Vagus Nerve Stimulation with Mild Stimulation Intensity Exerts Anti-Inflammatory and Neuroprotective Effects in Parkinson’s Disease Model Rats
Abstract
1. Introduction
2. Materials and Methods
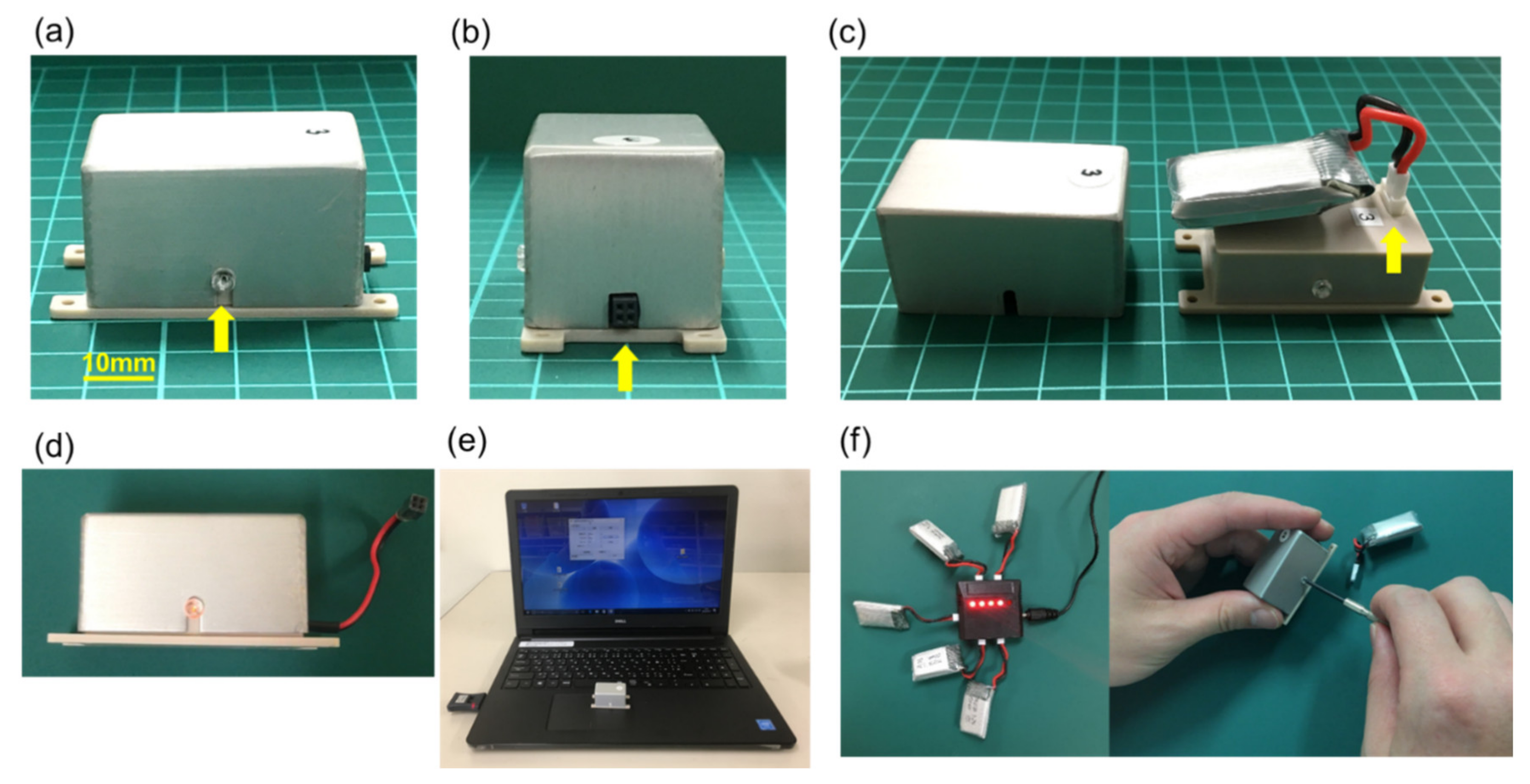
2.1. Electrical Stimulation System for Animal Studies
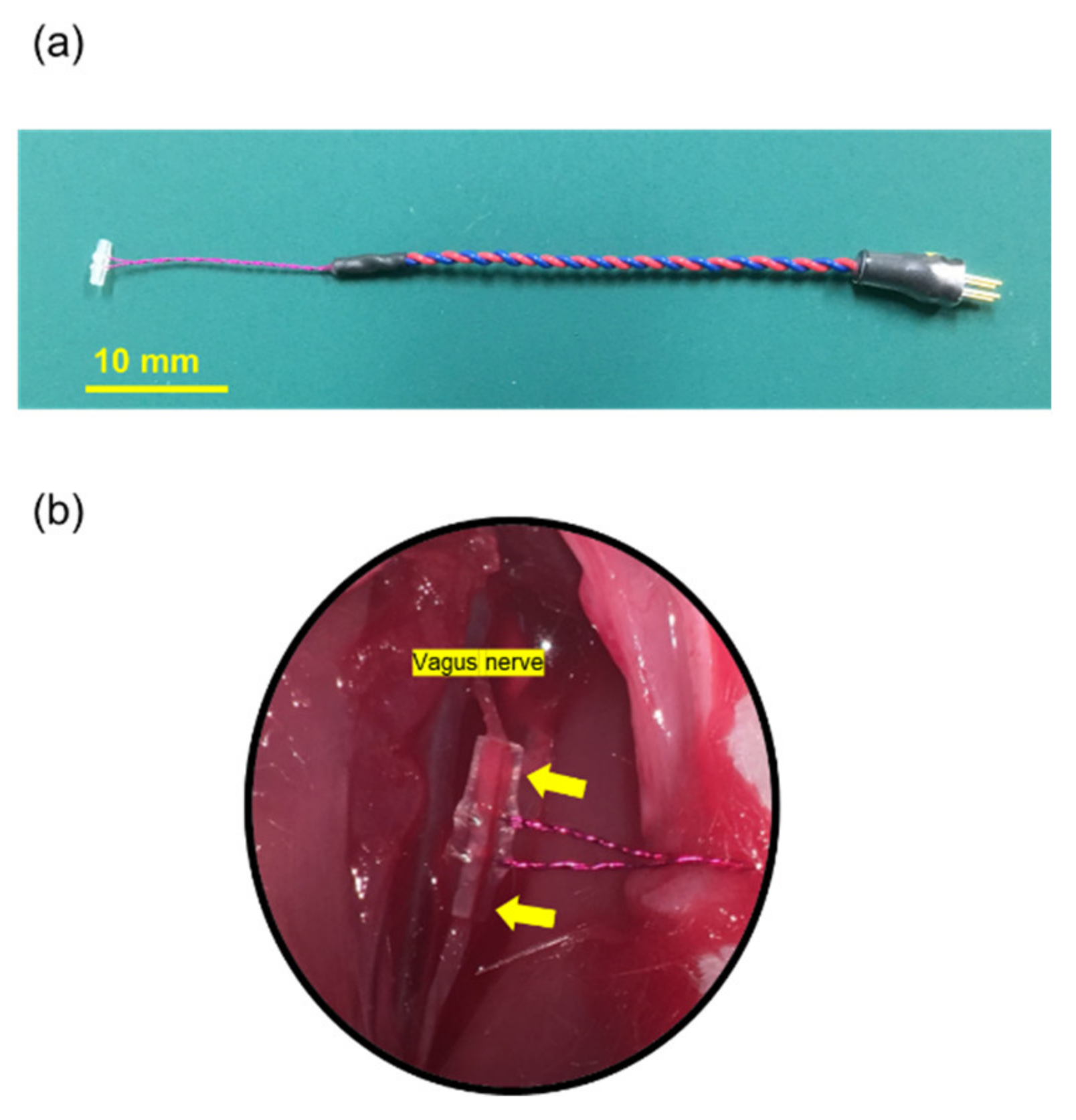
2.2. Electrode
2.3. Ethics Statement and Animals
2.4. Surgical Procedure
2.4.1. VNS Surgery
2.4.2. 6-OHDA Lesioning
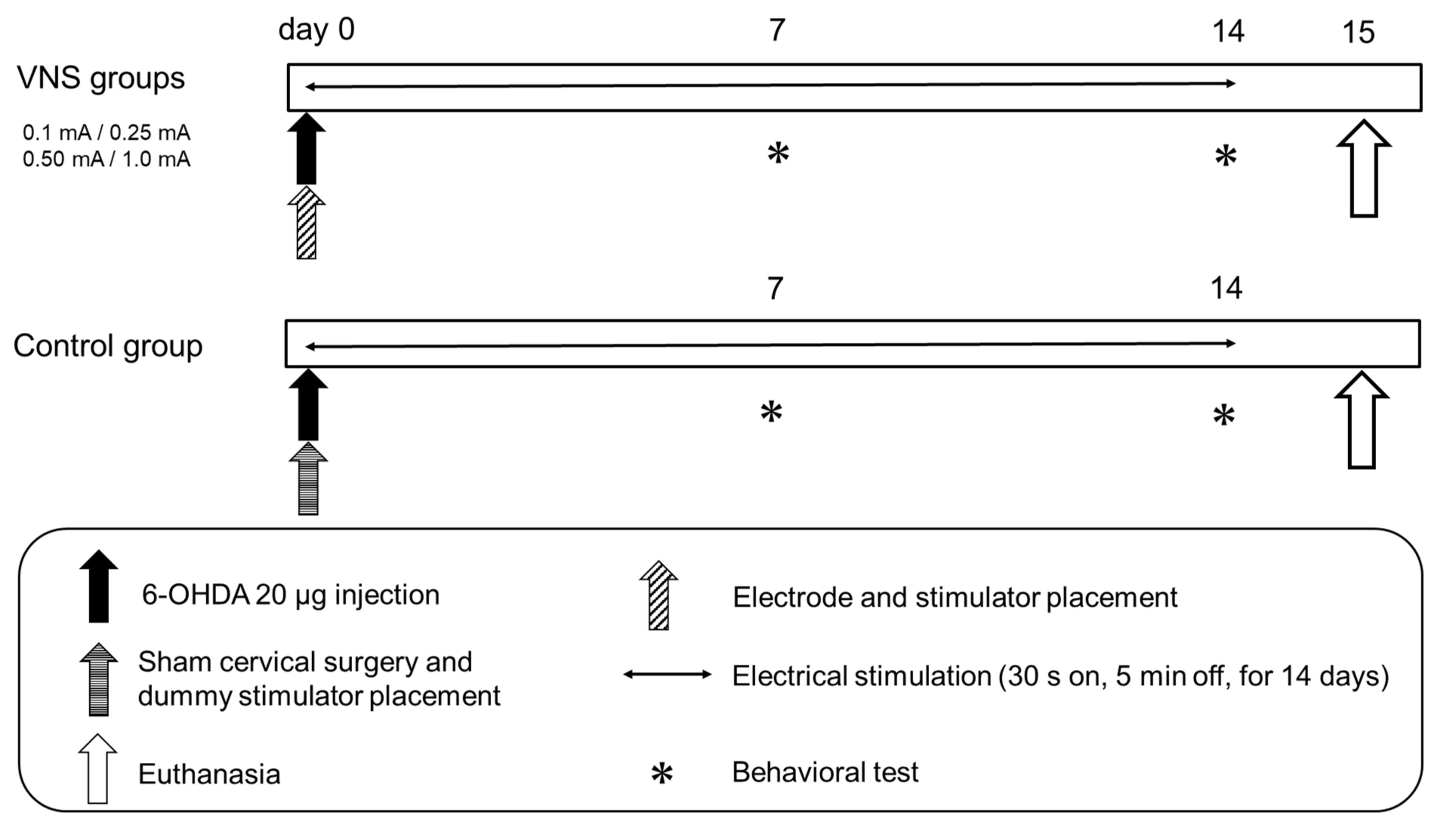
2.5. VNS Stimulation Parameters and Experimental Protocol
2.6. Behavioral Tests
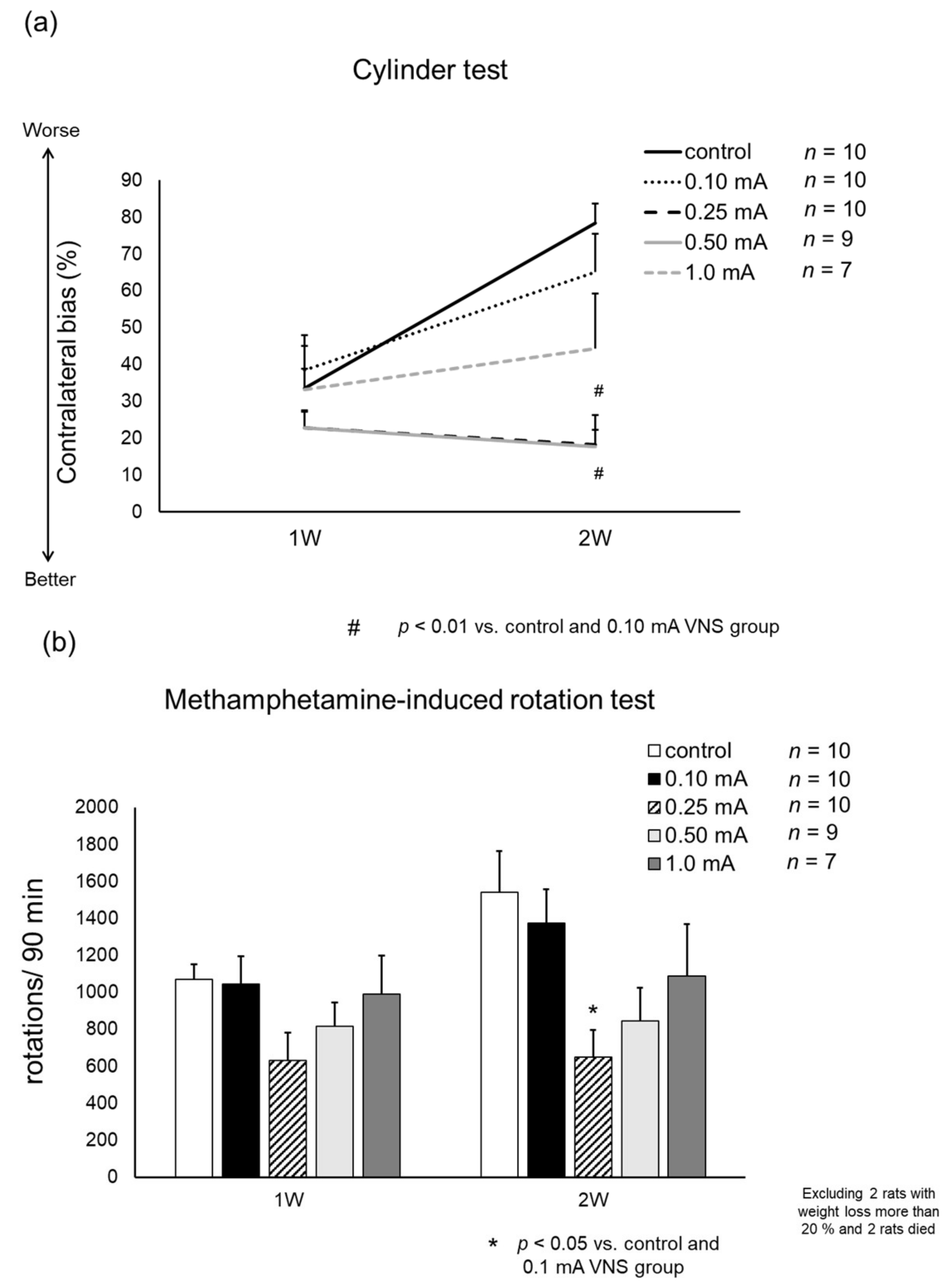
2.6.1. Cylinder Test
2.6.2. Methamphetamine-Induced Rotation Test
2.7. Fixation and Sectioning
2.8. Immunohistochemical Investigations
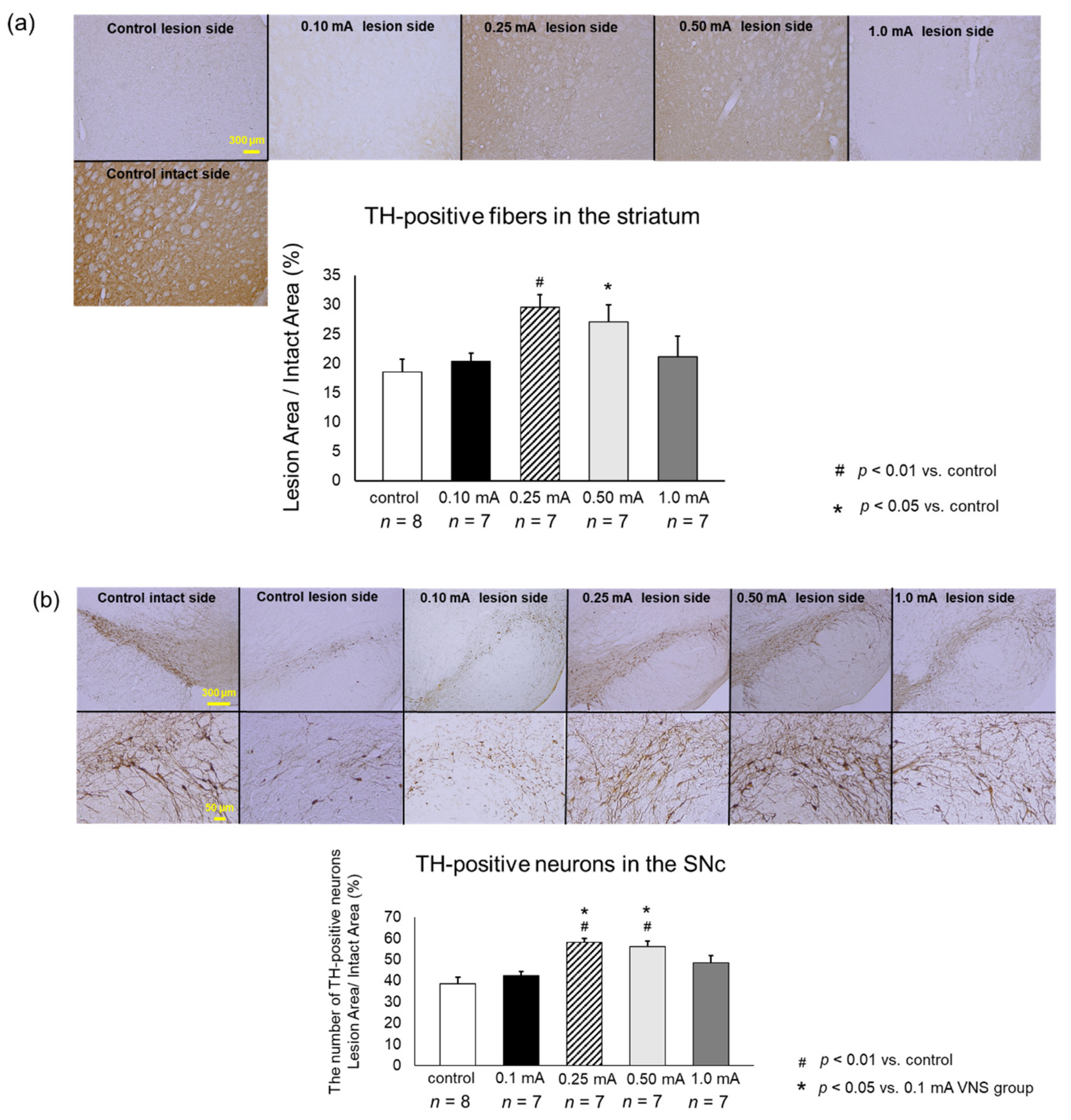
2.8.1. Tyrosine Hydroxylase (TH) Immunohistochemical Investigations
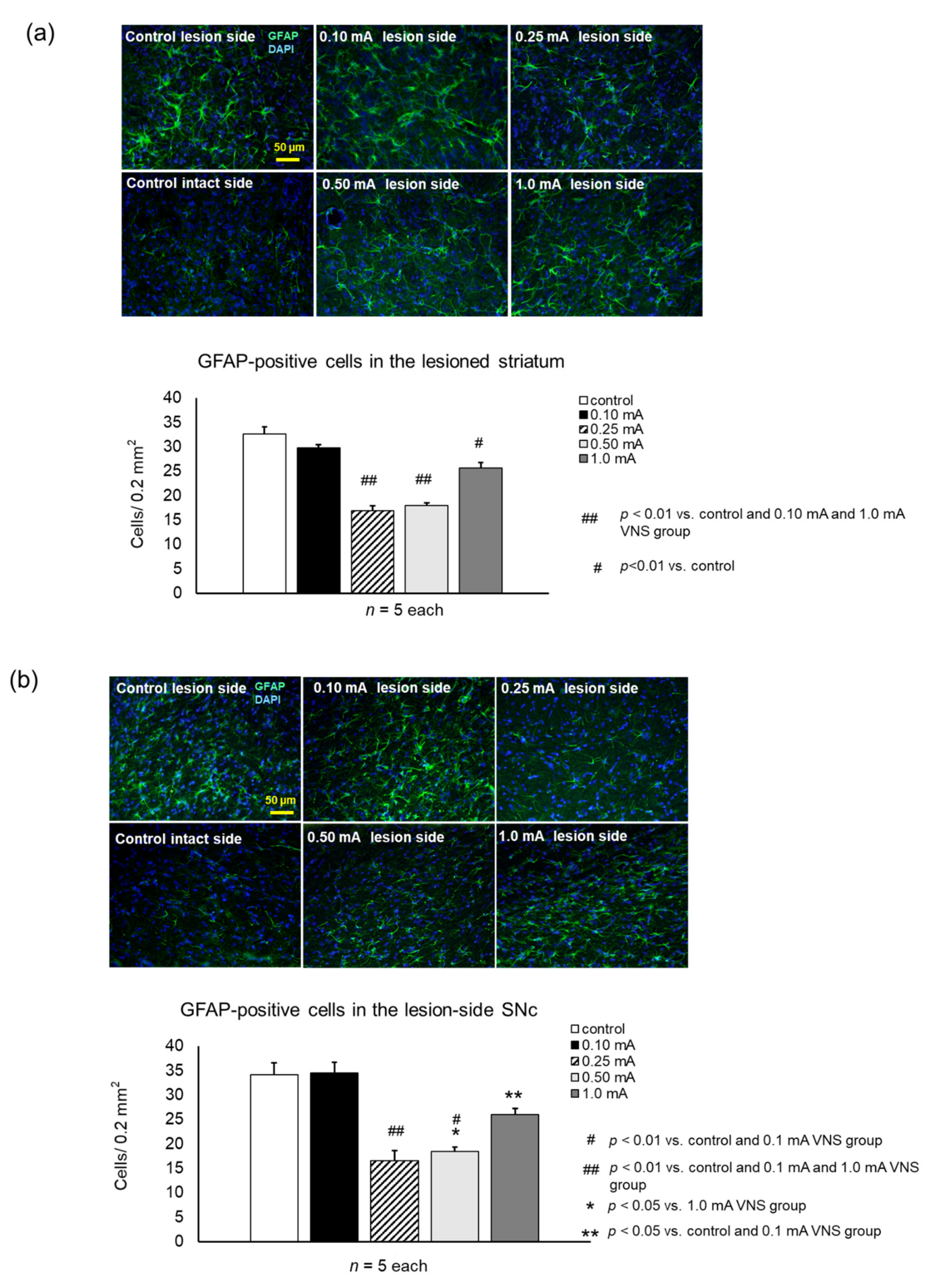
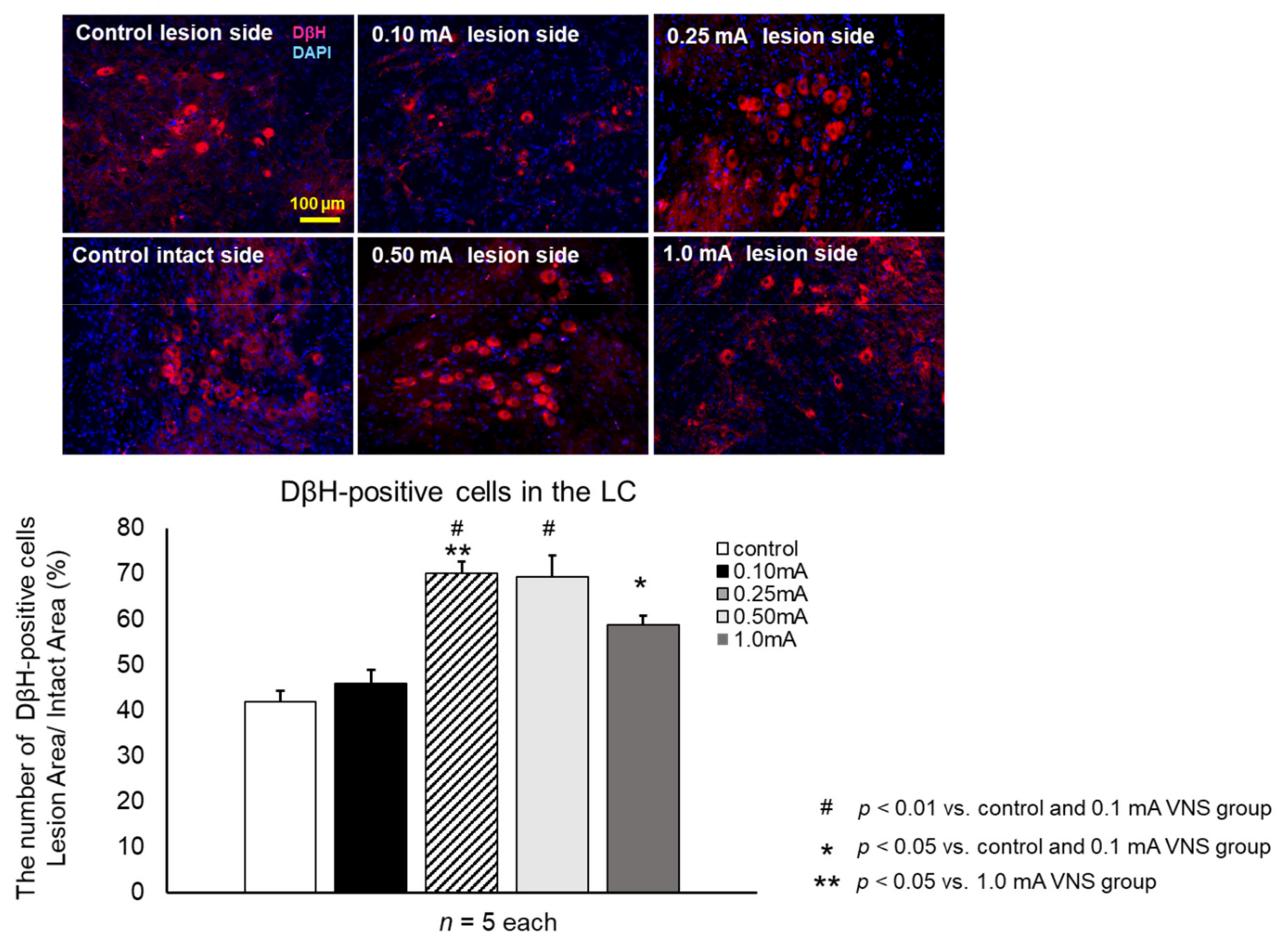
2.8.2. Fluorescent Immunostaining of Microglias, Astrocyte and Noradrenaline Neurons
2.9. Morphological Analyses
2.9.1. TH Immunostaining
2.9.2. Activation of Microglia and Astrocytes in Striatum and SNc
2.9.3. Preservation of Noradrenergic Neurons in the LC
2.10. Statistical Analyses
3. Results
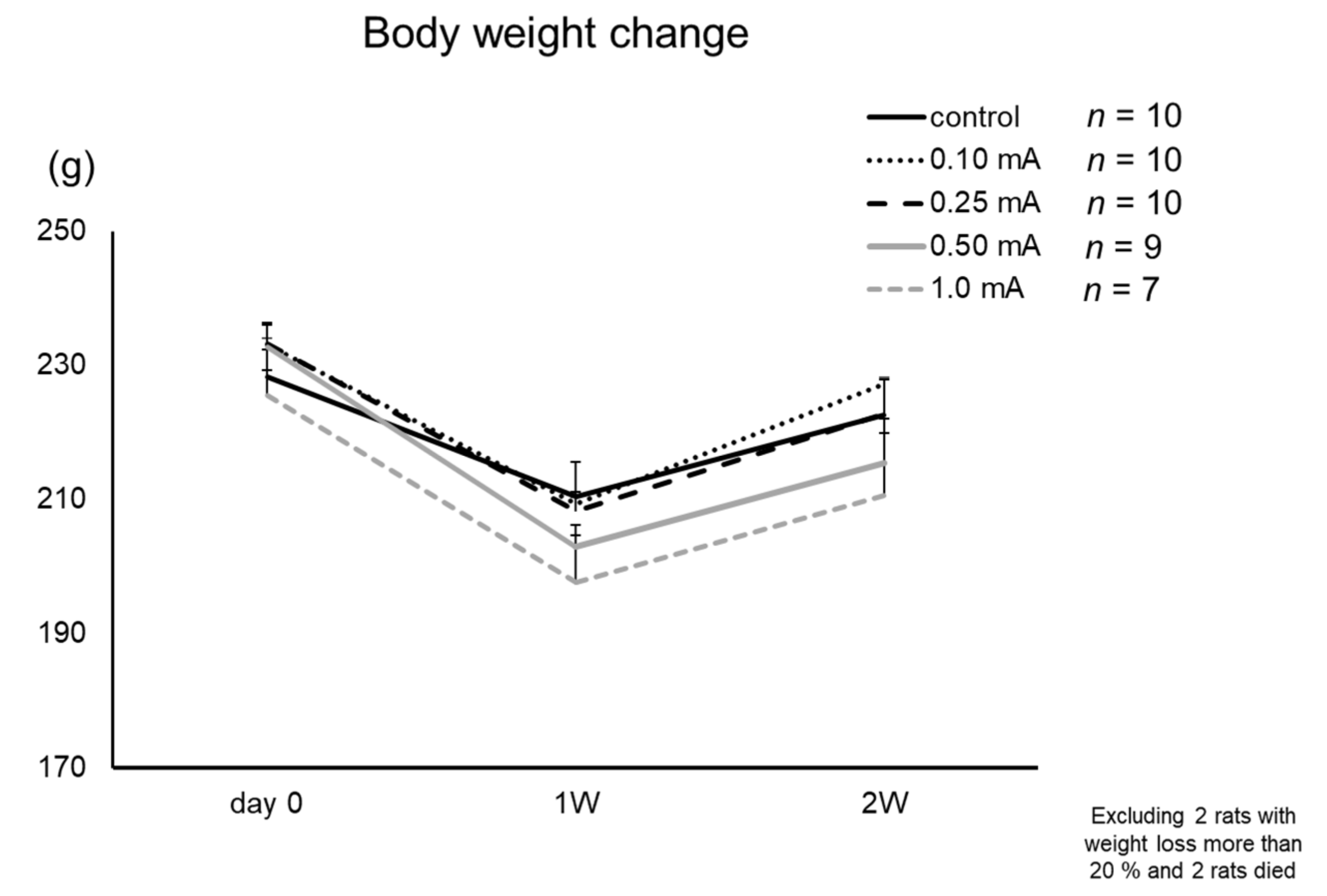
3.1. Body Weight
3.2. Behavioral Tests
3.2.1. Cylinder Test
3.2.2. Methamphetamine-Induced Rotation Test
3.3. Immunohistochemical Investigations
3.3.1. TH
3.3.2. Iba1
3.3.3. GFAP
3.3.4. DβH
4. Discussion
4.1. VNS in Clinical Settings
4.2. Anti-Inflammatory Effects of VNS
4.3. PD Pathogenesis and Inflammation
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Fidel, M.; Torrente, D.; Santos El-Bach, R.; Morales, L.; Gonzalez, J.; Barreto, G.E. Astrocytes Role in Parkinson: A Double-Edged Sword. Neurodegener. Dis. 2013. [Google Scholar] [CrossRef][Green Version]
- Chung, Y.C.; Ko, H.W.; Bok, E.; Park, E.S.; Huh, S.H.; Nam, J.H.; Jin, B.K. The role of neuroinflammation on the pathogenesis of Parkinson’s disease. BMB Rep. 2010, 43, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003, 60, 337–341. [Google Scholar] [CrossRef]
