Possible Association of Cholesterol as a Biomarker in Suicide Behavior
Abstract
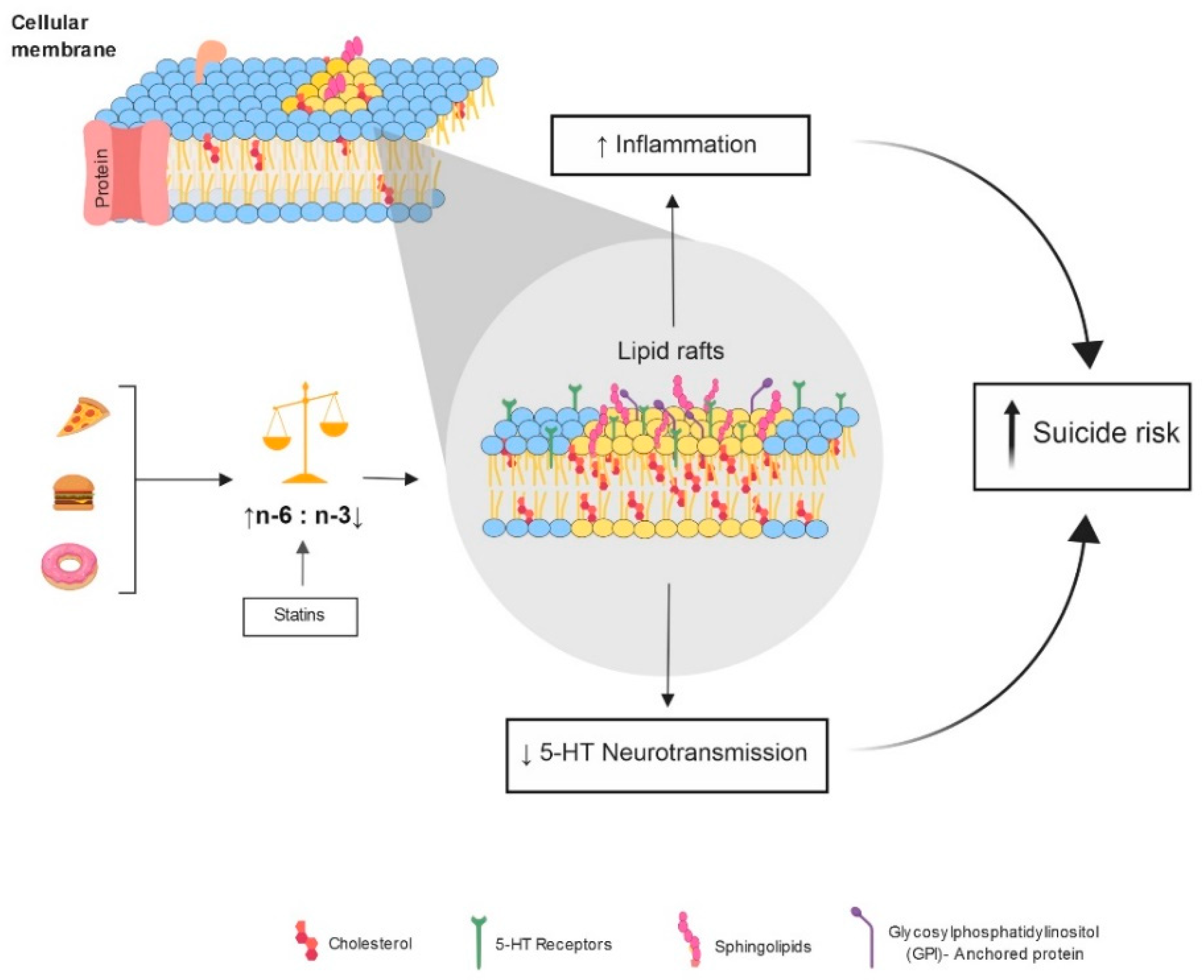
:1. Introduction
Cholesterol in CNS
2. Association between Cholesterol and Suicidality
3. Proposed Models Connecting Cholesterol Reduction with Suicide
3.1. Neuroinflammation
3.2. Serotonin
4. Genetics of Cholesterol Regulation and Suicide
5. Pharmacological Treatments That Alter Lipid Metabolism and the Possible Participation in Suicidal Behavior
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klonsky, E.D.; May, A.M.; Saffer, B.Y. Suicide, suicide attempts, and suicidal ideation. Annu. Rev. Clin. Psychol. 2016, 12, 307–330. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Preventing Suicide: A Global Imperative. 2012. Available online: http://www.who.int/mental_health/suicide-prevention/es (accessed on 3 May 2021).
