Differentiation of Adipose-Derived Stem Cells into Vascular Smooth Muscle Cells for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Human and Porcine Adipose-Derived Stem Cells
2.2. Differentiation of ASC
2.3. Western Blot Analysis
2.4. Immunofluorescent Staining
2.5. Collagen Lattice Contraction Assay
2.6. Statistical Analysis
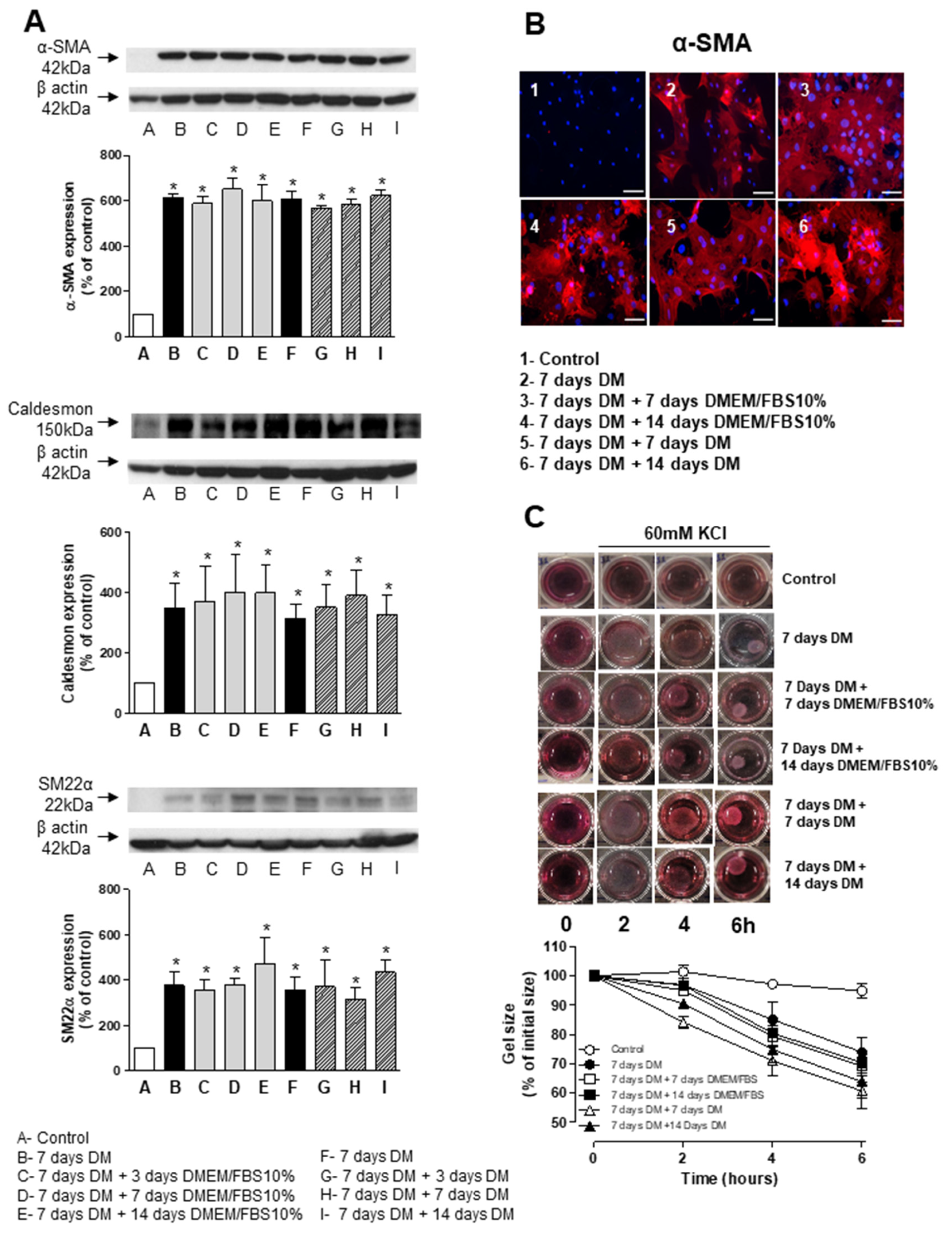
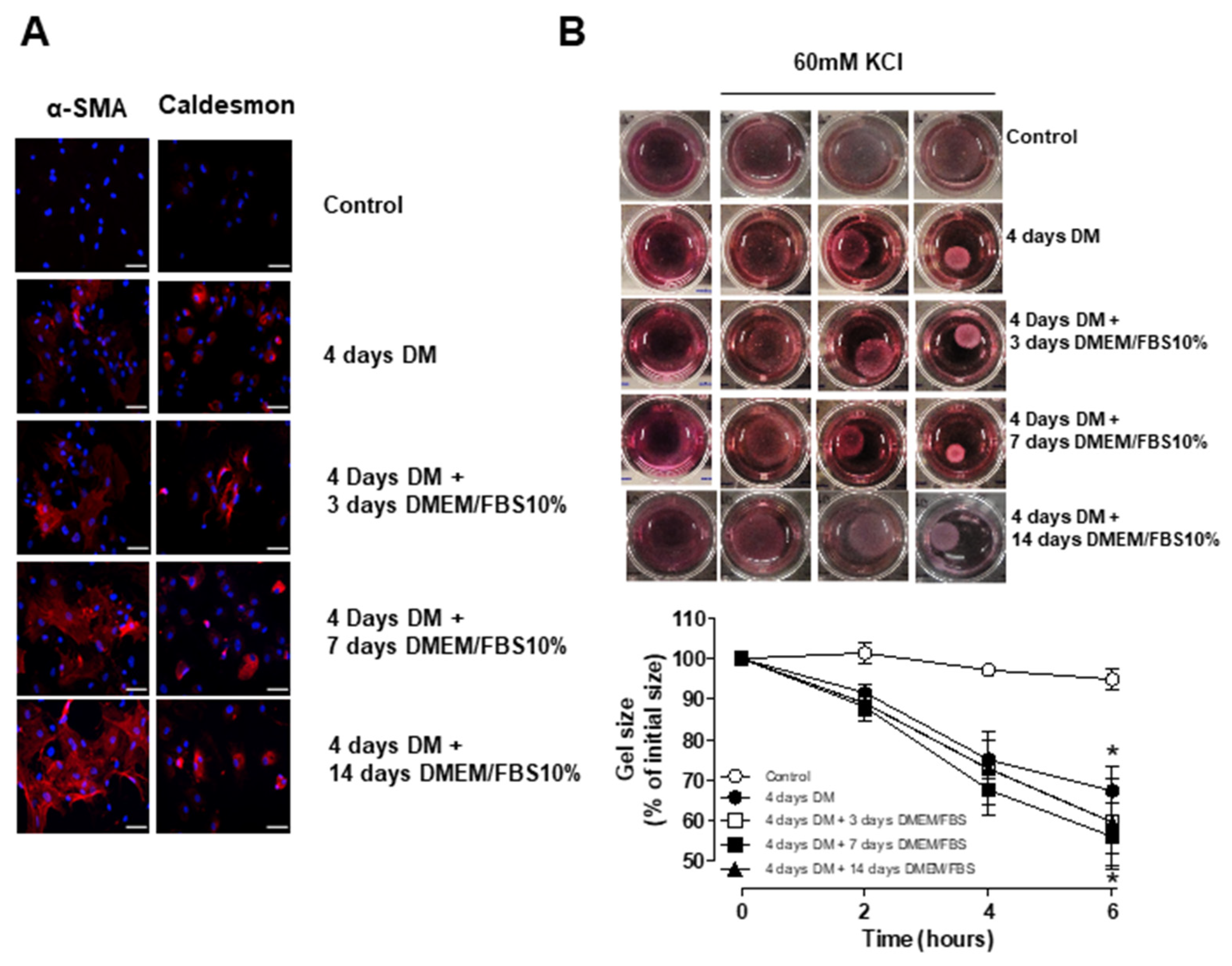
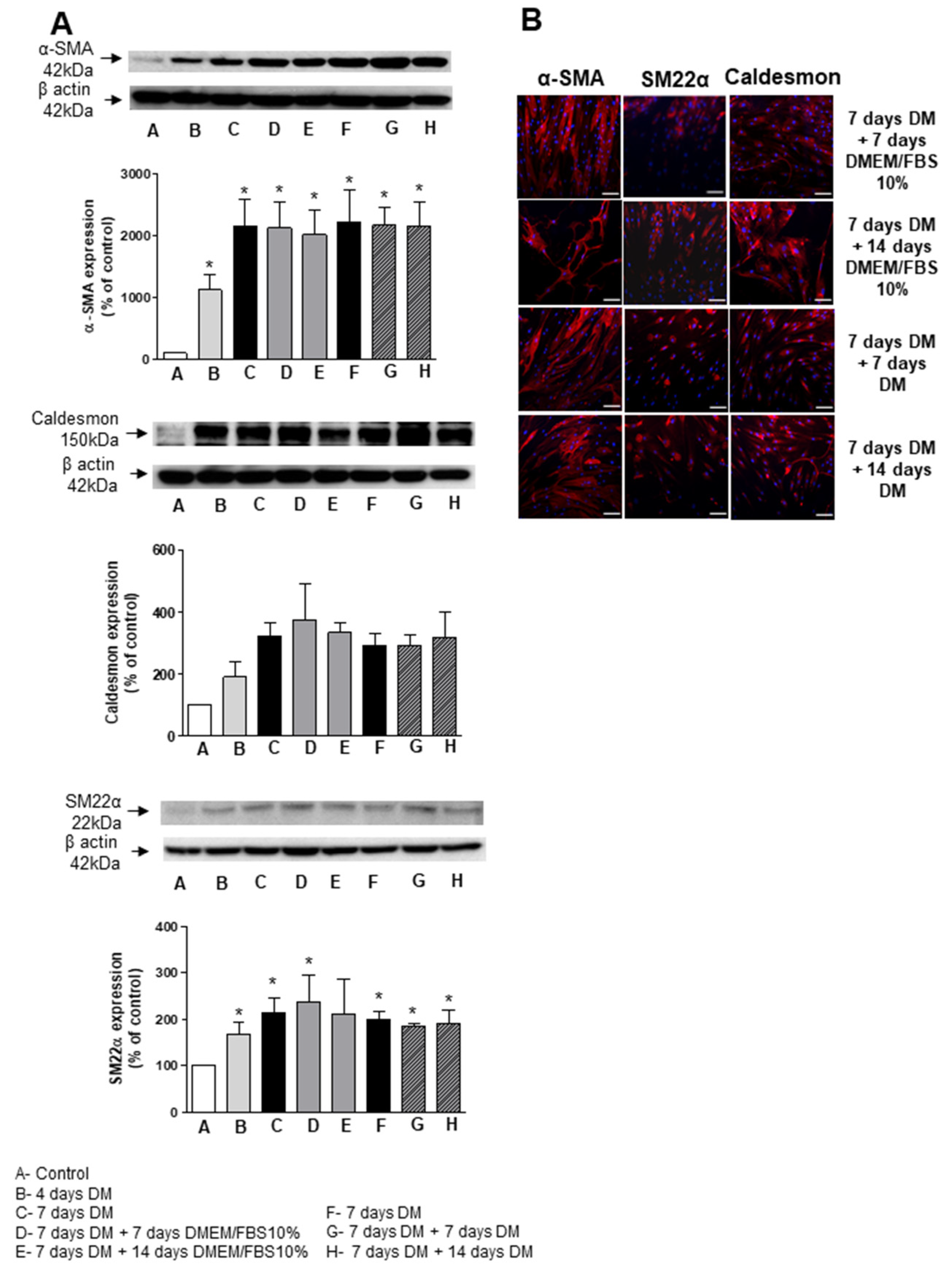
3. Results
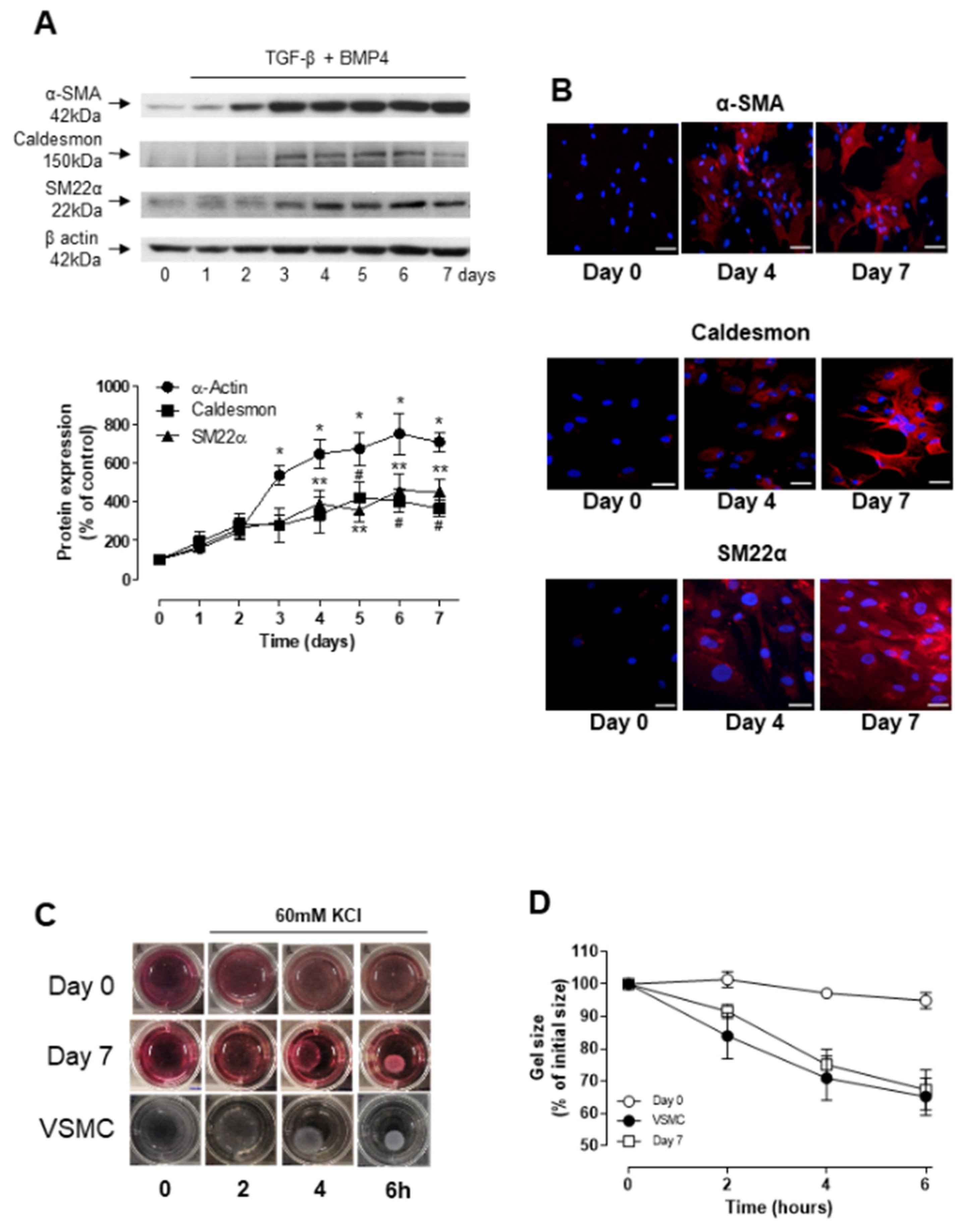
3.1. TGF-β- and BMP-4-Induced Differentiation of ASCs into Smooth Muscle Cell
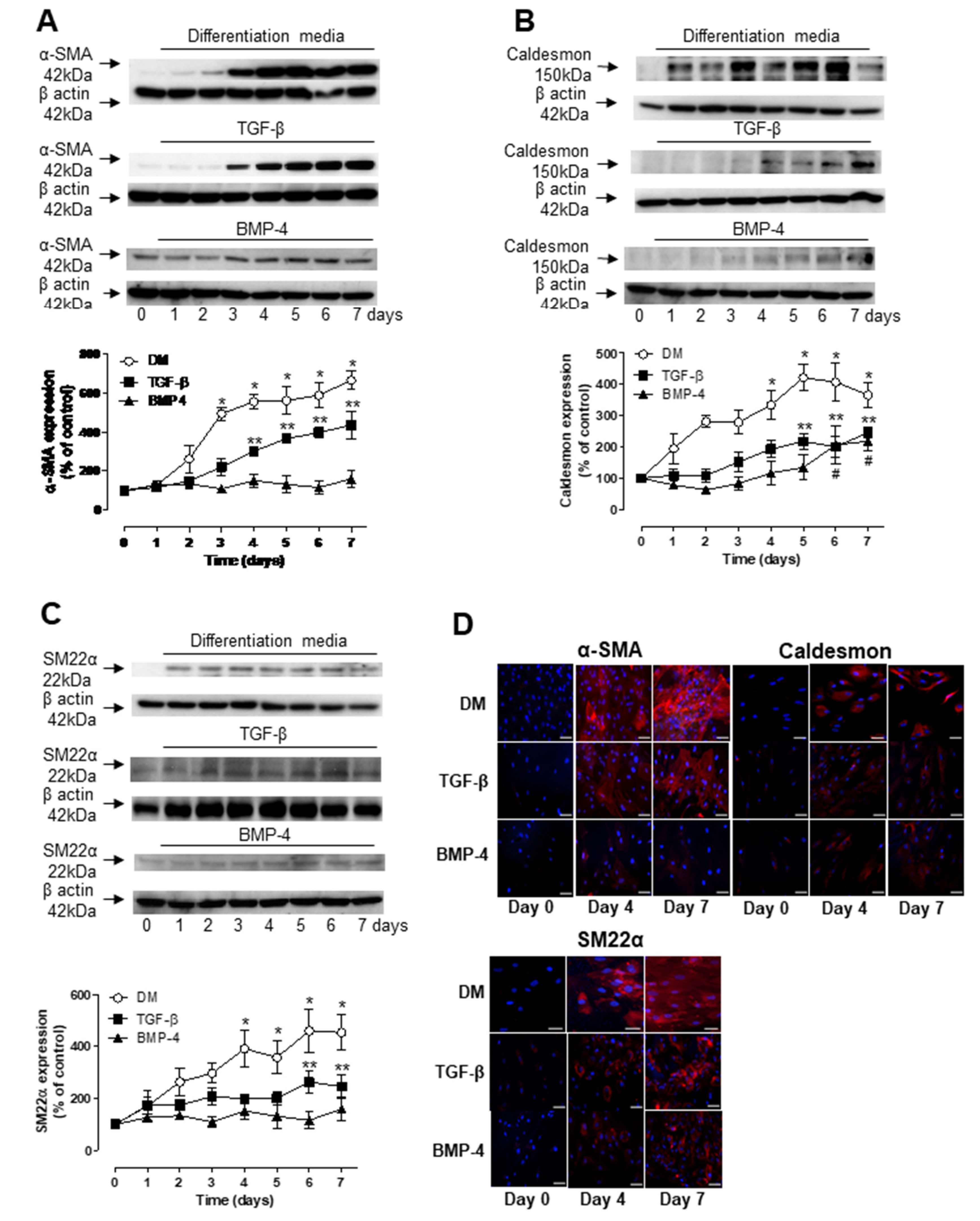
3.2. Role of TGF-β and BMP-4 in ASC Differentiation
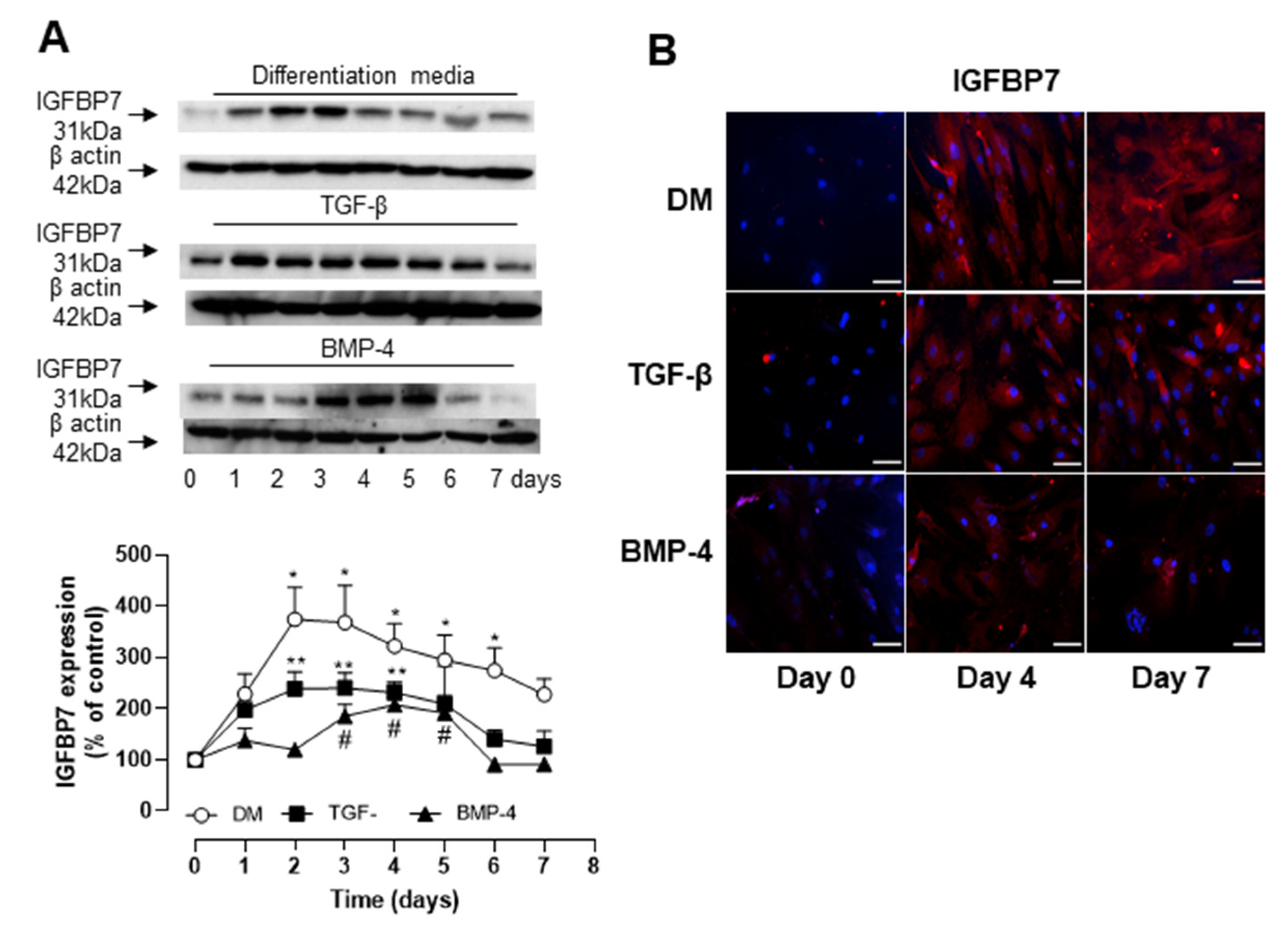
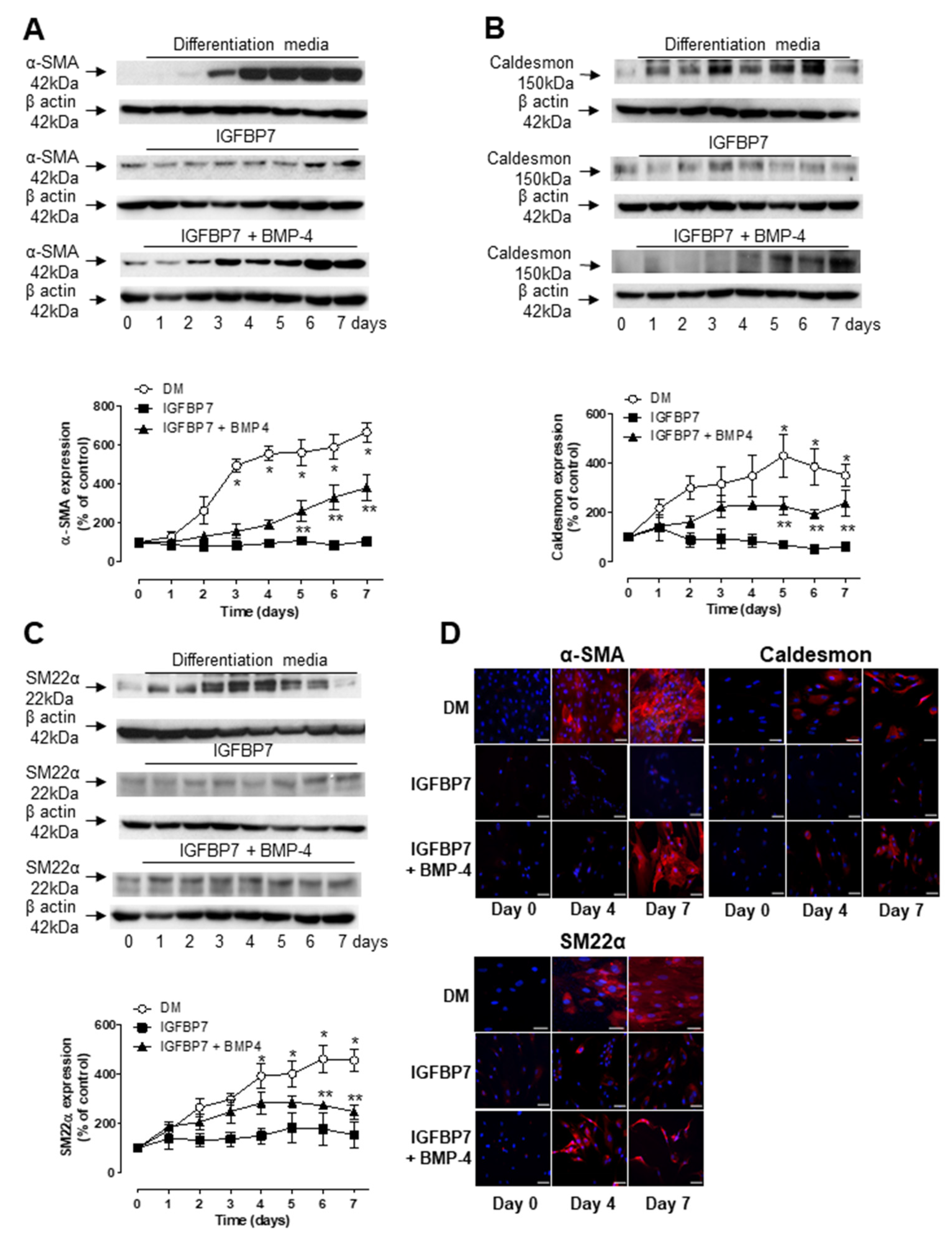
3.3. Role of IGFBP7 in ASC Differentiation
3.4. Stability and Long-Term Differentiation of ASC
3.5. Short-Term Differentiation of ASC into SMC
3.6. ASC Differentiation in Synthetic Surface
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKee, J.A.; Banik, S.S.; Boyer, M.J.; Hamad, N.M.; Lawson, J.H.; Niklason, L.E.; Counter, C.M. Human arteries engineered in vitro. EMBO Rep. 2003, 4, 633–638. [Google Scholar] [CrossRef] [Green Version]
