastases from Colorectal Cancers
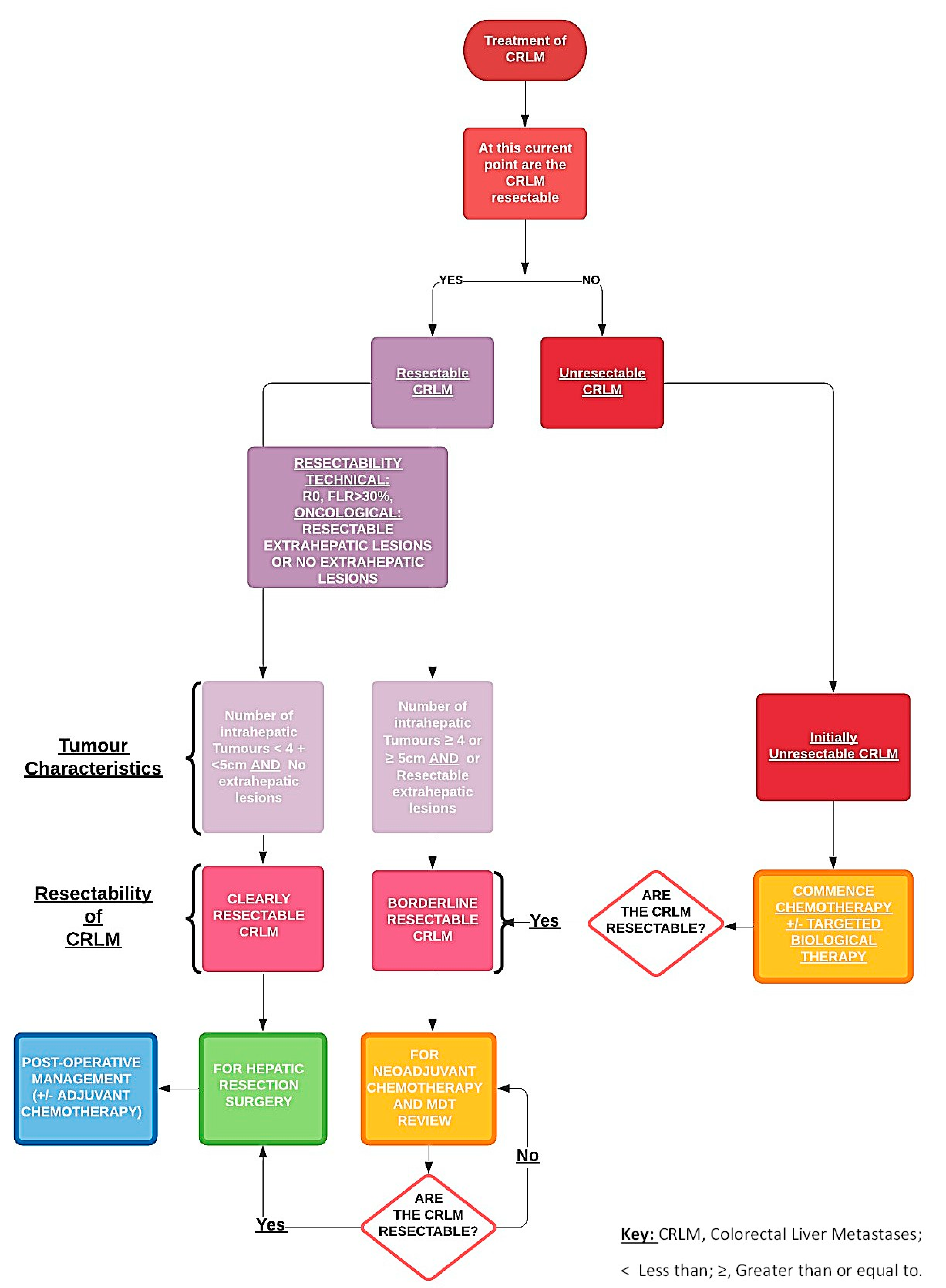
3.1. Initial Assessment
3.2. Perioperative Chemotherapy
3.3. Synchronous Disease
3.4. The Issue of Disappearing Liver Metastases
3.5. Patients with Initially Resectable Disease
3.6. Patients with Initially Unresectable Metastases
3.7. Adjuvant Treatment When Resectable
4. Biotherapies and Their Action Modes
4.1. Immunotherapy and Adoptive Cell Transfer
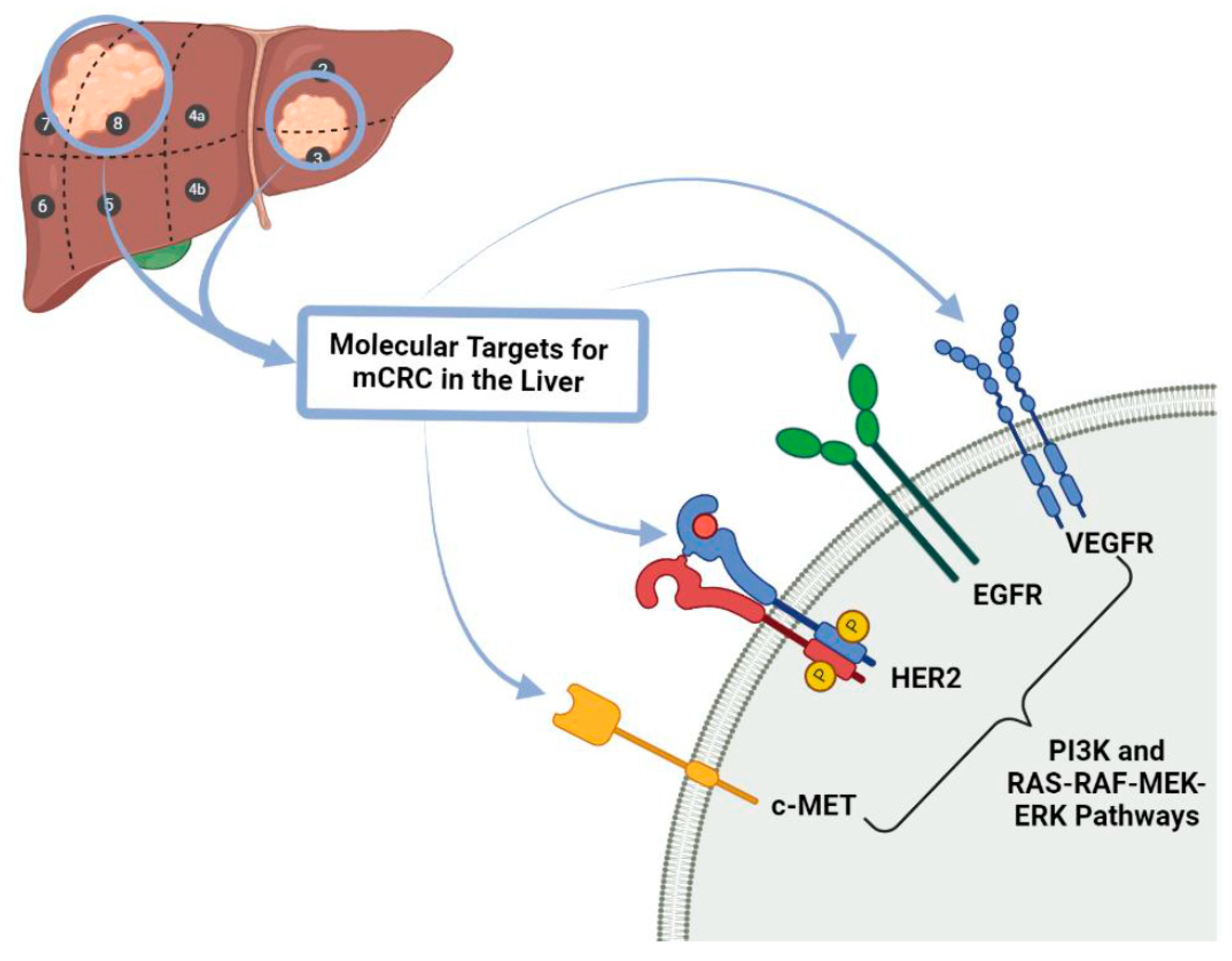
4.2. Targeted Biological Therapy
4.3. Future of Biotherapies for Liver Metastasis in Colorectal Cancer
4.3.1. Immunotherapy
4.3.2. Immunotherapy in Colorectal Liver Metastasis
5. Beyond the Treatment of CRC and Its Progression: Prevention of CRC Occurrence and Metastatic Recurrence Is of High Importance
5.1. Environment, Diet, Lifestyle, Microbiome, and Immune System Together Influence Pathogenic Mechanisms of Colorectal Carcinogenesis
5.1.1. Dietary Advice
5.1.2. Pre-Existing Conditions
5.1.3. Gut Microbiome Immunology and CRC
5.2. Molecular Pathology Research towards the Environment, Lifestyle, Microbiome, Immunity for Prevention, Treatment and Clinical Outcomes Regarding CRC Management
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saridaki, Z.; Souglakos, J. Genetic Alterations in Colorectal Cancer in Older Patients. In Management of Colorectal Cancers in Older People; Papamichael, D., Audisio, R., Eds.; Springer: London, UK, 2013; pp. 9–20. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Dhirendra, K.S.; Dwight, V.N.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tian, H. Current Development Status of MEK Inhibitors. Molecules 2017, 22, 1551. [Google Scholar] [CrossRef]
- Maverakis, E.; Tran, K.; Cheng, M.; Mitra, A.; Ogawa, H.; Shi, V.; Olney, L.; Kloxin, A. MEK inhibitors and their potential in the treatment of advanced melanoma: The advantages of combination therapy. Drug Des. Dev. Ther. 2015, 10, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Samir, B.; Arnab, G. Colorectal Liver Metastasis: Current Concepts. Indian J. Surg. 2020, 1–10. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef]
- Voizard, N.; Cerny, M.; Assad, A.; Billiard, J.-S.; Olivié, D.; Perreault, P.; Kielar, A.; Do, R.K.G.; Yokoo, T.; Sirlin, C.B.; et al. Assessment of hepatocellular carcinoma treatment response with LI-RADS: A pictorial review. Insights Imaging 2019, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tamandl, D.; Mang, T.; Ba-Ssalamah, A. Imaging of colorectal cancer—The clue to individualized treatment. Innov. Surg. Sci. 2020, 3, 3–15. [Google Scholar] [CrossRef]
- Venook, A.P.; Curley, S.A. Management of potentially resectable colorectal cancer liver metastases. World J. Gastrointest. Surg. 2020, 5, 138. [Google Scholar]
