Can Optimizing the Mechanical Environment Deliver a Clinically Significant Reduction in Fracture Healing Time?
Abstract
1. Introduction
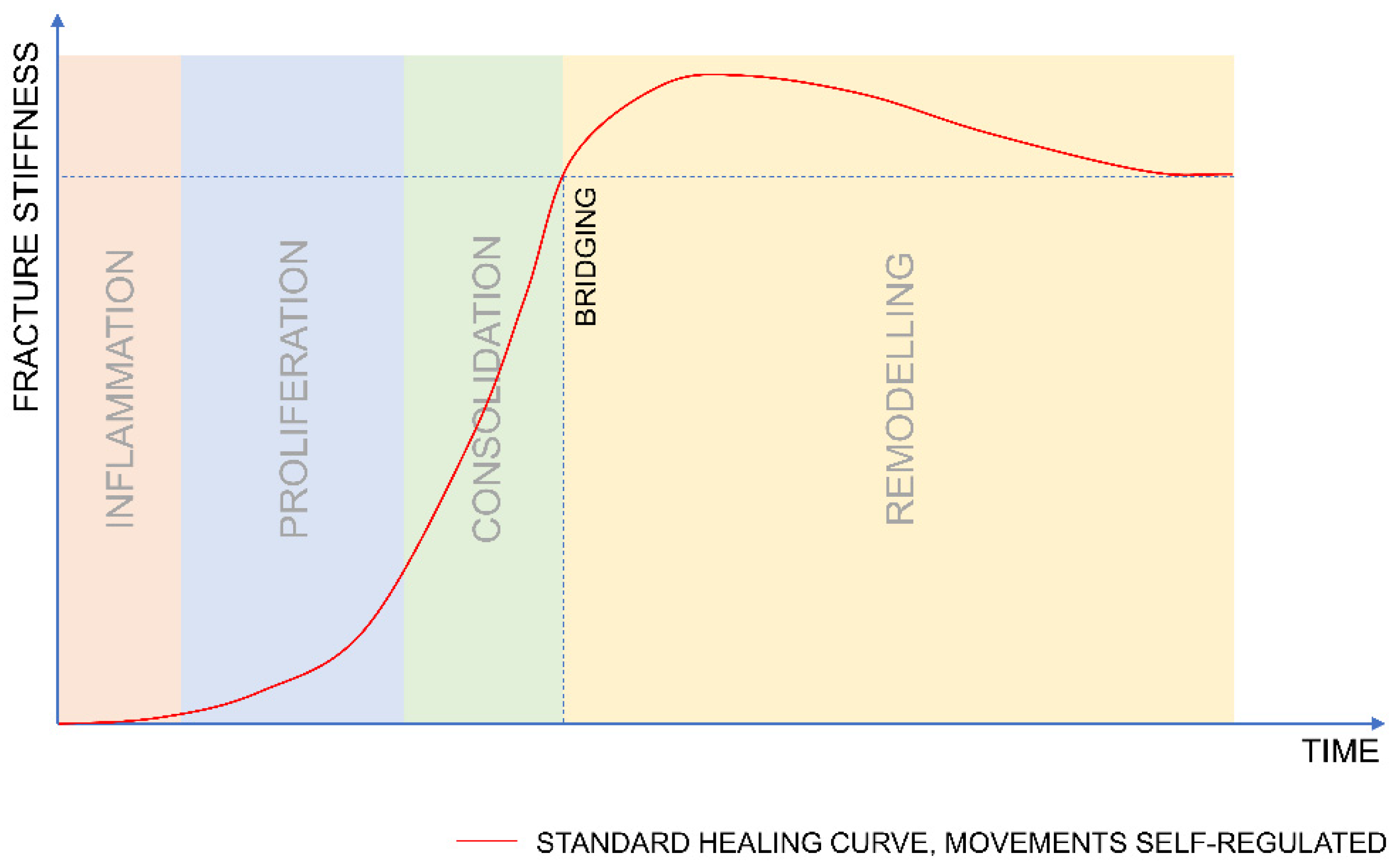