- McBane, J.E.; Sharifpoor, S.; Labow, R.S.; Ruel, M.; Suuronen, E.J.; Santerre, J.P. Tissue Engineering a Small Diameter Vessel Substitute: Engineering Constructs with Select Biomaterials and Cells. Curr. Vasc. Pharmacol. 2012, 10, 347–360. [Google Scholar] [CrossRef]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010, 5, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Ramseier, M.L.; Behn, A.W.; Yang, Y. Synthesis and characterization of polycaprolactone urethane hollow fiber membranes as small diameter vascular grafts. Mater. Sci. Eng. C 2016, 64, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Tzchori, I.; Falah, M.; Shteynberg, D.; Ashkenazi, D.L.; Loberman, Z.; Perry, L.; Flugelman, M.Y. Improved Patency of ePTFE Grafts as a Hemodialysis Access Site by Seeding Autologous Endothelial Cells Expressing Fibulin-5 and VEGF. Mol. Ther. 2018, 26, 1660–1668. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.J.; Ajji, A.; Mohebbi-Kalhori, D.; Rukhlova, M.; Hadjizadeh, A.; Bureau, M.N. Development of a compliant and cyto-compatible micro-fibrous polyethylene terephthalate vascular scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi-Kalhori, D.; Rukhlova, M.; Ajji, A.; Bureau, M.; Moreno, M.J. A novel automated cell-seeding device for tissue engineering of tubular scaffolds: Design and functional validation. J. Tissue Eng. Regen. Med. 2011, 6, 710–720. [Google Scholar] [CrossRef]
- Chang, S.; Song, S.; Lee, J.; Yoon, J.; Park, J.; Choi, S.; Park, J.-K.; Choi, K.; Choi, C. Phenotypic Modulation of Primary Vascular Smooth Muscle Cells by Short-Term Culture on Micropatterned Substrate. PLoS ONE 2014, 9, e88089. [Google Scholar] [CrossRef]
- Siepe, M.; Akhyari, P.; Lichtenberg, A.; Schlensak, C.; Beyersdorf, F. Stem cells used for cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. 2008, 34, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Riha, G.M.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Review:Application of Stem Cells for Vascular Tissue Engineering. Tissue Eng. 2005, 11, 1535–1552. [Google Scholar] [CrossRef]