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Dhir, M.; Sasson, A.R. Surgical Management of Liver Metastases from Colorectal Cancer. J. Oncol. Pr. 2016, 12, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Abe, T.; Oshita, A.; Sumi, Y.; Yano, T.; Okuda, H.; Kurayoshi, M.; Kobayashi, T.; Ohdan, H.; Noriyuki, T.; et al. Efficacy of upfront hepatectomy without neoadjuvant chemotherapy for resectable colorectal liver metastasis. World J. Surg. Oncol. 2021, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Paulatto, L.; Burgio, M.D.; Sartoris, R.; Beaufrère, A.; Cauchy, F.; Paradis, V.; Vilgrain, V.; Ronot, M. Colorectal liver metastases: Radiopathological correlation. Insights Imaging 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Stevenson, H.L.; Prats, M.M.; Sasatomi, E. Chemotherapy-induced Sinusoidal Injury (CSI) score: A novel histologic assess-ment of chemotherapy-related hepatic sinusoidal injury in patients with colorectal liver metastasis. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Gruenberger, T.; Glynne-Jones, R. Synchronous liver metastases in patients with rectal cancer: Can we establish which treatment first? Ther. Adv. Med. Oncol. 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Al Bandar, M.H.; Kim, N.K. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (Re-view). Oncol. Rep. 2017, 37, 2553–2564. [Google Scholar] [CrossRef]
- Benoist, S.; Brouquet, A.; Penna, C.; Julié, C.; Hajjam, M.E.; Chagnon, S.; Mitry, E.; Rougier, P.; Nordlinger, B. Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J. Clin. Oncol. 2006, 24, 3939–3945. [Google Scholar] [CrossRef]
- Symonds, L.K.; Cohen, S.A. Use of perioperative chemotherapy in colorectal cancer metastatic to the liver. Gastroenterol. Rep. 2019, 7, 301–311. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metas-tasis (New EPOC): Long-term results of a multicentre, randomized, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar]
- Wensink, E.; Bond, M.; Kucukkose, E.; May, A.; Vink, G.; Koopman, M.; Kranenburg, O.; Roodhart, J. A review of the sensitivity of metastatic colorectal cancer patients with deficient mismatch repair to stand-ard-of-care chemotherapy and monoclonal antibodies, with recommendations for future research. Cancer Treat. Rev. 2021, 95, 102174. [Google Scholar] [CrossRef] [PubMed]
- Poston, G.; Adam, R.; Byrne, B.; Esser, R.; Malik, H.; Wasan, H.; Xu, J. The role of cetuximab in converting initially unresectable colorectal cancer liver metastases for resection. Eur. J. Surg. Oncol. 2017, 43, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 1–30. [Google Scholar] [CrossRef]
- Ichida, H.; Mise, Y.; Ito, H.; Ishizawa, T.; Inoue, Y.; Takahashi, Y.; Shinozaki, E.; Yamaguchi, K.; Saiura, A. Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metas-tases. World J. Surg. Oncol. 2019, 17, 1–9. [Google Scholar] [CrossRef]
- De Greef, K.; Rolfo, C.; Russo, A.; Chapelle, T.; Bronte, G.; Passiglia, F.; Coelho, A.; Papadimitriou, K.; Peeters, M. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J. Gastroenterol. 2016, 22, 7215–7225. [Google Scholar] [CrossRef] [PubMed]
- Sabanathan, D.; Eslick, G.D.; Shannon, J. Use of Neoadjuvant Chemotherapy Plus Molecular Targeted Therapy in Colorectal Liver Metastases: A Systematic Review and Meta-analysis. Clin. Color. Cancer 2016, 15, e141–e147. [Google Scholar] [CrossRef]
- Villaruz, L.C.; Socinski, M.A. The clinical viewpoint: Definitions, limitations of RECIST, practical considerations of measure-ment. Clin. Cancer Res. 2013, 19, 2629–2636. [Google Scholar] [CrossRef]