- Baloyannis, S.J.; Costa, V.; Baloyannis, I.S. Morphological alterations of the synapses in the locus coeruleus in Parkinson’s disease. J. Neurol. Sci. 2006, 248, 35–41. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 774–783. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, S.H.; Chu, C.H.; Wang, S.J.; Oyarzabal, E.; Wilson, B.; Sanders, V.; Xie, K.; Wang, Q.; Hong, J.S. A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia 2015, 63, 1057–1072. [Google Scholar] [CrossRef]
- Gesi, M.; Soldani, P.; Giorgi, F.S.; Santinami, A.; Bonaccorsi, I.; Fornai, F. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci. Biobehav. Rev. 2000, 24, 655–668. [Google Scholar] [CrossRef]
- Vazey, E.M.; Aston-Jones, G. The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front. Behav. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Modglin, A.A.; Roosevelt, R.W.; Neese, S.L.; Jensen, R.A.; Browning, R.A.; Clough, R.W. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J. Neurotrauma 2005, 22, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.A.; Khodaparast, N.; Fayyaz, T.; Cheung, R.J.; Ahmed, S.S.; Vrana, W.A.; Rennaker, R.L., 2nd; Kilgard, M.P. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb. Cortex 2012, 22, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Engineer, N.D.; Riley, J.R.; Seale, J.D.; Vrana, W.A.; Shetake, J.A.; Sudanagunta, S.P.; Borland, M.S.; Kilgard, M.P. Reversing pathological neural activity using targeted plasticity. Nature 2011, 470, 101–104. [Google Scholar] [CrossRef]
- Kuwahara, K.; Sasaki, T.; Yasuhara, T.; Kameda, M.; Okazaki, Y.; Hosomoto, K.; Kin, I.; Okazaki, M.; Yabuno, S.; Kawauchi, S.; et al. Long-Term Continuous Cervical Spinal Cord Stimulation Exerts Neuroprotective Effects in Experimental Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Roof, R.L.; Schielke, G.P.; Ren, X.; Hall, E.D. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke 2001, 32, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Shinko, A.; Agari, T.; Kameda, M.; Yasuhara, T.; Kondo, A.; Tayra, J.T.; Sato, K.; Sasaki, T.; Sasada, S.; Takeuchi, H.; et al. Spinal cord stimulation exerts neuroprotective effects against experimental Parkinson’s disease. PLoS ONE 2014, 9, e101468. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Liu, K.; Agari, T.; Yasuhara, T.; Morimoto, J.; Okazaki, M.; Takeuchi, H.; Toyoshima, A.; Sasada, S.; Shinko, A.; et al. Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease. Exp. Neurol. 2016, 275, 220–231. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. The Amphetamine Induced Rotation Test: A Re-Assessment of Its Use as a Tool to Monitor Motor Impairment and Functional Recovery in Rodent Models of Parkinson’s Disease. J. Parkinson’s Dis. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Kadota, T.; Shingo, T.; Yasuhara, T.; Tajiri, N.; Kondo, A.; Morimoto, T.; Yuan, W.J.; Wang, F.; Baba, T.; Tokunaga, K.; et al. Continuous intraventricular infusion of erythropoietin exerts neuroprotective/rescue effects upon Parkinson’s disease model of rats with enhanced neurogenesis. Brain Res. 2009, 1254, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Zicca, A.; Barba, C.; Guerrini, R.; Genitori, L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia 2017, 58 (Suppl. 1), 85–90. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Blake, J. Mechanism of action of non-invasive cervical vagus nerve stimulation for the treatment of primary headaches. Am. J. Manag. Care 2017, 23, S312–S316. [Google Scholar] [PubMed]
- Ay, I.; Lu, J.; Ay, H.; Gregory Sorensen, A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci. Lett. 2009, 459, 147–151. [Google Scholar] [CrossRef]
- Ay, I.; Nasser, R.; Simon, B.; Ay, H. Transcutaneous Cervical Vagus Nerve Stimulation Ameliorates Acute Ischemic Injury in Rats. Brain Stimul. 2016, 9, 166–173. [Google Scholar] [CrossRef]
- Dawson, J.; Pierce, D.; Dixit, A.; Kimberley, T.J.; Robertson, M.; Tarver, B.; Hilmi, O.; McLean, J.; Forbes, K.; Kilgard, M.P.; et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 2016, 47, 143–150. [Google Scholar] [CrossRef]