- Buehrer, B.M.; Cheatham, B. Isolation and Characterization of Human Adipose-Derived Stem Cells for Use in Tissue Engineering. Methods Mol. Biol. 2013, 1001, 1–11. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Timper, K.; Seboek, D.; Eberhardt, M.; Linscheid, P.; Christ-Crain, M.; Keller, U.; Müller, B.; Zulewski, H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys. Res. Commun. 2006, 341, 1135–1140. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Suh, S.Y.; Bae, Y.C.; Jung, J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 328, 258–264. [Google Scholar] [CrossRef]
- Gimble, J.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, M.; Sesti, C.; Washington, I.M.; Du, L.; Dronadula, N.; Chin, M.T.; Stolz, D.B.; Davis, E.C.; Dichek, D.A. Transforming Growth Factor-β Signaling in Myogenic Cells Regulates Vascular Morphogenesis, Differentiation, and Matrix Synthesis. Arter. Thromb. Vasc. Biol. 2012, 32, e1–e11. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Park, J.S.; Chu, J.S.; Krakowski, A.; Luo, K.; Chen, D.J.; Li, S. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J. Biol. Chem. 2004, 279, 43725–43734. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yin, S.; Cen, L.; Liu, Q.; Liu, W.; Cao, Y.; Cui, L. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4. Tissue Eng. Part A 2010, 16, 1201–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagna, G.; Ku, M.M.; Nguyen, P.H.; Neuman, N.A.; Davis-Dusenbery, B.; Hata, A. Control of Phenotypic Plasticity of Smooth Muscle Cells by Bone Morphogenetic Protein Signaling through the Myocardin-related Transcription Factors. J. Biol. Chem. 2007, 282, 37244–37255. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Lechleider, R.J. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ. Res. 2004, 94, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Hoofnagle, M.H.; Kingston, P.A.; McCanna, M.E.; Owens, G.K. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am. J. Physiol. Cell. Physiol. 2004, 287, C1560–C1568. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.; Tian, W.; Xiang, B.; Yang, T.; Li, G.; Wang, J.; Gruwel, M.; Kashour, T.; Rendell, J.; et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An MR imaging study of rat hearts. Am. J. Physiol. Circ. Physiol. 2009, 297, H1020–H1031. [Google Scholar] [CrossRef]
- Pen, A.; Moreno, M.J.; Durocher, Y.; Deb-Rinker, P.; Stanimirovic, D.B. Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-beta signaling. Oncogene 2008, 27, 6834–6844. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.X.; Huang, S.; Zhang, Q.Q.; Liu, Y.; Zhang, D.M.; Guo, X.H.; Han, D.W. Insulin-like growth factor binding protein-7 induces ac-tivation and transdifferentiation of hepatic stellate cells in vitro. World J. Gastroenterol. 2009, 15, 3246–3253. [Google Scholar] [CrossRef]
- Salem, S.A.; Hwie, A.N.M.; Saim, A.; Kong, C.H.C.; Sagap, I.; Singh, R.; Yusof, M.R.; Zainuddin, Z.M.; Idrus, R.H. Human Adipose Tissue Derived Stem Cells as a Source of Smooth Muscle Cells in the Regeneration of Muscular Layer of Urinary Bladder Wall. Malays. J. Med. Sci. 2013, 20, 80–87. [Google Scholar]
- Chen, R.; Dean, D. Mechanical properties of stem cells from different sources during vascular smooth muscle cell differentiation. Mol. Cell Biomech. 2017, 14, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Liu, G.; Lin, G.; Yang, R.; Lue, T.F.; Lin, C.S. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tis-sue-derived stem cells. J. Sex. Med. 2009, 6, 967–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, J.; Takiyama, Y.; Honjyo, J.; Makino, Y.; Fujita, Y.; Tateno, M.; Haneda, M. Role of IGFBP7 in Diabetic Nephropathy: TGF-β1 Induces IGFBP7 via Smad2/4 in Human Renal Proximal Tubular Epithelial Cells. PLoS ONE. 2016, 11, e0150897. [Google Scholar] [CrossRef]
- Thery, M.; Racine, V.; Piel, M.; Pepin, A.; Dimitrov, A.; Chen, Y.; Sibarita, J.-B.; Bornens, M. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl. Acad. Sci. USA 2006, 103, 19771–19776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehne, T.; Saeed, M.; Higgins, C.B.; Gleason, K.; Krombach, G.A.; Weber, O.M.; Martin, A.J.; Turner, D.; Teitel, D.; Moore, P. Endovascular Stents in Pulmonary Valve and Artery in Swine: Feasibility Study of MR Imaging–guided Deployment and Postinterventional Assessment. Radiology 2003, 226, 475–481. [Google Scholar] [CrossRef]
- Heino, T.J.; Alm, J.J.; Moritz, N.; Aro, H.T. Comparison of the osteogenic capacity of minipig and human bone marrow-derived mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yogi, A.; Rukhlova, M.; Charlebois, C.; Tian, G.; Stanimirovic, D.B.; Moreno, M.J. Differentiation