- Aykan, N.F.; Özatlı, T. Objective response rate assessment in oncology: Current situation and future expectations. World J. Clin. Oncol. 2020, 11, 53–73. [Google Scholar] [CrossRef]
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA A Cancer J. Clin. 2018, 68, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Vatandoust, S.; Price, T.J.; Karapetis, C. Colorectal cancer: Metastases to a single organ. World J. Gastroenterol. 2015, 21, 11767–11776. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.W.T.; Laurence, J.M.; Pang, T.; Johnston, E.; Hollands, M.J.; Pleass, H.C.C.; Richardson, A.J. A systematic review of a liver-first approach in patients with colorectal cancer and synchronous colorec-tal liver metastases. HPB 2014, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sahlmann, C.-O.; Homayounfar, K.; Niessner, M.; Dyczkowski, J.; Conradi, L.-C.; Braulke, F.; Meller, B.; Beißbarth, T.; Ghadimi, B.M.; Meller, J.; et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer 2017, 123, 638–649. [Google Scholar] [CrossRef]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival following hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Wang, H.-W.; Jin, K.-M.; Li, J.; Xing, B.-C. Comparison of sequential, delayed and simultaneous resection strategies for synchronous colorectal liver metastases. BMC Surg. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Du Pasquier, C.; Roulin, D.; Bize, P.; Sempoux, C.; Rebecchini, C.; Montemurro, M.; Schäfer, M.; Halkic, N.; Demartines, N. Tumor response and outcome after reverse treatment for patients with synchronous colorectal liver metastasis: A cohort study. BMC Surg. 2020, 20, 78. [Google Scholar] [CrossRef]
- Feo, L.; Polcino, M.; Nash, G.M. Resection of the Primary Tumor in Stage IV Colorectal Cancer: When Is It Necessary? Surg. Clin. N. Am. 2017, 97, 657–669. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.T.W. Colorectal Cancer. Nat. Rev. Dis. Prim. 2015, 176, 100–106. [Google Scholar] [CrossRef]
- Basso, M.; Dadduzio, V.; Ardito, F.; Lombardi, P.; Strippoli, A.; Vellone, M.; Orlandi, A.; Rossi, S.; Cerchiaro, E.; Cassano, A.; et al. Conversion Chemotherapy for Technically Unresectable Colorectal Liver Metastases. Medicine 2016, 95, e3722. [Google Scholar] [CrossRef] [PubMed]
- Poultsides, G.A.; Bao, F.; Servais, E.L.; Hernandez-Boussard, T.; DeMatteo, R.P.; Allen, P.J.; Fong, Y.; Kemeny, N.E.; Saltz, L.B.; Klimstra, D.S.; et al. Pathologic response to pre-operative chemotherapy in colorectal liver metastases: Fibrosis, not necrosis, predicts outcome. Ann. Surg. Oncol. 2012, 19, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Langella, S.; Ardito, F.; Russolillo, N.; Panettieri, E.; Perotti, S.; Mele, C.; Giuliante, F.; Ferrero, A. Intraoperative Ultrasound Staging for Colorectal Liver Metastases in the Era of Liver-Specific Magnetic Resonance Imaging: Is It Still Worthwhile? J. Oncol. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Joo, I. The role of intraoperative ultrasonography in the diagnosis and management of focal hepatic lesions. Ultrasonography 2015, 34, 246–257. [Google Scholar] [CrossRef] [PubMed]
