Prognostic Significance of the Microbiome and Stromal Cells Phenotype in Esophagus Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Immunohistochemical Study
2.4. Quantitative PCR (qPCR)
2.5. 16S rRNA Gene Library Preparation and MiSeq Sequencing
2.6. Bioinformatics Treatment
Availability of Data
2.7. Statistical Analyses
3. Results
3.1. Clinical Samples
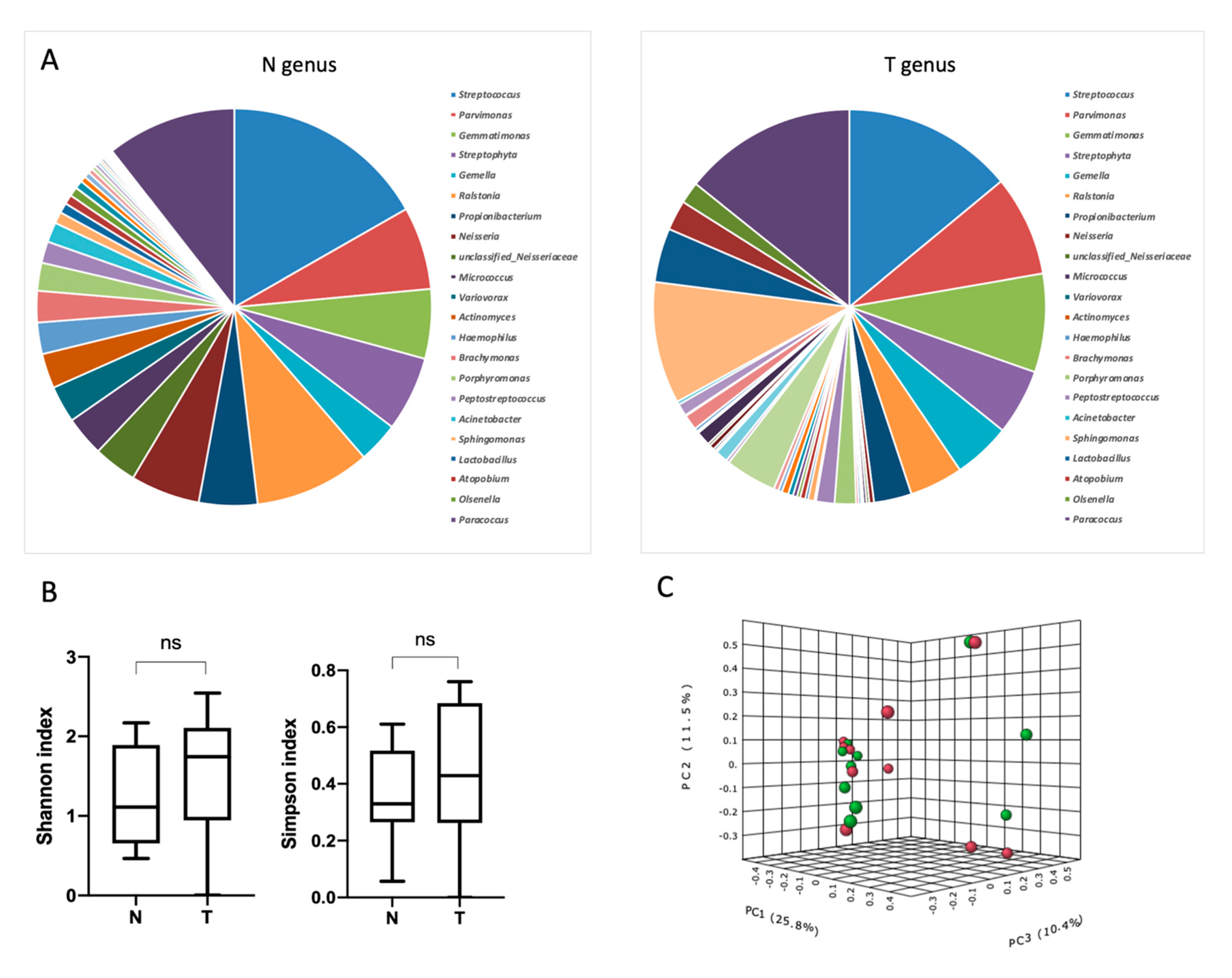
3.2. Characterization of Esophageal Bacterial Communities
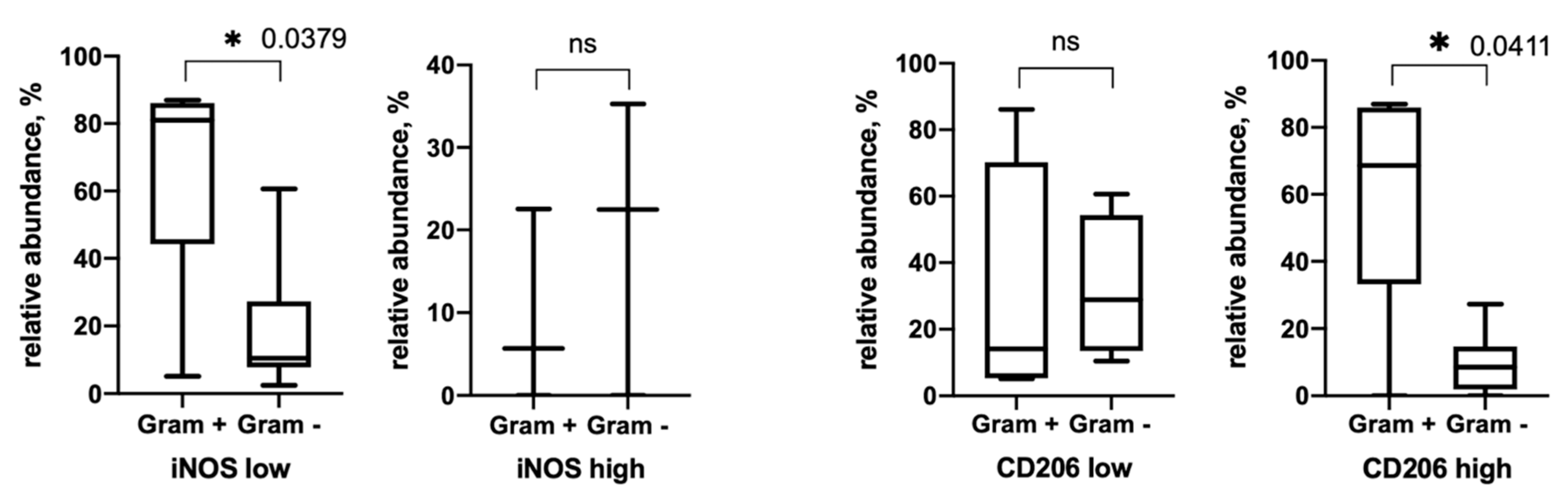
3.3. Gram+ and Gram− Bacteria Relative Abundance
3.4. Alpha-Diversity Depends on Tumor Stroma Phenotype
3.5. Correlation and Survival Analysis of Bacterial Burden and Stroma
3.6. Survival Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Glenn, T.F. Esophageal cancer. Facts, figures, and screening. Gastroenterol. Nurs. 2001, 24, 271–273; quiz 274–275. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, X.; Li, Y.; Tian, X.; Wang, Y.; Huang, M.; Ren, L.; Zhou, L.; Ding, Z.; Zhu, J.; et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Ann. Surg. Oncol. 2019, 26, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez, A.J.; Hoffmann, C.; Muir, A.B.; Dods, K.K.; Spergel, J.M.; Bushman, F.D.; Wang, M.L. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Snider, E.J.; Compres, G.; Freedberg, D.E.; Khiabanian, H.; Nobel, Y.R.; Stump, S.; Uhlemann, A.C.; Lightdale, C.J.; Abrams, J.A. Alterations to the Esophageal Microbiome Associated with Progression from Barrett’s Esophagus to Esophageal Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1687–1693. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.R.F.; Walker, A.W.; O’Donovan, M.; Parkhill, J.; Fitzgerald, R.C. A non-endoscopic device to sample the oesophageal microbiota: A case-control study. Lancet Gastroenterol. Hepatol. 2017, 2, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, K.; Haensch, W.; Roefzaad, C.; Schlag, P.M. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001, 61, 3932–3936. [Google Scholar]
- Kato, T.; Noma, K.; Ohara, T.; Kashima, H.; Katsura, Y.; Sato, H.; Komoto, S.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-Associated Fibroblasts Affect Intratumoral CD8(+) and FoxP3(+) T Cells Via IL6 in the Tumor Microenvironment. Clin. Cancer Res. 2018, 24, 4820–4833. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, L.; Li, P.; Song, M.; Qin, G.; Gao, Q.; Zhang, Z.; Yue, D.; Wang, D.; Nan, S.; et al. Dual TGF-beta and PD-1 blockade synergistically enhances MAGE-A3-specific CD8(+) T cell response in esophageal squamous cell carcinoma. Int. J. Cancer 2018, 143, 2561–2574. [Google Scholar] [CrossRef] [Green Version]