of Adipose-Derived Stem Cells into Vascular Smooth Muscle Cells for Tissue Engineering Applications. Biomedicines 2021, 9, 797. https://doi.org/10.3390/biomedicines9070797
Yogi A, Rukhlova M, Charlebois C, Tian G, Stanimirovic DB, Moreno MJ. Differentiation of Adipose-Derived Stem Cells into Vascular Smooth Muscle Cells for Tissue Engineering Applications. Biomedicines. 2021; 9(7):797. https://doi.org/10.3390/biomedicines9070797
Chicago/Turabian StyleYogi, Alvaro, Marina Rukhlova, Claudie Charlebois, Ganghong Tian, Danica B. Stanimirovic, and Maria J. Moreno. 2021. "Differentiation of Adipose-Derived Stem Cells into Vascular Smooth Muscle Cells for Tissue Engineering Applications" Biomedicines 9, no. 7: 797. https://doi.org/10.3390/biomedicines9070797
APA StyleYogi, A., Rukhlova, M., Charlebois, C., Tian, G., Stanimirovic, D. B., & Moreno, M. J. (2021). Differentiation of Adipose-Derived Stem Cells into Vascular Smooth Muscle Cells for Tissue Engineering Applications. Biomedicines, 9(7), 797. https://doi.org/10.3390/biomedicines9070797