- McKeown, E.; Nelson, D.; Johnson, E.K.; Maykel, J.A.; Stojadinovic, A.; Nissan, A.; Avital, I.; Brücher, B.; Steele, S.R. Current Approaches and Challenges for Monitoring Treatment Response in Colon and Rectal Cancer. J. Cancer 2014, 5, 31–43. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- De’Angelis, N.; Baldini, C.; Brustia, R.; Pessaux, P.; Sommacale, D.; Laurent, A.; Le Roy, B.; Tacher, V.; Kobeiter, H.; Luciani, A.; et al. Surgical and regional treatments for colorectal cancer metastases in older patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0230914. [Google Scholar] [CrossRef]
- Ikoma, N.; Raghav, K.; Chang, G. An Update on Randomized Clinical Trials in Metastatic Colorectal Carcinoma. Surg. Oncol. Clin. N. Am. 2017, 26, 667–687. [Google Scholar] [CrossRef]
- Yokota, T. Are KRAS/BRAF Mutations Potent Prognostic and/or Predictive Biomarkers in Colorectal Cancers? Anticancer Agents Med. Chem. 2012, 12, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Wang, G.Y.; He, J.L.; Wu, F.P.; Zhang, Y.N. Overall survival of patients with KRAS wild-type tumor treated with FOLFOX/FORFIRI±cetuximab as the first-line treatment for metastatic colorectal cancer A meta-analysis. Medicine 2017, 96, 2–7. [Google Scholar] [CrossRef]
- Chan, G.; Chee, C.E. Perioperative Chemotherapy for Liver Metastasis of Colorectal Cancer. Cancers 2020, 12, 3535. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, T. Conversion therapy combined with individualized surgical treatment strategy improves survival in patients with colorectal cancer liver metastases. Int. J. Clin. Exp. Pathol. 2021, 14, 314–321. [Google Scholar] [PubMed]
- Villard, C.; Habib, M.; Nordenvall, C.; Nilsson, P.; Jorns, C.; Sparrelid, E. Conversion therapy in patients with colorectal liver metastases. Eur. J. Surg. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, N. Treatment of colorectal liver metastases. World J. Surg. Oncol. 2011, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Kirstein, M.M. First-line molecular therapies in the treatment of metastatic colorectal cancer—A literature-based review of phases II and III trials. Innov. Surg. Sci. 2018, 3, 85–86. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Tsuji, A.; Ohori, H.; Yamaguchi, T.; Matsuura, M.; Nishioka, A.; Makiyama, A.; Noura, S.; Kochi, M.; Sagawa, T.; Kotaka, M.; et al. Safety analysis of the randomized phase II study of FOLFOXIRI plus cetuximab versus FOLFOXIRI plus bevacizumab as the first-line treatment in metastatic colorectal cancer with RAS wild-type tumors: The DEEPER trial (JAC-CRO CC-13). J. Clin. Oncol. 2021, 39, 86. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. 2011, 22, 1535–1546. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Kozuch, P.S. Impact of KRAS Mutations on Management of Colorectal Carcinoma. Pathol. Res. Int. 2011, 2011, 1–11. [Google Scholar] [CrossRef][Green Version]
- Sotelo, M.; García-Paredes, B.; Aguado, C.; Sastre, J.; Díaz-Rubio, E. Role of cetuximab in first-line treatment of metastatic colorectal cancer. World J. Gastroenterol. 2014, 20, 4208–4219. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, L.; Zhang, X.; Gao, G.; Shen, L.; Hu, J.; Yang, M.; Liu, B.; Qian, X. FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis. Medicine 2018, 97, 1–8. [Google Scholar] [CrossRef]