- Ichihara, F.; Kono, K.; Takahashi, A.; Kawaida, H.; Sugai, H.; Fujii, H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. 2003, 9, 4404–4408. [Google Scholar] [PubMed]
- Vacchelli, E.; Semeraro, M.; Enot, D.P.; Chaba, K.; Poirier Colame, V.; Dartigues, P.; Perier, A.; Villa, I.; Rusakiewicz, S.; Gronnier, C.; et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget 2015, 6, 20840–20850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovaleva, O.V.; Rashidova, M.A.; Samoilova, D.V.; Podlesnaya, P.A.; Mochalnikova, V.V.; Gratchev, A. Immunosuppressive Phenotype of Esophagus Tumors Stroma. Anal. Cell Pathol. 2020, 2020, 5424780. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Wang, X.; Li, F.; Li, S.; Li, M.; Peng, H.; Yang, L.; Liu, C.; Pang, L.; et al. Prognostic impact of tumor-associated macrophage infiltration in esophageal cancer: A meta-analysis. Future Oncol. 2019, 15, 2303–2317. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, K.; Liu, J.H.; Jiang, X.L.; Wang, X.L.; Chen, Y.Z.; Li, S.G.; Zou, H.; Pang, L.J.; Liu, C.X.; et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Peters, J.H.; Nieman, D.; Sharma, M.; Watson, T.; Yu, J. Macrophage subtype predicts lymph node metastasis in oesophageal adenocarcinoma and promotes cancer cell invasion in vitro. Br. J. Cancer 2015, 113, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Maniecki, M.B.; Etzerodt, A.; Ulhoi, B.P.; Steiniche, T.; Borre, M.; Dyrskjot, L.; Orntoft, T.F.; Moestrup, S.K.; Moller, H.J. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int. J. Cancer 2012, 131, 2320–2331. [Google Scholar] [CrossRef]
- Kovaleva, O.; Podlesnaya, P.; Rashidova, M.; Samoilova, D.; Petrenko, A.; Zborovskaya, I.; Mochalnikova, V.; Kataev, V.; Khlopko, Y.; Plotnikov, A.; et al. Lung Microbiome Differentially Impacts Survival of Patients with Non-Small Cell Lung Cancer Depending on Tumor Stroma Phenotype. Biomedicines 2020, 8, 349. [Google Scholar] [CrossRef]