2. The Course of Fracture Healing
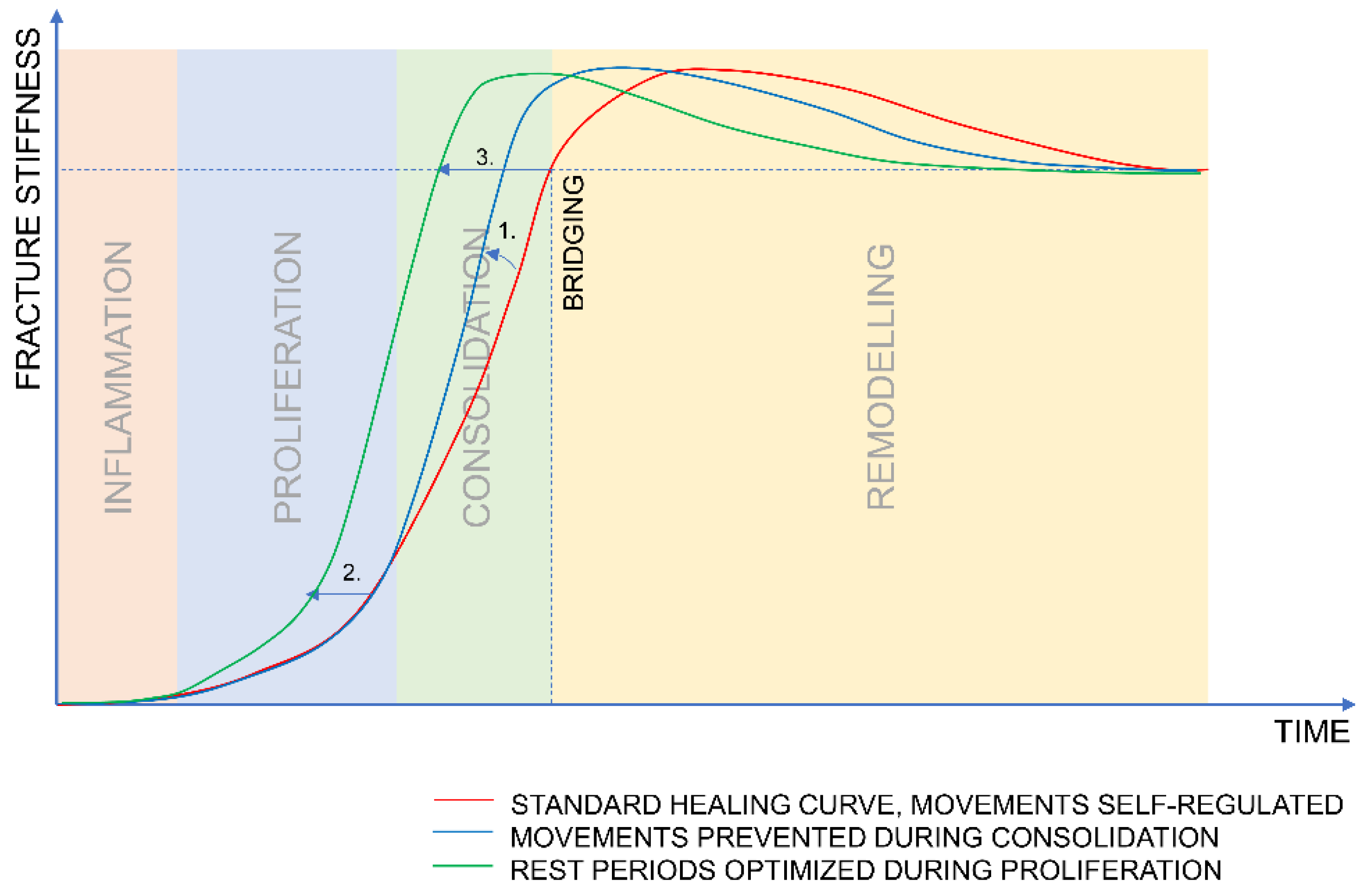
2.1. When Should Mechanical Stimulation Be Applied?
2.1.1. Inflammation
2.1.2. Proliferation
2.1.3. Consolidation