- Comunanza, V.; Bussolino, F. Therapy for Cancer: Strategy of Combining Anti-Angiogenic and Target Therapies. Front. Cell Dev. Biol. 2017, 5, 101. [Google Scholar] [CrossRef]
- Ohhara, Y.; Fukuda, N.; Takeuchi, S.; Honma, R.; Shimizu, Y.; Kinoshita, I.; Dosaka-Akita, H. Role of targeted therapy in metastatic colorectal cancer. World J. Gastrointest. Oncol. 2016, 8, 642–655. [Google Scholar] [CrossRef]
- Shuford, R.A.; Cairns, A.L.; Moaven, O. Precision Approaches in the Management of Colorectal Cancer: Current Evidence and Latest Advancements Towards Individualizing the Treatment. Cancers 2020, 12, 3481. [Google Scholar] [CrossRef]
- Markman, J.L.; Shiao, S.L. Impact of the immune system and immunotherapy in colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.; Murphy, A. The emerging role of immunotherapy in colorectal cancer. Ann. Transl. Med. 2016, 4, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, P. Improving antitumor efficacy via combinatorial regimens of oncolytic virotherapy. Mol. Cancer 2020, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Morise, Z.; Tanaka, C.; Hayashi, T.; Ikeda, Y.; Maeda, K.; Masumori, K.; Koide, Y.; Katsuno, H.; Tanahashi, Y.; et al. Repeat hepatectomy with systemic chemotherapy might improve survival of recurrent liver metastasis from colorectal cancer—A retrospective observational study. World J. Surg. Oncol. 2019, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lintoiu-Ursut, B.; Tulin, A.; Constantinoiu, S. Recurrence after hepatic resection in colorectal cancer liver metastasis—Review article. J. Med. Life 2015, 8, 12–14. [Google Scholar] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- De Cicco, P.; Ercolano, G.; Ianaro, A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front. Immunol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Bocanegra, A.; Blanco, E.; Fernandez-Hinojal, G.; Arasanz, H.; Chocarro, L.; Zuazo, M.; Morente, P.; Vera, R.; Escors, D.; Kochan, G. PD-L1 in Systemic Immunity: Unraveling Its Contribution to PD-1/PD-L1 Blockade Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef]
- Saint-Jean, M.; Knol, A.-C.; Volteau, C.; Quéreux, G.; Peuvrel, L.; Brocard, A.; Pandolfino, M.-C.; Saiagh, S.; Nguyen, J.-M.; Bedane, C.; et al. Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes in Advanced Melanoma Patients. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Reinoso, A.; Nehme-Álvarez, D.; Domínguez-Alonso, C.; Álvarez-Vallina, L. Synthetic TILs: Engineered Tu-mor-Infiltrating Lymphocytes with Improved Therapeutic Potential. Front. Oncol. 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Qiu, H.; Zhang, M.; Sun, L.; Peng, P.; Yu, Q.; Yuan, X. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 2016, 8, 3980–4000. [Google Scholar] [CrossRef]