- Conway Morris, A.; Gadsby, N.; McKenna, J.P.; Hellyer, T.P.; Dark, P.; Singh, S.; Walsh, T.S.; McAuley, D.F.; Templeton, K.; Simpson, A.J.; et al. 16S pan-bacterial PCR can accurately identify patients with ventilator-associated pneumonia. Thorax 2017, 72, 1046–1048. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UCHIME2: Improved chimera prediction for amplicon sequencing. BioRxiv 2016, 074252. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Bini, E.J.; Yang, L.; Zhou, M.; Francois, F.; Blaser, M.J. Bacterial biota in the human distal esophagus. Proc. Natl. Acad. Sci. USA 2004, 101, 4250–4255. [Google Scholar] [CrossRef] [Green Version]
- Fillon, S.A.; Harris, J.K.; Wagner, B.D.; Kelly, C.J.; Stevens, M.J.; Moore, W.; Fang, R.; Schroeder, S.; Masterson, J.C.; Robertson, C.E.; et al. Novel device to sample the esophageal microbiome—The esophageal string test. PLoS ONE 2012, 7, e42938. [Google Scholar] [CrossRef]
- Harris, J.K.; Fang, R.; Wagner, B.D.; Choe, H.N.; Kelly, C.J.; Schroeder, S.; Moore, W.; Stevens, M.J.; Yeckes, A.; Amsden, K.; et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE 2015, 10, e0128346. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, R.; Hou, G.; Ming, W.; Fan, T.; Chen, L.; Zhang, L.; Jiang, W.; Wang, W.; Lu, Z.; et al. Characterization of the Esophageal Microbiota and Prediction of the Metabolic Pathways Involved in Esophageal Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 268. [Google Scholar] [CrossRef]
- Lin, E.W.; Karakasheva, T.A.; Hicks, P.D.; Bass, A.J.; Rustgi, A.K. The tumor microenvironment in esophageal cancer. Oncogene 2016, 35, 5337–5349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rao, Y.; Guo, X.; Liu, N.; Liu, S.; Wen, P.; Li, S.; Li, Y. Oral Microbiome in Patients with Oesophageal Squamous Cell Carcinoma. Sci. Rep. 2019, 9, 19055. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, D.; Malekzadeh, R.; Ploner, A.; Shakeri, R.; Sotoudeh, M.; Fahimi, S.; Nasseri-Moghaddam, S.; Kamangar, F.; Abnet, C.C.; Winckler, B.; et al. Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Sci. Rep. 2015, 5, 8820. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.I.; Yoon, J.; Sul, W.J.; Kim, J.F.; Kim, J. Alterations in Gastric Microbial Communities Are Associated with Risk of Gastric Cancer in a Korean Population: A Case-Control Study. Cancers 2020, 12, 2619. [Google Scholar] [CrossRef]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Dalmasso, G.; Cougnoux, A.; Delmas, J.; Darfeuille-Michaud, A.; Bonnet, R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes 2014, 5, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, T.; Fowler, V.G., Jr.; Shelburne, S.A., 3rd. Invasive gram-positive bacterial infection in cancer patients. Clin. Infect. Dis. 2014, 59 (Suppl. 