Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naïve Patients with Major Depression in Young Adulthood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Data
2.3. Serum and Plasma Separation
2.4. Measurement of Biomarkers
2.5. Statistical Analysis
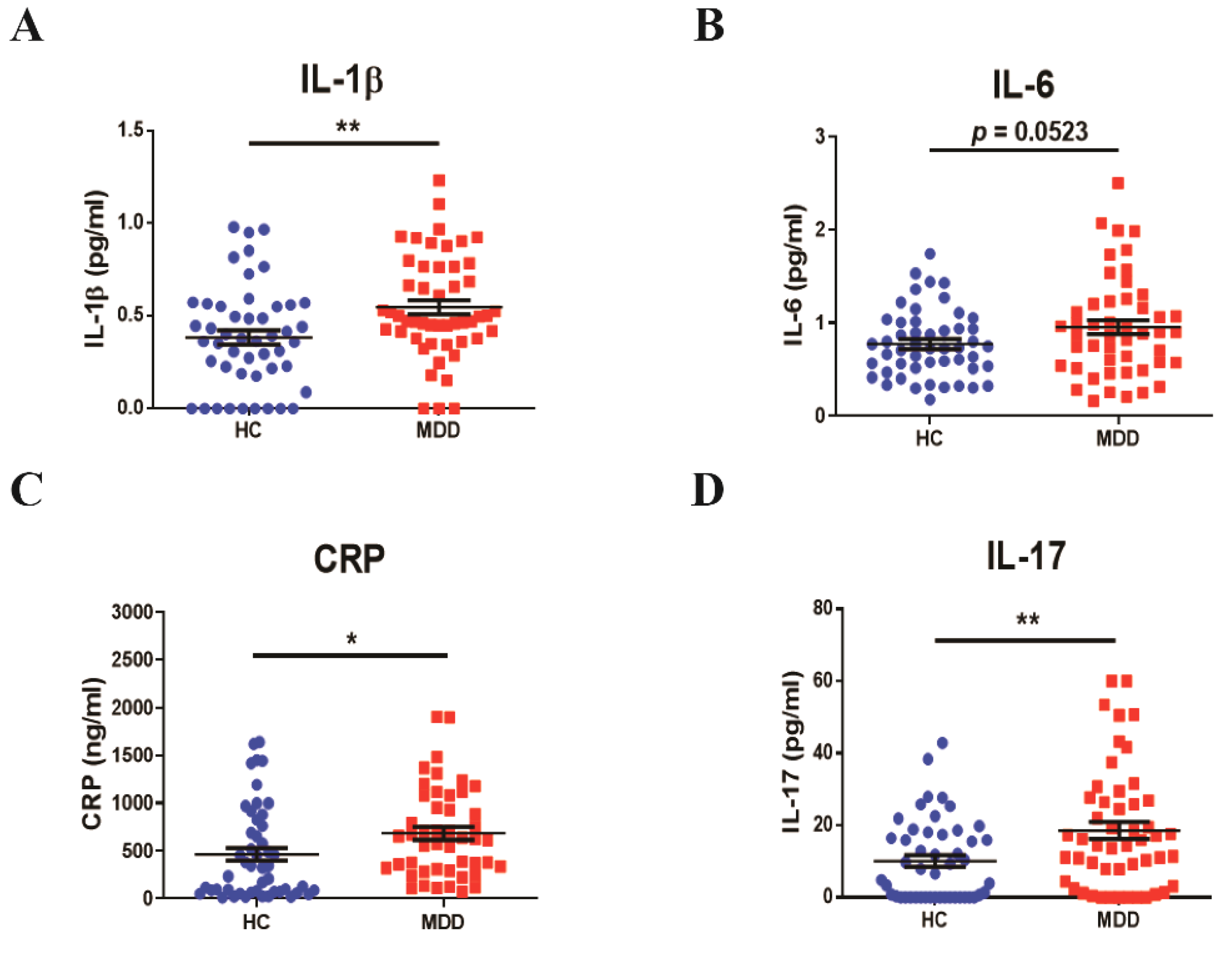
3. Results
3.1. Profiles of Baseline Inflammatory Cytokines in Young Adult Patients with MDD
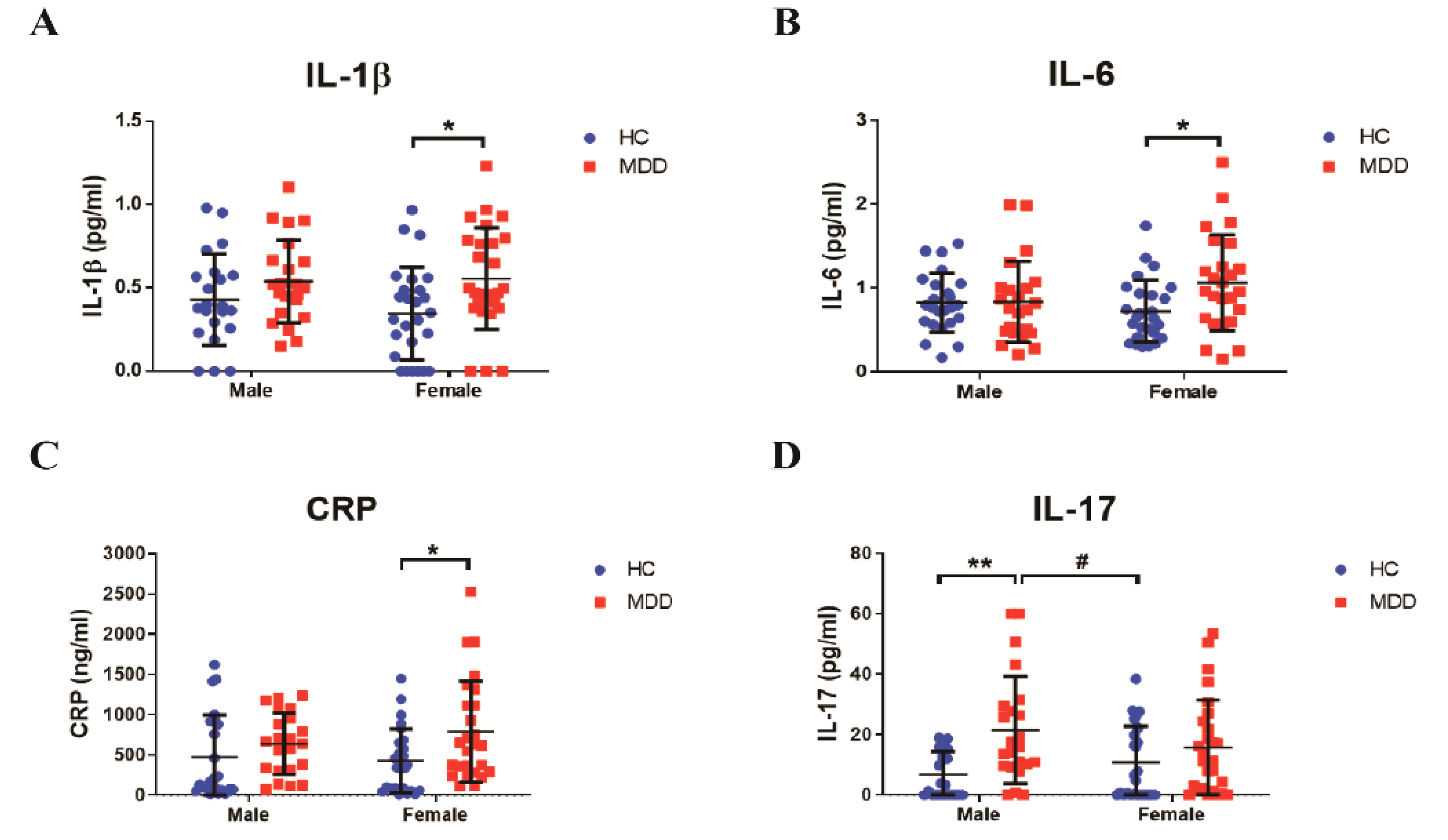
3.2. Sex Differences in Inflammatory Response in Young Adult Patients with MDD
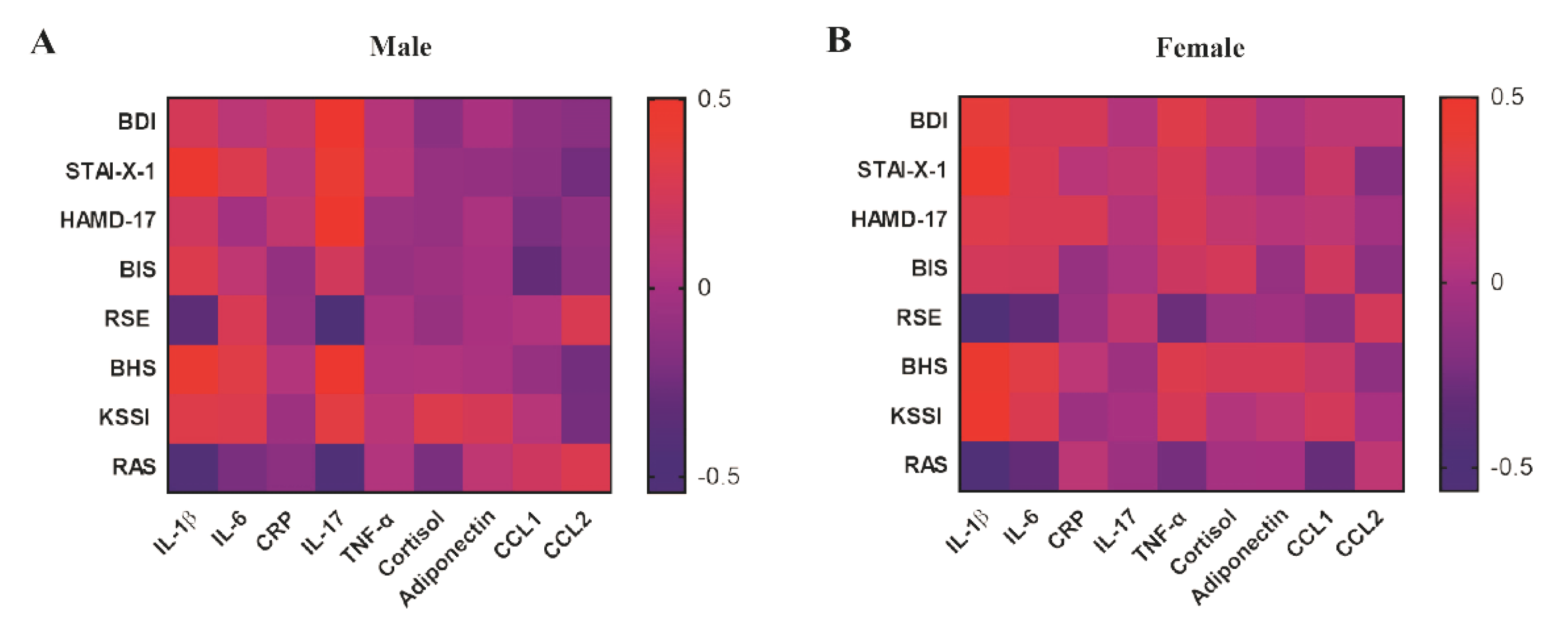
3.3. Correlation between Depressive Symptoms and Inflammatory Biomarkers in Young Adulthood
3.4. Predictable Diagnostic Marker from the Baseline of the Inflammatory Biomarkers in Young Adult Patients with MDD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDI | Beck Depression Inventory |
| BHS | Beck Hopelessness Scale |
| BIS | Barratt Impulsiveness Scale |
| HAMD-17 | Hamilton Rating Scale for Depression-17 |
| HC | healthy control |
| KSI | KAIST Scale for Suicidal Ideation |
| MDD | major depressive disorder |
| RAS | Resilience Appraisal Scale |
| RSE | Rosenberg Self-Esteem Scale |
| STAI-X-1 | State-Trait Anxiety Inventory-X-1 |
| SSRI | Serotonin Selective Reuptake Inhibitor |
References
- Kendler, K.S.; Gardner, C.O.; Prescott, C.A. Toward a comprehensive developmental model for major depression in women. Am. J. Psychiatry 2002, 159, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.S.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Borges, G.; Bromet, E.J.; Bruffaerts, R.; de Girolamo, G.; de Graaf, R.; Gureje, O.; et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the who world mental health surveys. Lancet 2007, 370, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.J. Depression is the leading cause of disability around the world. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef]
- Eaton, W.W.; Shao, H.; Nestadt, G.; Lee, H.B.; Bienvenu, O.J.; Zandi, P. Population-based study of first onset and chronicity in major depressive disorder. Arch. Gen. Psychiatry 2008, 65, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Mojtabai, R.; Olfson, M.; Han, B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 2016, 138, e20161878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Klein, D.N.; Glenn, C.R.; Kosty, D.B.; Seeley, J.R.; Rohde, P.; Lewinsohn, P.M. Predictors of first lifetime onset of major depressive disorder in young adulthood. J. Abnorm. Psychol. 2013, 122, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, J.L.; Garber, J. The prevention of depressive symptoms in children and adolescents: A meta-analytic review. J. Consult. Clin. Psychol. 2006, 74, 401–415. [Google Scholar] [CrossRef] [Green Version]