- Hallam, S.; Escorcio-Correia, M.; Soper, R.; Schultheiss, A.; Hagemann, T. Activated macrophages in the tumour microenvi-ronment—Dancing to the tune of TLR and NF-κB. J. Pathol. 2009, 219, 143–152. [Google Scholar] [CrossRef]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- Martinelli, E.; Morgillo, F.; Troiani, T.; Ciardiello, F. Cancer resistance to therapies against the EGFR-RAS-RAF pathway: The role of MEK. Cancer Treat. Rev. 2017, 53, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Inoue, A.; Hangai, S.; Saijo, S.; Negishi, H.; Nishio, J.; Taniguchi, T. The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the sup-pression of liver metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, 14097–14102. [Google Scholar] [CrossRef] [PubMed]
- Milette, S.; Sicklick, J.K.; Lowy, A.M.; Brodt, P. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin. Cancer Res. 2017, 23, 6390–6399. [Google Scholar] [CrossRef]
- Lingling, Z.; Jiewei, L.; Li, W.; Danli, Y.; Jie, Z.; Wen, L.; Dan, P.; Lei, P.; Qinghua, Z. Molecular regulatory network of PD-1/PD-L1 in non-small cell lung cancer. Pathol. Res. Pr. 2020, 216, 152852. [Google Scholar] [CrossRef]
- Tai, D.; Choo, S.P.; Chew, V. Rationale of Immunotherapy in Hepatocellular Carcinoma and Its Potential Biomarkers. Cancers 2019, 11, 1926. [Google Scholar] [CrossRef]
- Koi, M.; Carethers, J.M. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncol. 2017, 13, 1633–1647. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.Y.; Baek, J.Y.; Cha, Y.J.; Ahn, J.B.; Kim, H.S.; Kim, T.W. A phase II study of avelumab monotherapy in patients with mismatch repair-deficient/microsatellite instabil-ity-high or POLE-mutated metastatic or unresectable colorectal cancer. Cancer Res. Treat. 2020, 52, 1135–1144. [Google Scholar] [PubMed]
- Zarour, L.R.; Anand, S.; Billingsley, K.G.; Bisson, W.H.; Cercek, A.; Clarke, M.F.; Coussens, L.M.; Gast, C.E.; Geltzeiler, C.; Hansen, L.; et al. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.P.; Sforza, V.; Ciardiello, D.; Napolitano, S.; Della Corte, C.M.; Morgillo, F.; Raucci, A.; et al. Present and future of metastatic colorectal cancer treatment: A review of new candidate targets. World J. Gastroenterol. 2017, 23, 4675–4688. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Gol Golshani, Y.Z. Advances in immunotherapy for colorectal cancer: A review. Therap. Adv. Gastroenterol. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Vogel, A.; Hofheinz, R.; Kubicka, S.; Arnold, D. Treatment decisions in metastatic colorectal cancer—Beyond first and second line combination therapies. Cancer Treat. Rev. 2017, 59, 54–60. [Google Scholar] [CrossRef]
- Combo, K.K.; Bekaii-Saab, T. A Comprehensive Review of Sequencing and Combination Strategies of Targeted Agents in Metastatic Colorectal Cancer. Oncologist 2018, 23, 25–34. [Google Scholar]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Re-pair—Deficient/Microsatellite Instability—High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Jia, L.; Zheng, Z.; Tran, E.; Robbins, P.F.; Rosenberg, S.A. Single-Cell Transcriptome Analysis Reveals Gene Signatures Associated with T-cell Persistence Following Adoptive Cell Therapy. Cancer Immunol. Res. 2019, 7, 1824–1836. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Robbins, P.F. Targeting neoantigens for cancer immunotherapy. Int. Immunol. 2016, 28, 365–370. [Google Scholar] [CrossRef]