5), S331–S334. [Google Scholar] [CrossRef] [Green Version]




| Category | All Cases |
|---|---|
| Age | |
| ≤60 | 26 (54%) |
| >60 | 22 (46%) |
| Gender | |
| male | 36 (75%) |
| female | 12 (25%) |
| Stage | |
| I–II | 25 (52%) |
| III–IV | 23 (48%) |
| Nodal status | |
| N- | 25 (52%) |
| N+ | 23 (48%) |
| Histologic grade | |
| G1/2 | 38 (79%) |
| G3 | 10 (21%) |
| # | Histology | TNM | Stage | Grade |
|---|---|---|---|---|
| 1 | SCC | T4N3M0 | III | G3 |
| 2 | SCC | T4N0M0 | III | G2 |
| 3 | SCC | T3N0M0 | II | G2 |
| 4 | SCC | T4NxMx | IV | G3 |
| 5 | SCC | T3N0M0 | II | G1 |
| 6 | SCC | T1N0M0 | I | G2 |
| 7 | SCC | T2N2M0 | III | G2 |
| 8 | SCC | T3N0M0 | II | G3 |
| 9 | SCC | T3N1M0 | III | G2 |
| 10 | SCC | T2N0M0 | II | G3 |
| Shannon Index ± SD | Simpson Index ± SD | |||||
|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | |
| CD68 | 2029 ± 0.15 | 1084 ± 0.35 | 0.0556 | 0.7650 ± 0.03 | 0.4889 ± 0.14 | 0.1508 |
| CD163 | 1674 ± 0.30 | 1282 ± 0.39 | 0.5167 | 0.6552 ± 0.11 | 0.5612 ± 0.10 | 0.5167 |
| CD206 | 1493 ± 0.24 | 1599 ± 0.38 | 0.4762 | 0.6690 ± 0.08 | 0.5990 ± 0.13 | 0.7619 |
| PU.1 | 1953 ± 0.14 | 0.962 ± 0.31 | 0.1143 | 0.7643 ± 0.03 | 0.4210 ± 0.15 | 0.1143 |
| PD-L1 | 1717 ± 0.36 | 1316 ± 0.25 | 0.2571 | 0.6731 ± 0.11 | 0.5195 ± 0.06 | 0.1167 |
| iNOS | 1752 ± 0.24 | 1101 ± 0.39 | 0.1833 | 0.6783 ± 0.06 | 0.5073 ± 0.21 | 0.6667 |
| CD3 | 1874 ± 0.09 | 1081 ± 0.31 | 0.2571 | 0.7434 ± 0.02 | 0.4524 ± 0.11 | 0.2571 |
| CD8 | 1970 ± 0.12 | 0.593 ± 0.30 | 0.0167 * | 0.7642 ± 0.02 | 0.3069 ± 0.15 | 0.0167 * |
| FOXP3 | 1584 ± 0.29 | 1447 ± 0.41 | 0.8889 | 0.6244 ± 0.10 | 0.6374 ± 0.11 | 0.5333 |
| Bacterial Burden vs. Age | Bacterial Burden vs. Gender | Bacterial Burden Vs. Grade | Bacterial Burden vs. Stage | Bacterial Burden vs. n | |
|---|---|---|---|---|---|
| Spearman r | |||||
| r | 0.03016 | −0.01079 | 0.1418 | 0.09904 | 0.01886 |
| 95% confidence interval | −0.2674 to 0.3225 | −0.3050 to 0.2853 | −0.1600 to 0.4194 | −0.2020 to 0.3830 | −0.2778 to 0.3123 |
| p value | |||||