- Orsolini, L.; Latini, R.; Pompili, M.; Serafini, G.; Volpe, U.; Vellante, F.; Fornaro, M.; Valchera, A.; Tomasetti, C.; Fraticelli, S.; et al. Understanding the complex of suicide in depression: From research to clinics. Psychiatry Investig. 2020, 17, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Cash, S.J.; Bridge, J.A. Epidemiology of youth suicide and suicidal behavior. Curr. Opin. Pediatr. 2009, 21, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuppili, P.P.; Nabhinani, N. Age and major depressive disorder: What factors should be investigated? Lancet Psychiatry 2018, 5, 784. [Google Scholar] [CrossRef] [Green Version]
- Rethorst, C.D.; Toups, M.S.; Greer, T.L.; Nakonezny, P.A.; Carmody, T.J.; Grannemann, B.D.; Huebinger, R.M.; Barber, R.C.; Trivedi, M.H. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol. Psychiatry 2013, 18, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Bui, E.; Rogers, A.H.; Walton, Z.E.; Ross, R.; Zeng, M.; Nadal-Vicens, M.; Mischoulon, D.; Baker, A.W.; Keshaviah, A.; et al. Inflammatory cytokines in major depressive disorder: A case-control study. Aust. N. Z. J. Psychiatry 2017, 51, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Giuliani, F. The role of inflammation in depression and fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naive patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Swiergiel, A.H.; de Beaurepaire, R. Cytokines as mediators of depression: What can we learn from animal studies? Neurosci. Biobehav. Rev. 2005, 29, 891–909. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, Y.; Zheng, W.; Liu, W.; Wang, C.; Lan, X.; Deng, X.; Xu, Y.; Zhang, B.; Ning, Y. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl. Psychiatry 2020, 10, 246. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with c-reactive protein, il-1, and il-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 2018, 23, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derry, H.M.; Padin, A.C.; Kuo, J.L.; Hughes, S.; Kiecolt-Glaser, J.K. Sex differences in depression: Does inflammation play a role? Curr. Psychiatry Rep. 2015, 17, 78. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV Axis i Disorders Research Version (Scid-I); New York State Psychiatric Institue Biometrics Research: New York, NY, USA, 1996. [Google Scholar]