- Khodaparast, N.; Kilgard, M.P.; Casavant, R.; Ruiz, A.; Qureshi, I.; Ganzer, P.D.; Rennaker, R.L., 2nd; Hays, S.A. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil. Neural Repair 2016, 30, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.A.; Khodaparast, N.; Hulsey, D.R.; Ruiz, A.; Sloan, A.M.; Rennaker, R.L., 2nd; Kilgard, M.P. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014, 45, 3097–3100. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Tan, A.A.; Duke, A.; Neese, S.L.; Clough, R.W.; Browning, R.A.; Jensen, R.A. Recovery of function after vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. J. Neurotrauma 2006, 23, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, D.T.; Schmid, A.N.; Kim, L.J.; Abe, C.M.; Trieu, J.L.; Choua, C.; Hays, S.A.; Kilgard, M.P.; Rennaker, R.L. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J. Neurotrauma 2016, 33, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lin, J.; Lin, J.; Kui, G.; Zhang, J.; Yu, Y. Neuroprotective effects of vagus nerve stimulation on traumatic brain injury. Neural Regen. Res. 2014, 9, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.L.; Cornelison, L.E.; Blankenship, B.A.; Durham, P.L. Vagus nerve stimulation inhibits trigeminal nociception in a rodent model of episodic migraine. Pain Rep. 2017, 2, e628. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Grazzi, L.; de Tommaso, M.; Pierangeli, G.; Martelletti, P.; Rainero, I.; Dorlas, S.; Geppetti, P.; Ambrosini, A.; Sarchielli, P.; et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study. Neurology 2018, 91, e364–e373. [Google Scholar] [CrossRef]
- Merrill, C.A.; Jonsson, M.A.; Minthon, L.; Ejnell, H.; Silander, H.C.; Blennow, K.; Karlsson, M.; Nordlund, A.; Rolstad, S.; Warkentin, S.; et al. Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J. Clin. Psychiatry 2006, 67, 1171–1178. [Google Scholar] [CrossRef]