2.1.4. Remodelling
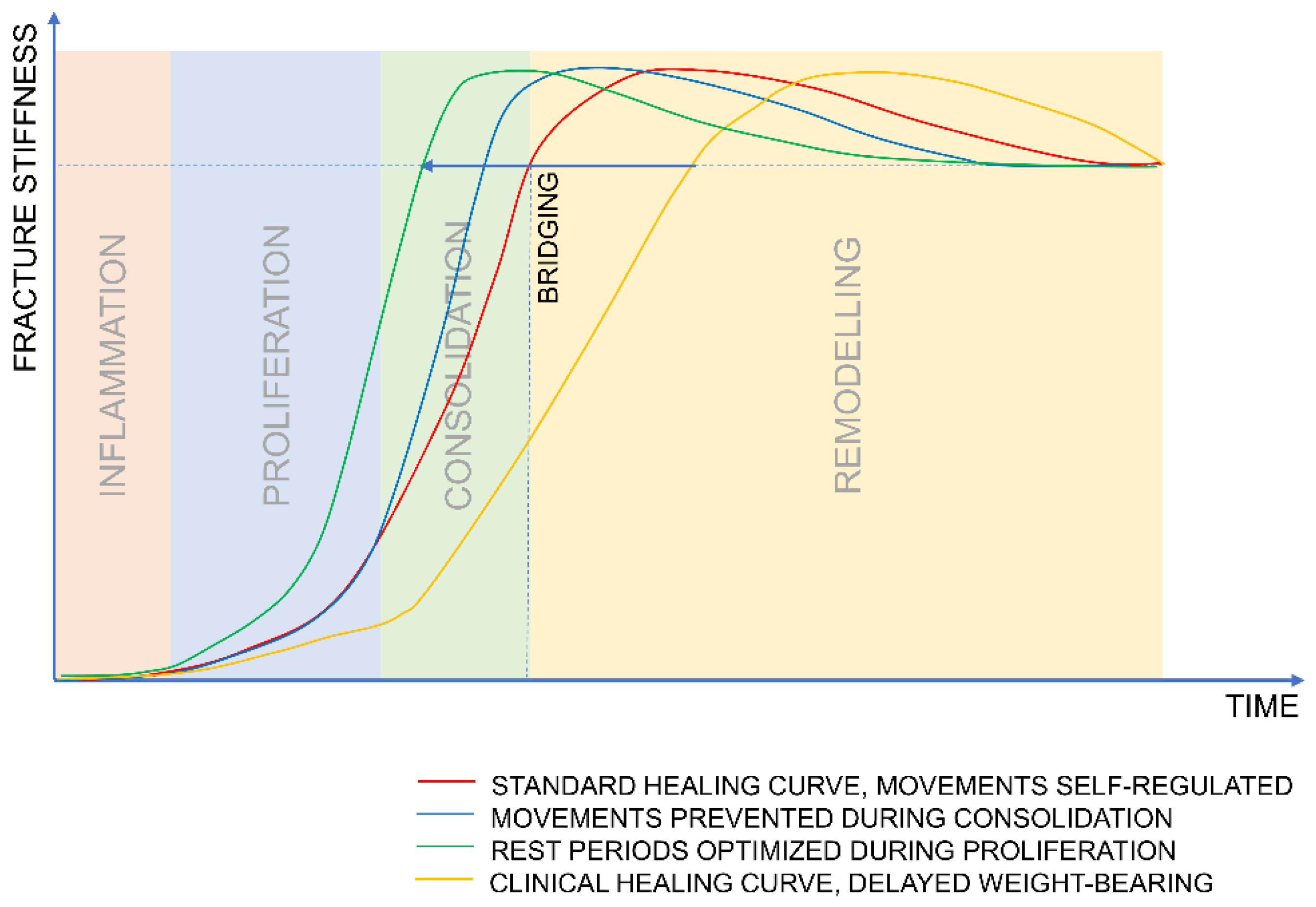
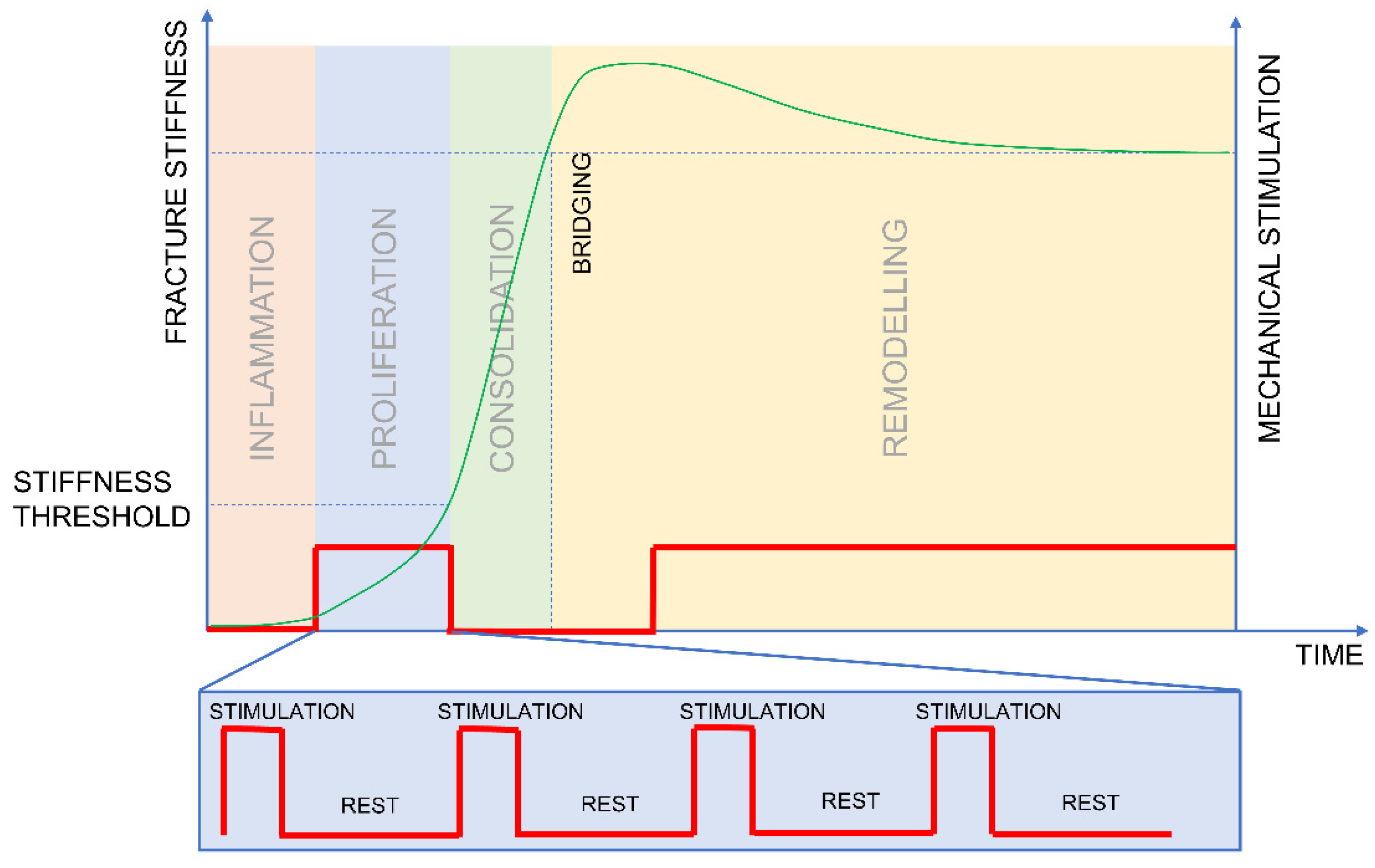
2.2. How Should Stimulation Be Applied?
3. Hypothesis
- The mechanical stimulation should be applied predominantly during the proliferative phase to boost callus formation and should be stopped soon after the deflection of the healing curve to allow for consolidation.