- Beck, A.T. Depression: Clinical, Experimental, and Theoretical Aspects; Harper & Row: New York, NY, USA, 1967. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, M. Society and the Adolescent Self-Image; Princeton University Press: Princeton, NJ, USA, 1965; 326p. [Google Scholar]
- Barratt, E.S. Impulsiveness and aggression. In Violence and Mental Disorder Developments in Risk Assessment; Monahan, J., Steadman, H.J., Eds.; The University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Beck, A.T.; Weissman, A.; Lester, D.; Trexler, L. The measurement of pessimism: The hopelessness scale. J. Consult. Clin. Psychol. 1974, 42, 861–865. [Google Scholar] [CrossRef]
- Shim, G.; Jeong, B. Predicting suicidal ideation in college students with mental health screening questionnaires. Psychiatry Investig. 2018, 15, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Gooding, P.; Tarrier, N. Suicide risk in schizophrenia: Explanatory models and clinical implications, the schematic appraisal model of suicide (sams). Psychol. Psychother 2008, 81, 55–77. [Google Scholar] [CrossRef]
- Kennis, M.; Gerritsen, L.; van Dalen, M.; Williams, A.; Cuijpers, P.; Bockting, C. Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 321–338. [Google Scholar] [CrossRef] [Green Version]
- Hacimusalar, Y.; Esel, E. Suggested biomarkers for major depressive disorder. Noro Psikiyatr. Ars. 2018, 55, 280–290. [Google Scholar] [CrossRef]
- Zisook, S.; Lesser, I.; Stewart, J.W.; Wisniewski, S.R.; Balasubramani, G.K.; Fava, M.; Gilmer, W.S.; Dresselhaus, T.R.; Thase, M.E.; Nierenberg, A.A.; et al. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 2007, 164, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Obrosky, S.; George, C. The course of major depressive disorder from childhood to young adulthood: Recovery and recurrence in a longitudinal observational study. J. Affect. Disord. 