- Xiao, L.; Cen, D.; Gan, H.; Sun, Y.; Huang, N.; Xiong, H.; Xu, X.H. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. 2019, 27, 1114–1125. [Google Scholar] [CrossRef]
- Macrae, F.A. Colorectal cancer: Epidemiology, risk factors, and protective factors. Uptodate Com 2016, 3, 1–28. [Google Scholar]
- Xu, T.; Zhang, Y.; Zhang, J.; Qi, C.; Liu, D.; Wang, Z.; Li, Y.; Ji, C.; Li, J.; Lin, X.; et al. Germline Profiling and Molecular Characterization of Early Onset Metastatic Colorectal Cancer. Front. Oncol. 2020, 10, 568911. [Google Scholar] [CrossRef] [PubMed]
- Preisler, L.; Habib, A.; Shapira, G.; Kuznitsov-Yanovsky, L.; Mayshar, Y.; Carmel-Gross, I.; Malcov, M.; Azem, F.; Shomron, N.; Kariv, R.; et al. Heterozygous APC germline mutations impart predisposition to colorectal cancer. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Cercek, A.; Kemel, Y.; Mandelker, D.; Stewart, C.; Arnold, A.G.; Sheehan, M.; Yaeger, R.; Segal, N.H.; Varghese, A.M.; Saltz, L.B.; et al. Prevalence of germline genetic alterations in colorectal cancer patients. J. Clin. Oncol. 2018, 36, 1577. [Google Scholar] [CrossRef]
- Gong, R.; He, Y.; Liu, X.Y.; Wang, H.Y.; Sun, L.Y.; Yang, X.H.; Shao, J.Y. Mutation spectrum of germline cancer susceptibility genes among unselected Chinese colorectal cancer pa-tients. Cancer Manag. Res. 2019, 11, 3721–3739. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Lopes, G.; Stern, M.C.; Temin, S.; Sharara, A.I.; Cervantes, A.; Costas-Chavarri, A.; Engineer, R.; Hamashima, C.; Ho, G.F.; Huitzil, F.D.; et al. Early Detection for Colorectal Cancer: ASCO Resource-Stratified Guideline. J. Glob. Oncol. 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Lim, C.Y.S.; Laidsaar-Powell, R.C.; Young, J.M.; Kao, S.C.; Zhang, Y.; Butow, P. Colorectal cancer survivorship: A systematic review and thematic synthesis of qualitative research. Eur. J. Cancer Care 2021, e13421. [Google Scholar] [CrossRef]
- Pietrzyk, Ł. Food properties and dietary habits in colorectal cancer prevention and development. Int. J. Food Prop. 2017, 20, 2323–2343. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar]
- Klepp, P.; Brackmann, S.; Cvancarova, M.; Hoivik, M.L.; Hovde, M.; Henriksen, M.; Huppertz-Hauss, G.; Bernklev, T.; Hoie, O.; Kempski-Monstad, I.; et al. Risk of colorectal cancer in a population-based study 20 years after diagnosis of ulcerative colitis: Results from the IBSEN study. BMJ Open Gastroenterol. 2020, 7, e000361. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2017, 57, 1–24. [Google Scholar] [CrossRef]
- Fukui, H.; Xu, X.; Miwa, H. Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, S.; Jia, J.; Huang, L.; Li, F.; Jin, F.; Wang, Y. Intestinal Microbiota-Associated Metabolites: Crucial Factors in the Effectiveness of Herbal Medicines and Diet Therapies. Front. Physiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier function and metabolic/liver diseases. Liver Res. 2020, 4, 81–87. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Pamer, E.G. Microbiota-Mediated Inflammation and Antimicrobial Defense in the Intestine. Annu. Rev. Immunol. 2015, 33, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome Dependent Regulation of Tregs and Th17 Cells in Mucosa. Front. Immunol. 2019, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immu-notherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Sarvizadeh, M.; Ghasemi, F.; Tavakoli, F.; Khatami, S.S.; Razi, E.; Sharifi, H.; Biouki, N.M.; Taghizadeh, M. Vaccines for colorectal cancer: An update. J. Cell. Biochem. 2018, 120, 8815–8828. [Google Scholar] [CrossRef] [PubMed]