- Aguglia, A.; Solano, P.; Parisi, V.M.; Asaro, P.; Caprino, M.; Trabucco, A.; Amerio, A.; Amore, M.; Serafini, G. Predictors of relapse in high lethality suicide attempters: A six-month prospective study. J. Affect. Disord. 2020, 271, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.W.; Halford, R.W.; Ramirez, D.M.; Shah, R.; Kotti, T. Cholesterol 24-hydroxylase: An enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 2009, 78, 1017–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, N.; Sinha, V.K. A study of serum lipid profile and serum apolipoproteins A1 and B in Indian male violent criminal offenders. Crim. Behav. Ment. Health 2006, 16, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; Kim, T.W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, E.; Mechawar, N.; Turecki, G. Cholesterol and phospholipids in frontal cortex and synaptosomes of suicide completers: Relationship with endosomal lipid trafficking genes. J. Psychiatr. Res. 2013, 47, 272–279. [Google Scholar] [CrossRef] [PubMed]
- da Graça Cantarelli, M.; Tramontina, A.C.; Leite, M.C.; Gonçalves, C.A. Potential neurochemical links between cholesterol and suicidal behavior. Psychiatry Res. 2014, 220, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, E.; Chen, G.G.; Cruceanu, C.; Mechawar, N.; Turecki, G. Analysis of oxysterols and cholesterol in prefrontal cortex of suicides. Int. J. Neuropsychopharmacol. 2013, 16, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Ding, Y.; Wu, F.; Xie, G.; Hou, J.; Mao, P. Serum lipid levels and suicidality: A meta-analysis of 65 epidemiological studies. J. Psychiatry Neurosci. 2016, 41, 56–69. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, X.; Sun, Q.; Zou, R.; Li, Z.; Liu, S. Association between serum lipid concentrations and attempted suicide in patients with major depressive disorder: A meta-analysis. PLoS ONE 2020, 15, e0243847. [Google Scholar] [CrossRef] [PubMed]
- Suneson, K.; Asp, M.; Träskman-Bendz, L.; Westrin, Å.; Ambrus, L.; Lindqvist, D. Low total cholesterol and low-density lipoprotein associated with aggression and hostility in recent suicide attempters. Psychiatry Res. 2019, 273, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Lester, D. Serum cholesterol levels and suicide: A meta-analysis. Suicide Life Threat. Behav. 2002, 32, 333–346. [Google Scholar] [CrossRef]
- Troisi, A. Cholesterol in coronary heart disease and psychiatric disorders: Same or opposite effects on morbidity risk? Neurosci. Biobehav. Rev. 2009, 33, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakostas, G.I.; Ongür, D.; Iosifescu, D.V.; Mischoulon, D.; Fava, M. Cholesterol in mood and anxiety disorders: Review of the literature and new hypotheses. Eur. Neuropsychopharmacol. 2004, 14, 135–142. [Google Scholar] [CrossRef]
- Golomb, B.A. Cholesterol and violence: Is there a connection? Ann. Intern. Med. 1998, 128, 478–487. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Manuck, S.B.; Matthews, K.A. Lowering cholesterol concentrations and mortality: A quantitative review of primary prevention trials. BMJ 1990, 301, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Peto, R.; Collins, R.; MacMahon, S.; Lu, J.; Li, W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ 1991, 303, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysokiński, A.; Strzelecki, D.; Kłoszewska, I. Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab. Syndr. 2015, 9, 168–176. [Google Scholar] [CrossRef]
- Aguglia, A.; Solano, P.; Giacomini, G.; Caprino, M.; Conigliaro, C.; Romano, M.; Aguglia, E.; Serafini, G.; Amore, M. The association between dyslipidemia and lethality of suicide attempts: A case-control study. Front. Psychiatry 2019, 10, 70. [Google Scholar] [CrossRef]