- Huston, J.M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yuan, R.; Rosas-Ballina, M.; Ashok, M.; Goldstein, R.S.; Chavan, S.; Pavlov, V.A.; et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007, 35, 2762–2768. [Google Scholar] [CrossRef]
- Mihaylova, S.; Killian, A.; Mayer, K.; Pullamsetti, S.S.; Schermuly, R.; Rosengarten, B. Effects of anti-inflammatory vagus nerve stimulation on the cerebral microcirculation in endotoxinemic rats. J. Neuroinflamm. 2012, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, H.; Li, Z.F.; Xiang, H.; Liu, W.; Li, J.G. Vagus nerve stimulation attenuates intestinal epithelial tight junctions disruption in endotoxemic mice through alpha7 nicotinic acetylcholine receptors. Shock 2013, 40, 144–151. [Google Scholar] [CrossRef]
- Kong, S.S.; Liu, J.J.; Hwang, T.C.; Yu, X.J.; Zhao, M.; Zhao, M.; Yuan, B.X.; Lu, Y.; Kang, Y.M.; Wang, B.; et al. Optimizing the parameters of vagus nerve stimulation by uniform design in rats with acute myocardial infarction. PLoS ONE 2012, 7, e42799. [Google Scholar] [CrossRef] [PubMed]
- Uitterdijk, A.; Yetgin, T.; te Lintel Hekkert, M.; Sneep, S.; Krabbendam-Peters, I.; van Beusekom, H.M.; Fischer, T.M.; Cornelussen, R.N.; Manintveld, O.C.; Merkus, D.; et al. Vagal nerve stimulation started just prior to reperfusion limits infarct size and no-reflow. Basic Res. Cardiol. 2015, 110, 508. [Google Scholar] [CrossRef]
- Boland, C.; Collet, V.; Laterre, E.; Lecuivre, C.; Wittebole, X.; Laterre, P.F. Electrical vagus nerve stimulation and nicotine effects in peritonitis-induced acute lung injury in rats. Inflammation 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Stakenborg, N.; Wolthuis, A.M.; Gomez-Pinilla, P.J.; Farro, G.; Di Giovangiulio, M.; Bosmans, G.; Labeeuw, E.; Verhaegen, M.; Depoortere, I.; D’Hoore, A.; et al. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2017, 29. [Google Scholar] [CrossRef]