| p (two-tailed) | 0.8405 | 0.9426 | 0.3416 | 0.5078 | 0.8998 |
| Bacterial Burden vs. CD68 | Bacterial Burden vs. CD163 | Bacterial Burden vs. CD206 | Bacterial Burden vs. CD204 | Bacterial Burden vs. PU1 | |
| Spearman r | |||||
| r | −0.08035 | 0.003585 | −0.3976 | −0.1854 | −0.1761 |
| 95% confidence interval | −0.3668 to 0.2200 | −0.2919 to 0.2984 | −0.6200 to −0.1161 | −0.4556 to 0.1162 | −0.4480 to 0.1256 |
| p value | |||||
| p (two-tailed) | 0.5914 | 0.9809 | 0.0056 * | 0.2123 | 0.2365 |
| Bacterial Burden vs. PD-L1 | Bacterial Burden vs. iNOS | Bacterial Burden vs. CD3 | Bacterial Burden vs. CD8 | Bacterial Burden vs. FoxP3 | |
| Spearman r | |||||
| r | 0.2535 | −0.2953 | 0.07555 | 0.09317 | −0.08894 |
| 95% confidence interval | −0.04507 to 0.5104 | −0.5431 to −0.0001028 | −0.2246 to 0.3626 | −0.2077 to 0.3779 | −0.3743 to 0.2118 |
| p value | |||||
| p (two-tailed) | 0.0856 | 0.0439 * | 0.6138 | 0.5334 | 0.5522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovaleva, O.; Podlesnaya, P.; Rashidova, M.; Samoilova, D.; Petrenko, A.; Mochalnikova, V.; Kataev, V.; Khlopko, Y.; Plotnikov, A.; Gratchev, A. Prognostic Significance of the Microbiome and Stromal Cells Phenotype in Esophagus Squamous Cell Carcinoma. Biomedicines 2021, 9, 743. https://doi.org/10.3390/biomedicines9070743
Kovaleva O, Podlesnaya P, Rashidova M, Samoilova D, Petrenko A, Mochalnikova V, Kataev V, Khlopko Y, Plotnikov A, Gratchev A. Prognostic Significance of the Microbiome and Stromal Cells Phenotype in Esophagus Squamous Cell Carcinoma. Biomedicines. 2021; 9(7):743. https://doi.org/10.3390/biomedicines9070743
Chicago/Turabian StyleKovaleva, Olga, Polina Podlesnaya, Madina Rashidova, Daria Samoilova, Anatoly Petrenko, Valeria Mochalnikova, Vladimir Kataev, Yuri Khlopko, Andrey Plotnikov, and Alexei Gratchev. 2021. "Prognostic Significance of the Microbiome and Stromal Cells Phenotype in Esophagus Squamous Cell Carcinoma" Biomedicines 9, no. 7: 743. https://doi.org/10.3390/biomedicines9070743
APA StyleKovaleva, O., Podlesnaya, P., Rashidova, M., Samoilova, D., Petrenko, A., Mochalnikova, V., Kataev, V., Khlopko, Y., Plotnikov, A., & Gratchev, A. (2021). Prognostic Significance of the Microbiome and Stromal Cells Phenotype in Esophagus Squamous Cell Carcinoma. Biomedicines, 9(7), 743. https://doi.org/10.3390/biomedicines9070743