- Alvarez, J.C.; Cremniter, D.; Gluck, N.; Quintin, P.; Leboyer, M.; Berlin, I.; Therond, P.; Spreux-Varoquaux, O. Low serum cholesterol in violent but not in non-violent suicide attempters. Psychiatry Res. 2000, 95, 103–108. [Google Scholar] [CrossRef]
- Atmaca, M.; Kuloglu, M.; Tezcan, E.; Ustundag, B. Serum leptin and cholesterol values in violent and non-violent suicide attempters. Psychiatry Res. 2008, 158, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Mufti, R.M.; Balon, R.; Arfken, C.L. Low cholesterol and violence. Psychiatr. Serv. 1998, 49, 221–224. [Google Scholar] [CrossRef]
- Steegmans, P.H.; Fekkes, D.; Hoes, A.W.; Bak, A.A.; van der Does, E.; Grobbee, D.E. Low serum cholesterol concentration and serotonin metabolism in men. BMJ 1996, 312, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kułak-Bejda, A.; Bejda, G.; Lech, M.; Waszkiewicz, N. Are lipids possible markers of suicide behaviors? J. Clin. Med. 2021, 10, 333. [Google Scholar] [CrossRef]
- Bartoli, F.; Di Brita, C.; Crocamo, C.; Clerici, M.; Carrà, G. Lipid profile and suicide attempt in bipolar disorder: A meta-analysis of published and unpublished data. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 90–95. [Google Scholar] [CrossRef]
- Park, S.; Yi, K.K.; Na, R.; Lim, A.; Hong, J.P. No association between serum cholesterol and death by suicide in patients with schizophrenia, bipolar affective disorder, or major depressive disorder. Behav. Brain Funct. 2013, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Koponen, H.; Kautiainen, H.; Leppänen, E.; Mäntyselkä, P.; Vanhala, M. Association between suicidal behaviour and impaired glucose metabolism in depressive disorders. BMC Psychiatry 2015, 15, 163. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.H.; Kang, E.S.; Fava, M.; Mischoulon, D.; Nierenberg, A.A.; Yu, B.H.; Lee, D.; Jeon, H.J. Serum lipids, recent suicide attempt and recent suicide status in patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 113–118. [Google Scholar] [CrossRef]
- Sankaranarayanan, A.; Pratt, R.; Anoop, A.; Smith, A.; Espinoza, D.; Ramachandran, P.; Tirupati, S. Serum lipids and suicidal risk among patients with schizophrenia spectrum disorders: Systematic review and meta-analysis. Acta Psychiatr. Scand. 2021, 144, 125–152. [Google Scholar] [CrossRef]
- Jones, B.D.M.; Farooqui, S.; Kloiber, S.; Husain, M.O.; Mulsant, B.H.; Husain, M.I. Targeting metabolic dysfunction for the treatment of mood disorders: Review of the evidence. Life 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Blassberg, R.; Macrae, J.I.; Briscoe, J.; Jacob, J. Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum. Mol. Genet. 2016, 25, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Stransky, A.; Tierney, E. Cognitive and behavioral aspects of Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160c, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, A.; Mensi, R.; Mhalla, A.; Hallara, I.; Neffati, F.; Douki, W.; Najjar, M.F.; Gaha, L. Dyslipidemia and suicidal risk in patients with psychiatric disorders. Encephale 2018, 44, 315–320. [Google Scholar] [CrossRef]
- Sublette, M.E. Lipids and suicide risk. Curr. Top. Behav. Neurosci. 2020, 46, 155–177. [Google Scholar]
- Engström, K.; Saldeen, A.S.; Yang, B.; Mehta, J.L.; Saldeen, T. Effect of fish oils containing different amounts of EPA, DHA, and antioxidants on plasma and brain fatty acids and brain nitric oxide synthase activity in rats. Upsala J. Med. Sci. 2009, 114, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bilici, M.; Efe, H.; Köroğlu, M.A.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef]