- Barella, L.F.; Miranda, R.A.; Franco, C.C.; Alves, V.S.; Malta, A.; Ribeiro, T.A.; Gravena, C.; Mathias, P.C.; de Oliveira, J.C. Vagus nerve contributes to metabolic syndrome in high-fat diet-fed young and adult rats. Exp. Physiol. 2015, 100, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.B.; Xu, C.; Tang, Q.Q.; Shen, W.X.; Zhou, J.Z.; Chen, J.D.; Wang, Y.P. Effects and mechanisms of auricular vagus nerve stimulation on high-fat-diet--induced obese rats. Nutrition 2015, 31, 1416–1422. [Google Scholar] [CrossRef]
- Onuora, S. Rheumatoid arthritis: Vagus nerve stimulation reduces RA severity in patients. Nat. Rev. Rheumatol. 2016, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Rau, G.; Starr, P.A. Hemorrhagic complications of microelectrode-guided deep brain stimulation. Stereotact. Funct. Neurosurg. 2003, 80, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, A.J.; Simpson, R.K., Jr. Risks of common complications in deep brain stimulation surgery: Management and avoidance. J. Neurosurg. 2014, 120, 132–139. [Google Scholar] [CrossRef]
- Yoo, P.B.; Lubock, N.B.; Hincapie, J.G.; Ruble, S.B.; Hamann, J.J.; Grill, W.M. High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J. Neural Eng. 2013, 10, 026003. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, F.; Pedersen, N.L.; Tillander, A.; Ludvigsson, J.F.; Ekbom, A.; Svenningsson, P.; Chen, H.; Wirdefeldt, K. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 2017, 88, 1996–2002. [Google Scholar] [CrossRef]
- Hays, S.A.; Rennaker, R.L.; Kilgard, M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013, 207, 275–299. [Google Scholar] [CrossRef]
- Farrand, A.Q.; Helke, K.L.; Gregory, R.A.; Gooz, M.; Hinson, V.K.; Boger, H.A. Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain Stimul. 2017, 10, 1045–1054. [Google Scholar] [CrossRef]
- Groves, D.A.; Bowman, E.M.; Brown, V.J. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett. 2005, 379, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006, 1119, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Krahl, S.E.; Clark, K.B.; Smith, D.C.; Browning, R.A. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 1998, 39, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, A.E.; Caso, J.R.; García-Bueno, B.; Leza, J.C.; Madrigal, J.L. Dual effects of noradrenaline on astroglial production of chemokines and pro-inflammatory mediators. J. Neuroinflamm. 