- When stimulation is applied, sufficient resting time should be provided between the stimulatory events to allow for callus formation.
4. Discussion
4.1. What Evidence Contradicts the Hypothesis?
4.2. How Does Clinical Treatment Compare?
4.3. Design of an Experiment
- Group 1—mimics clinical scenario—no stimulation until callus formation is visualized on an X-ray. The stimulation should be applied as batches of 100 cycles applied every hour, yielding 1200 cycles over 12 h. This protocol should mimic a simplified clinical scenario; therefore, no stimulation is applied overnight.
- Group 2—inverse/reverse dynamization group—stimulation applied from the fifth postoperative day until the stiffness reaches the threshold of 750 N/mm. The stimulation should be applied as batches of 100 cycles applied every hour, yielding 1200 cycles over 12 h; no stimulation is applied overnight.
- Group 3—prolonged resting periods—stimulation applied from the fifth postoperative day until the stiffness reaches the threshold of 750 N/mm. The stimulation should be applied once a day in a batch of 1200 cycles.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamagishi, M.; Yoshimura, Y. The biomechanics of fracture healing. JBJS 1955, 37, 1035–1068. [Google Scholar] [CrossRef]
- Claes, L.; Reusch, M.; Göckelmann, M.; Ohnmacht, M.; Wehner, T.; Amling, M.; Beil, F.T.; Ignatius, A. Metaphyseal fracture healing follows similar biomechanical rules as diaphyseal healing. J. Orthop. Res. 2011, 29, 425–432. [Google Scholar] [CrossRef]
- McKibbin, B. The biology of fracture healing in long bones. J. Bone Jt. Surg. Br. Vol. 1978, 60, 150–162. [Google Scholar] [CrossRef]
- Epari, D.R.; Schell, H.; Bail, H.J.; Duda, G.N. Instability prolongs the chondral phase during bone healing in sheep. Bone 2006, 38, 864–870. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Perren, S.M.; Cordey, J. The concept of interfragmentary strain. In Current Concepts of Internal Fixation of Fractures; Uhthoff, H.K., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1980; pp. 63–77. [Google Scholar]
- Claes, L.E.; Heigele, C.A.; Neidlinger-Wilke, C.; Kaspar, D.; Seidl, W.; Margevicius, K.J.; Augat, P. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 1998, 355, S132–S147. [Google Scholar] [CrossRef] [PubMed]
- Windolf, M.; Ernst, M.; Schwyn, R.; Perren, S.M.; Mathis, H.; Wilke, M.R.; Richards, R.G. A Biofeedback System for Continuous Monitoring of Bone Healing. BIODEVICES 2014, 243–248. [Google Scholar] [CrossRef]
- Klein, P.; Schell, H.; Streitparth, F.; Heller, M.; Kassi, J.P.; Kandziora, F.; Bragulla, H.; Haas, N.P.; Duda, G.N. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J. Orthop. Res. 2003, 21, 662–669. [Google Scholar] [CrossRef]
- Claes, L.; Blakytny, R.; Besse, J.; Bausewein, C.; Ignatius, A.; Willie, B. Late dynamization by reduced fixation stiffness enhances fracture healing in a rat femoral osteotomy model. J. Orthop. Trauma 2011, 25, 169–174. [Google Scholar] [CrossRef]
- Claes, L.; Blakytny, R.; Göckelmann, M.; Schoen, M.; Ignatius, A.; Willie, B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J. Orthop. Res. 2009, 27, 22–27. [Google Scholar] [CrossRef]