- Kotan, V.O.; Sarandol, E.; Kirhan, E.; Ozkaya, G.; Kirli, S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: A 24-week follow-up study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1284–1290. [Google Scholar] [CrossRef]
- Daray, F.M.; Mann, J.J.; Sublette, M.E. How lipids may affect risk for suicidal behavior. J. Psychiatr. Res. 2018, 104, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Aguglia, A.; Giacomini, G.; Montagna, E.; Amerio, A.; Escelsior, A.; Capello, M.; Cutroneo, L.; Ferretti, G.; Scafidi, D.; Costanza, A.; et al. Meteorological variables and suicidal behavior: Air pollution and apparent temperature are associated with high-lethality suicide attempts and male gender. Front. Psychiatry 2021, 12, 653390. [Google Scholar] [CrossRef]
- De Berardis, D.; Marini, S.; Piersanti, M.; Cavuto, M.; Perna, G.; Valchera, A.; Mazza, M.; Fornaro, M.; Iasevoli, F.; Martinotti, G.; et al. The relationships between cholesterol and suicide: An update. ISRN Psychiatry 2012, 2012, 387901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.H.; Kim, Y.K. Potential peripheral biological predictors of suicidal behavior in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Kodas, E.; Vancassel, S.; Lejeune, B.; Guilloteau, D.; Chalon, S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: Critical role of developmental stage. J. Lipid Res. 2002, 43, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, V.V.; Rao, K.V.; Sastry, P.S. Regulation of diacylglycerol kinase in rat brain membranes by docosahexaenoic acid. Neurosci. Lett. 1994, 179, 171–174. [Google Scholar] [CrossRef]
- Czysz, A.H.; Rasenick, M.M. G-protein signaling, lipid rafts and the possible sites of action for the antidepressant effects of n-3 polyunsaturated fatty acids. CNS Neurol. Disord. Drug Targets 2013, 12, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Birger, M.; Swartz, M.; Cohen, D.; Alesh, Y.; Grishpan, C.; Kotelr, M. Aggression: The testosterone-serotonin link. Isreal Med. Assoc. J. 2003, 5, 653–658. [Google Scholar]
- Pompili, M.; Serafini, G.; Innamorati, M.; Möller-Leimkühler, A.M.; Giupponi, G.; Girardi, P.; Tatarelli, R.; Lester, D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 583–600. [Google Scholar] [CrossRef]
- Lalovic, A.; Sequeira, A.; DeGuzman, R.; Chawky, N.; Lesage, A.; Seguin, M.; Turecki, G. Investigation of completed suicide and genes involved in cholesterol metabolism. J. Affect. Disord. 2004, 79, 25–32. [Google Scholar] [CrossRef]
- Asellus, P.; Nordström, P.; Nordström, A.L.; Jokinen, J. CSF apolipoprotein E in attempted suicide. J. Affect. Disord. 2018, 225, 246–249. [Google Scholar] [CrossRef]
- Asellus, P.; Nordström, P.; Nordström, A.L.; Jokinen, J. Plasma apolipoprotein E and severity of suicidal behaviour. J. Affect. Disord. 2016, 190, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Mahley, R.W. Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón-Garcidueñas, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤40 years of age. Environ. Res. 2018, 164, 475–487. [Google Scholar] [CrossRef]
- Willour, V.L.; Seifuddin, F.; Mahon, P.B.; Jancic, D.; Pirooznia, M.; Steele, J.; Schweizer, B.; Goes, F.S.; Mondimore, F.M.; Mackinnon, D.F.; et al. A genome-wide association study of attempted suicide. Mol. Psychiatry 2012, 17, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yoshikawa, A.; Meltzer, H.Y. Replication of rs300774, a genetic biomarker near ACP1, associated with suicide attempts in patients with schizophrenia: Relation to brain cholesterol biosynthesis. J. Psychiatr. Res. 2017, 94, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.B.; Brasil Rocha, P.M.; Neves, F.S.; Miranda, D.M.; Correa, H. ACP1 gene polymorphism associated with suicide attempt type in bipolar disorder patients. Psychiatry Investig. 2017, 14, 909–910. [Google Scholar] [CrossRef] [Green Version]