2013, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Polak, P.E.; Kalinin, S.; Feinstein, D.L. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain A J. Neurol. 2011, 134, 665–677. [Google Scholar] [CrossRef]
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.K.; et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063. [Google Scholar] [CrossRef]
- Hays, S.A.; Khodaparast, N.; Ruiz, A.; Sloan, A.M.; Hulsey, D.R.; Rennaker, R.L., 2nd; Kilgard, M.P. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 2014, 25, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Borland, M.S.; Vrana, W.A.; Moreno, N.A.; Fogarty, E.A.; Buell, E.P.; Sharma, P.; Engineer, C.T.; Kilgard, M.P. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul. 2016, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Cracchiolo, M.; Ahmed, U.; Mughrabi, I.; Gabalski, A.; Daytz, A.; Rieth, L.; Becker, L.; Datta-Chaudhuri, T.; Al-Abed, Y.; et al. Quantitative estimation of nerve fiber engagement by vagus nerve stimulation using physiological markers. Brain Stimul. 2020, 13, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.A.; Danaphongse, T.T.; Abe, S.T.; Stevens, M.E.; Ezhil, V.; Seyedahmadi, A.; Adcock, K.S.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. High intensity VNS disrupts VNS-mediated plasticity in motor cortex. Brain Res. 2021, 1756, 147332. [Google Scholar] [CrossRef]
- Braak, H.; Rub, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Petrovitch, H.; White, L.R.; Masaki, K.H.; Tanner, C.M.; Curb, J.D.; Grandinetti, A.; Blanchette, P.L.; Popper, J.S.; Ross, G.W. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 2001, 57, 456–462. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef]
- Killinger, B.A.; Madaj, Z.; Sikora, J.W.; Rey, N.; Haas, A.J.; Vepa, Y.; Lindqvist, D.; Chen, H.; Thomas, P.M.; Brundin, P.; et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Verlinden, T.J.; Rijkers, K.; Hoogland, G.; Herrler, A. Morphology of the human cervical vagus nerve: Implications for vagus nerve stimulation treatment. Acta Neurol. Scand. 2016, 133, 173–182. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678.e23. [Google Scholar] [CrossRef]