- Bartnikowski, N.; Claes, L.E.; Koval, L.; Glatt, V.; Bindl, R.; Steck, R.; Ignatius, A.; Schuetz, M.A.; Epari, D.R. Modulation of fixation stiffness from flexible to stiff in a rat model of bone healing. Acta Orthop. 2017, 88, 217–222. [Google Scholar] [CrossRef]
- Goodship, A.; Kenwright, J. The influence of induced micromovement upon the healing of experimental tibial fractures. J. Bone Jt. Surg. Br. Vol. 1985, 67, 650–655. [Google Scholar] [CrossRef]
- Goodship, A.E.; Cunningham, J.L.; Kenwright, J. Strain rate and timing of stimulation in mechanical modulation of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S105–S115. [Google Scholar] [CrossRef]
- Hente, R.; Füchtmeier, B.; Schlegel, U.; Ernstberger, A.; Perren, S. The influence of cyclic compression and distraction on the healing of experimental tibial fractures. J. Orthop. Res. 2004, 22, 709–715. [Google Scholar] [CrossRef]
- Hente, R.; Perren, S. Mechanical Stimulation of Fracture Healing–Stimulation of Callus by Improved Recovery. Acta Chir. Orthop. Traumatol. Cech. 2018, 85, 385–391. [Google Scholar]
- Tufekci, P.; Tavakoli, A.; Dlaska, C.; Neumann, M.; Shanker, M.; Saifzadeh, S.; Steck, R.; Schuetz, M.; Epari, D. Early mechanical stimulation only permits timely bone healing in sheep. J. Orthop. Res. 2018, 36, 1790–1796. [Google Scholar] [CrossRef]
- Wolf, S.; Janousek, A.; Pfeil, J.; Veith, W.; Haas, F.; Duda, G.; Claes, L. The effects of external mechanical stimulation on the healing of diaphyseal osteotomies fixed by flexible external fixation. Clin. Biomech. 1998, 13, 359–364. [Google Scholar] [CrossRef]
- Augat, P.; Burger, J.; Schorlemmer, S.; Henke, T.; Peraus, M.; Claes, L. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J. Orthop. Res. 2003, 21, 1011–1017. [Google Scholar] [CrossRef]
- Augat, P.; Merk, J.; Wolf, S.; Claes, L. Mechanical Stimulation by External Application of Cyclic Tensile Strains Does Not Effectively Enhance Bone Healing. J. Orthop. Trauma 2001, 15, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.E.; Van Rhijn, M.; Tami, I.; Corveleijn, R.; Schneider, E.; Ito, K. Shear Does Not Necessarily Inhibit Bone Healing. Clin. Orthop. Relat. Res. 2006, 443, 307–314. [Google Scholar] [CrossRef]
- Augat, P.; Merk, J.; Ignatius, A.; Margevicius, K.; Bauer, G.; Rosenbaum, D.; Claes, L. Early, full weightbearing with flexible fixation delays fracture healing. Clin. Orthop. Relat. Res. 1996, 328, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Cunningham, J.; Goodship, A.; O’connor, B.; Kenwright, J. Measuring stiffness can define healing of tibial fractures. J. Bone Jt. Surg. Br. Vol. 1994, 76, 389–394. [Google Scholar] [CrossRef]
- Claes, L.; Cunningham, J. Monitoring the mechanical properties of healing bone. Clin. Orthop. Relat. Res. 2009, 467, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Epari, D.; Wehner, T.; Ignatius, A.; Schuetz, M.; Claes, L. A case for optimising fracture healing through inverse dynamization. Med. Hypotheses 2013, 81, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Miller, M.; Ivkovic, A.; Liu, F.; Parry, N.; Griffin, D.; Vrahas, M.; Evans, C. Improved healing of large segmental defects in the rat femur by reverse dynamization in the presence of bone morphogenetic protein-2. J. Bone Jt. Surg. Am. Vol. 2012, 94, 2063. [Google Scholar] [CrossRef]