| Names of Combined Chemotherapy Regimens | Components of Combined Chemotherapy |
|---|---|
| FOLFOX | Folinic Acid, Fluorouracil and Oxaliplatin. |
| FOLFORI | 5-Fluorouracil and High-Dose Leucovorin |
| FOLFIRI | Folinic Acid, Fluorouracil and Irinotecan |
| FOLFOXIRI | Folinic Acid, Fluorouracil and Oxaliplatin. |
| CAPOX or XELOX | Oxaliplatin and Capecitabine |
| Types of Therapy | Definition | Examples of Type of Therapy |
|---|---|---|
| “Classical” Cytotoxic Chemotherapies | Therapies that can be delivered intravenously or orally. It can be given during the neoadjuvant, adjuvant or palliative setting and can be given either systemically or regionally. | FOLFOX (also known as Oxaliplatin de Gramont or OxMdG, which means modified Oxaliplatin de Gramont) (Folinic acid, fluorouracil and oxaliplatin) FOLFORI (5-Fluorouracil and High-Dose Leucovorin) FOLFIRI (Folinic acid, fluorouracil and irinotecan) FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) CAPOX (capecitabine plus oxaliplatin) or XELOX (xeloda® = capecitabine plus oxaliplatine) |
| Targeted Therapies | Therapies that target specific molecules, including receptors, proteins, genes which impair the development and propagation of tumour growth. | Epidermal Growth Factor Receptor Inhibitor: Cetuximab and Panitumumab Tyrosine Kinase Inhibitor: Regorafenib BRAF Inhibitors: Encorafenib |
| Anti-angiogenic Therapies | Therapies against which targets the protein (VEGF) that promotes vessel development and growth in order to facilitate transportation of nutrients to the tumour in order for tumour growth. | Bevacizumab Ramucirumab Ziv-aflibercept |
| Biotherapies | Therapies that utilize and facilitate a patient’s own immune system in recognizing and killing present cancer cells, e.g., immunotherapies. | Monoclonal Antibodies CAR T-Cell Therapy Immune Checkpoint Inhibitors Cancer Vaccines Immunomodulators |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osei-Bordom, D.-C.; Kamarajah, S.; Christou, N. Colorectal Cancer, Liver Metastases and Biotherapies. Biomedicines 2021, 9, 894. https://doi.org/10.3390/biomedicines9080894
Osei-Bordom D-C, Kamarajah S, Christou N. Colorectal Cancer, Liver Metastases and Biotherapies. Biomedicines. 2021; 9(8):894. https://doi.org/10.3390/biomedicines9080894
Chicago/Turabian StyleOsei-Bordom, Daniel-Clement, Sivesh Kamarajah, and Niki Christou. 2021. "Colorectal Cancer, Liver Metastases and Biotherapies" Biomedicines 9, no. 8: 894. https://doi.org/10.3390/biomedicines9080894
APA StyleOsei-Bordom, D.-C., Kamarajah, S., & Christou, N. (2021). Colorectal Cancer, Liver Metastases and Biotherapies. Biomedicines, 9(8), 894. https://doi.org/10.3390/biomedicines9080894