- Anaya Chavez, Y.; Martinez, B. Genome-wide association studies (gwas) and its contribution to the genetic of asthma. Rev. Salud Uninorte 2010, 26, 223–231. [Google Scholar]
- Erlangsen, A.; Appadurai, V.; Wang, Y.; Turecki, G.; Mors, O.; Werge, T.; Mortensen, P.B.; Starnawska, A.; Børglum, A.D.; Schork, A.; et al. Genetics of suicide attempts in individuals with and without mental disorders: A population-based genome-wide association study. Mol. Psychiatry 2020, 25, 2410–2421. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Zhu, W.; Ding, C.; Dai, W.; Su, X.; Dai, W.; Xiao, J.; Xing, Z.; Huang, X. Effects of olanzapine treatment on lipid profiles in patients with schizophrenia: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 17028. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhao, X.M.; Deng, C.; Zeng, Y.P.; Hu, C.H. Simvastatin improves olanzapine-induced dyslipidemia in rats through inhibiting hepatic mTOR signaling pathway. Acta Pharmacol. Sin. 2019, 40, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Marín-Medina, A.; Ruíz-Hidalgo, G.; Blé-Castillo, J.L.; Zetina-Esquivel, A.M.; Zamora, R.M.; Juárez-Rojop, I.E.; Díaz-Zagoya, J.C. Combined effect of diosgenin along with ezetimibe or atorvastatin on the fate of labelled bile acid and cholesterol in hypercholesterolemic rats. Int. J. Environ. Res. Public Health 2019, 16, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, K.M.; Kaplan, B.J. Lipophilic statin use and suicidal ideation in a sample of adults with mood disorders. Crisis 2014, 35, 278–282. [Google Scholar] [CrossRef]
- Harro, J. Animals, anxiety, and anxiety disorders: How to measure anxiety in rodents and why. Behav. Brain Res. 2018, 352, 81–93. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Castro, T.B.; Genis-Mendoza, A.D.; León-Escalante, D.I.; Hernández-Díaz, Y.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; López-Narváez, M.L.; Marín-Medina, A.; Nicolini, H.; Castillo-Avila, R.G.; et al. Possible Association of Cholesterol as a Biomarker in Suicide Behavior. Biomedicines 2021, 9, 1559. https://doi.org/10.3390/biomedicines9111559
González-Castro TB, Genis-Mendoza AD, León-Escalante DI, Hernández-Díaz Y, Juárez-Rojop IE, Tovilla-Zárate CA, López-Narváez ML, Marín-Medina A, Nicolini H, Castillo-Avila RG, et al. Possible Association of Cholesterol as a Biomarker in Suicide Behavior. Biomedicines. 2021; 9(11):1559. https://doi.org/10.3390/biomedicines9111559
Chicago/Turabian StyleGonzález-Castro, Thelma Beatriz, Alma Delia Genis-Mendoza, Dulce Ivannia León-Escalante, Yazmín Hernández-Díaz, Isela Esther Juárez-Rojop, Carlos Alfonso Tovilla-Zárate, María Lilia López-Narváez, Alejandro Marín-Medina, Humberto Nicolini, Rosa Giannina Castillo-Avila, and et al. 2021. "Possible Association of Cholesterol as a Biomarker in Suicide Behavior" Biomedicines 9, no. 11: 1559. https://doi.org/10.3390/biomedicines9111559
APA StyleGonzález-Castro, T. B., Genis-Mendoza, A. D., León-Escalante, D. I., Hernández-Díaz, Y., Juárez-Rojop, I. E., Tovilla-Zárate, C. A., López-Narváez, M. L., Marín-Medina, A., Nicolini, H., Castillo-Avila, R. G., & Ramos-Méndez, M. Á. (2021). Possible Association of Cholesterol as a Biomarker in Suicide Behavior. Biomedicines, 9(11), 1559. https://doi.org/10.3390/biomedicines9111559