2016, 203, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.; Hicks, B.M.; Foster, K.T.; McGue, M.; Iacono, W.G. Age of onset and course of major depressive disorder: Associations with psychosocial functioning outcomes in adulthood. Psychol. Med. 2015, 45, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Stegenga, B.T.; Kamphuis, M.H.; King, M.; Nazareth, I.; Geerlings, M.I. The natural course and outcome of major depressive disorder in primary care: The predict-nl study. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Raison, C.L.; Miller, A.H. Is depression an inflammatory disorder? Curr. Psychiatry Rep. 2011, 13, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Vogelzangs, N.; Duivis, H.E.; Beekman, A.T.; Kluft, C.; Neuteboom, J.; Hoogendijk, W.; Smit, J.H.; de Jonge, P.; Penninx, B.W. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 2012, 2, e79. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Dong, X.; Chen, J. Adiponectin and depression: A meta-analysis. Biomed. Rep. 2015, 3, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Dienes, K.A.; Hazel, N.A.; Hammen, C.L. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology 2013, 38, 927–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Davami, M.H.; Baharlou, R.; Ahmadi Vasmehjani, A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M.R. Elevated il-17 and tgf-beta serum levels: A positive correlation between t-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic Clin. Neurosci. 2016, 7, 137–142. [Google Scholar] [PubMed]
- Jha, M.K.; Miller, A.H.; Minhajuddin, A.; Trivedi, M.H. Association of t and non-t cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology 2018, 95, 1–7. [Google Scholar] [CrossRef]
- Beurel, E.; Harrington, L.E.; Jope, R.S. Inflammatory t helper 17 cells promote depression-like behavior in mice. IBiol. Psychiatry 2013, 73, 622–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Suh, Y.H.; Chang, K.A. Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol. Brain 2021, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Labaka, A.; Goni-Balentziaga, O.; Lebena, A.; Perez-Tejada, J. Biological sex differences in depression: A systematic review. IBiol. Res. Nurs. 2018, 20, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Sacher, J. Stress, sex hormones, inflammation, and major depressive disorder: Extending social signal transduction theory of depression to account for sex differences in mood disorders. Psychopharmacology 2019, 236, 3063–3079. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Muniz, G.A.; Wood, S.K. Sex differences in the inflammatory consequences of stress: Implications for pharmacotherapy. J. Pharmacol. Exp. Ther. 2020, 375, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Thornton, L.M.; Gardner, C.O. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. Am. J. Psychiatry 2000, 157, 1243–1251. [Google Scholar] [CrossRef]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2016, 18, 447–457. [Google Scholar] [PubMed]
- Leuner, B.; Mendolia-Loffredo, S.; Shors, T.J. Males and females respond differently to controllability and antidepressant treatment. IBiol. Psychiatry 2004, 56, 964–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodes, G.E.; Hill-Smith, T.E.; Suckow, R.F.; Cooper, T.B.; Lucki, I. Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J. Pharmacol. Exp. Ther. 2010, 332, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar]
- Lopez Molina, M.A.; Jansen, K.; Drews, C.; Pinheiro, R.; Silva, R.; Souza, L. Major depressive disorder symptoms in male and female young adults. Psychol. Health Med. 2014, 19, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Pollak, Y.; Yirmiya, R. Cytokine-induced changes in mood and behaviour: Implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int. J. Neuropsychopharmacol. 2002, 5, 389–399. [Google Scholar] [CrossRef]
- Vogelzangs, N.; de Jonge, P.; Smit, J.H.; Bahn, S.; Penninx, B.W. Cytokine production capacity in depression and anxiety. Transl. Psychiatry 2016, 6, e825. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Ormstad, H.; Aass, H.C.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kucma, M.; Styczen, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Lipid peroxidation and immune biomarkers are associated with major depression and its phenotypes, including treatment-resistant depression and melancholia. Neurotox. Res. 2018, 33, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total | Male | Female | ||||
|---|---|---|---|---|---|---|
| HC | MDD | HC | MDD | HC | MDD | |
| Demographic information | ||||||
| Number (n) | 50 | 50 | 24 | 23 | 26 | 27 |
| Age (SD, year) | 24.80 ± 3.22 | 24.18 ± 3.74 | 24.17 ± 2.24 | 24.52 ± 2.86 | 25.52 ± 3.86 | 24.19 ± 3.69 |
| BMI (SD) | 21.83 ± 4.77 | 22.68 ± 4.04 | 22.60 ± 4.87 | 24.03 ± 3.78 | 20.89 ± 5.01 | 23.01 ± 4.28 |
| Smoking (%) | 7 (18%) | 17 (34%) | 7 (26%) | 12 (52%) | 0 (0%) | 5 (19%) |
| Non-smoking (%) | 43 (82%) | 33 (66%) | 17 (74%) | 11 (48%) | 26 (100%) | 22 (81%) |