- Farrand, A.Q.; Verner, R.S.; McGuire, R.M.; Helke, K.L.; Hinson, V.K.; Boger, H.A. Differential effects of vagus nerve stimulation paradigms guide clinical development for Parkinson’s disease. Brain Stimul. 2020, 13, 1323–1332. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kin, I.; Sasaki, T.; Yasuhara, T.; Kameda, M.; Agari, T.; Okazaki, M.; Hosomoto, K.; Okazaki, Y.; Yabuno, S.; Kawauchi, S.; et al. Vagus Nerve Stimulation with Mild Stimulation Intensity Exerts Anti-Inflammatory and Neuroprotective Effects in Parkinson’s Disease Model Rats. Biomedicines 2021, 9, 789. https://doi.org/10.3390/biomedicines9070789
Kin I, Sasaki T, Yasuhara T, Kameda M, Agari T, Okazaki M, Hosomoto K, Okazaki Y, Yabuno S, Kawauchi S, et al. Vagus Nerve Stimulation with Mild Stimulation Intensity Exerts Anti-Inflammatory and Neuroprotective Effects in Parkinson’s Disease Model Rats. Biomedicines. 2021; 9(7):789. https://doi.org/10.3390/biomedicines9070789
Chicago/Turabian StyleKin, Ittetsu, Tatsuya Sasaki, Takao Yasuhara, Masahiro Kameda, Takashi Agari, Mihoko Okazaki, Kakeru Hosomoto, Yosuke Okazaki, Satoru Yabuno, Satoshi Kawauchi, and et al. 2021. "Vagus Nerve Stimulation with Mild Stimulation Intensity Exerts Anti-Inflammatory and Neuroprotective Effects in Parkinson’s Disease Model Rats" Biomedicines 9, no. 7: 789. https://doi.org/10.3390/biomedicines9070789
APA StyleKin, I., Sasaki, T., Yasuhara, T., Kameda, M., Agari, T., Okazaki, M., Hosomoto, K., Okazaki, Y., Yabuno, S., Kawauchi, S., Kuwahara, K., Morimoto, J., Kin, K., Umakoshi, M., Tomita, Y., Tajiri, N., Borlongan, C. V., & Date, I. (2021). Vagus Nerve Stimulation with Mild Stimulation Intensity Exerts Anti-Inflammatory and Neuroprotective Effects in Parkinson’s Disease Model Rats. Biomedicines, 9(7), 789. https://doi.org/10.3390/biomedicines9070789