| Consumption of Alcohol (%, three times a week) | 2 (4%) | 8 (16%) | 1 (4%) | 4 (17%) | 1 (4%) | 4 (15%) |
| Non-frequent consumption (%, under two times a week) | 48 (96%) | 42 (84%) | 23 (96%) | 20 (83%) | 25 (96%) | 23 (85%) |
| Education (SD, years) | 15.60 ± 1.61 | 14.90 ± 1.55 | 15.67 ± 2.04 | 14.83 ± 1.83 | 15.62 ± 1.19 | 15.00 ± 1.31 |
| Clinical information | ||||||
| Age of onset (SD, year) | NA | 19.76 ± 5.67 | NA | 20 ± 4.12 | NA | 18.91 ± 6.09 |
| Number of episodes (SD) | NA | 2.43 ± 1.08 | NA | 2.39 ± 1.09 | NA | 2.48 ± 1.12 |
| BDI | 1.54 ± 1.64 | 26.44 ± 8.91 **** | 55.35 ± 1.51 | 26.84 ± 1.29 **** | 1.81 ± 1.74 | 25.85 ± 9.57 **** |
| STAI-X-1 | 31.73 ± 5.91 | 56.84 ± 8.87 **** | 31.73 ± 5.79 | 57.24 ± 1.38 **** | 31.74 ± 6.18 | 56.09 ± 8.77 **** |
| HAMD-17 | NA | 23.32 ± 6.94 | NA | 23.16 ± 1.04 | NA | 23.22 ± 8.35 |
| BIS | 55.40 ± 9.60 | 70.68 ± 12.04 **** | 55.35 ± 7.48 | 69.20 ± 10.34 **** | 55.44 ± 11.17 | 71.78 ± 13.24 **** |
| RSE | 34.16 ± 4.20 | 20.00 ± 5.47 **** | 34.94 ± 4.01 | 19.40 ± 6.21 **** | 33.42 ± 4.35 | 20.44 ± 4.93 **** |
| BHS | 1.16 ± 1.54 | 12.53 ±5.54 **** | 1.17 ± 1.58 | 13.75 ± 5.34 **** | 1.16 ± 1.54 | 11.63 ± 5.60 **** |
| KSI | 0.57 ± 2.06 | 32.41 ±25.03 **** | 0.67 ± 2.83 | 35.33 ± 28.79 **** | 0.47 ± 0.96 | 30.41 ± 22.61 **** |
| RAS | 49.65 ± 5.71 | 32.62 ± 7.54 **** | 50.61 ± 4.98 | 33.93 ± 7.30 **** | 48.74 ± 3.62 | 31.73 ± 7.73 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, J.-H.; Chang, K.-A. Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naïve Patients with Major Depression in Young Adulthood. Biomedicines 2021, 9, 708. https://doi.org/10.3390/biomedicines9070708
Kim J, Kim J-H, Chang K-A. Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naïve Patients with Major Depression in Young Adulthood. Biomedicines. 2021; 9(7):708. https://doi.org/10.3390/biomedicines9070708
Chicago/Turabian StyleKim, Jinho, Jong-Hoon Kim, and Keun-A Chang. 2021. "Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naïve Patients with Major Depression in Young Adulthood" Biomedicines 9, no. 7: 708. https://doi.org/10.3390/biomedicines9070708
APA StyleKim, J., Kim, J.-H., & Chang, K.-A. (2021). Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naïve Patients with Major Depression in Young Adulthood. Biomedicines, 9(7), 708. https://doi.org/10.3390/biomedicines9070708