- Fountain, S.; Windolf, M.; Henkel, J.; Tavakoli, A.; Schuetz, M.A.; Hutmacher, D.W.; Epari, D.R. Monitoring Healing Progression and Characterizing the Mechanical Environment in Preclinical Models for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 47–57. [Google Scholar] [CrossRef]
- Hankemeier, S.; Grässel, S.; Plenz, G.; Spiegel, H.; Bruckner, P.; Probst, A. Alteration of fracture stability influences chondrogenesis, osteogenesis and immigration of macrophages. J. Orthop. Res. 2001, 19, 531–538. [Google Scholar] [CrossRef]
- Glatt, V.; Samchukov, M.; Cherkashin, A.; Iobst, C. Reverse Dynamization Accelerates Bone-Healing in a Large-Animal Osteotomy Model. JBJS 2020, 10, 2106. [Google Scholar]
- Gardner, T.; Evans, M.; Simpson, H. Temporal variation of applied inter fragmentary displacement at a bone fracture in harmony with maturation of the fracture callus. Med. Eng. Phys. 1998, 20, 480–484. [Google Scholar] [CrossRef]
- Willie, B.M.; Blakytny, R.; Glöckelmann, M.; Ignatius, A.; Claes, L. Temporal variation in fixation stiffness affects healing by differential cartilage formation in a rat osteotomy model. Clin. Orthop. Relat. Res. 2011, 469, 3094. [Google Scholar] [CrossRef] [PubMed]
- Barcik, J.; Ernst, M.; Balligand, M.; Dlaska, C.E.; Drenchev, L.; Todorov, S.; Gueorguiev, B.; Skulev, H.; Zeiter, S.; Epari, D.; et al. Continuous Monitoring of Fracture Healing to Analyze Short-Term Response of Bone Repair Tissue to Mechanical Stimulation; EORS European Orthopaedic Research Society: Maastricht, The Netherlands, 2019; FP76-5522. [Google Scholar]
- Bishop, N. Mechanical Stimulation of Bone Healing; Technische Universitaet Hamburg-Harburg; Shaker Verlag: Aachen, Germany, 2007. [Google Scholar]
- Meyers, N.; Schülke, J.; Ignatius, A.; Claes, L. Novel systems for the application of isolated tensile, compressive, and shearing stimulation of distraction callus tissue. PLoS ONE 2017, 12, e0189432. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M. Fracture healing: Fracture healing understood as the result of a fascinating cascade of physical and biological interactions. Part II. Acta Chir. Orthop. Traumatol. Cech. 2015, 82, 13–21. [Google Scholar]
- Müller, C.W.; Pfeifer, R.; Meier, K.; Decker, S.; Reifenrath, J.; Gösling, T.; Wesling, V.; Krettek, C.; Hurschler, C.; Krämer, M. A novel shape memory plate osteosynthesis for noninvasive modulation of fixation stiffness in a rabbit tibia osteotomy model. BioMed Res. Int. 2015, 2015, 652940. [Google Scholar] [CrossRef]
- Gardner, M.J.; Ricciardi, B.F.; Wright, T.M.; Bostrom, M.P.; van der Meulen, M.C. Pause insertions during cyclic in vivo loading affect bone healing. Clin. Orthop. Relat. Res. 2008, 466, 1232–1238. [Google Scholar] [CrossRef][Green Version]
- Potter, B.K. From Bench to Bedside: How stiff is too stiff? Far-cortical locking or dynamic locked plating may obviate the question. Clin. Orthop. Relat. Res. 2016, 474, 1571–1573. [Google Scholar] [CrossRef]
- Bottlang, M.; Doornink, J.; Fitzpatrick, D.C.; Madey, S.M. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J. Bone Jt. Surg. Am. Vol. 2009, 91, 1985. [Google Scholar] [CrossRef]
- O’Loughlin, P.F.; Morr, S.; Bogunovic, L.; Kim, A.D.; Park, B.; Lane, J.M. Selection and development of preclinical models in fracture-healing research. JBJS 2008, 90, 79–84. [Google Scholar] [CrossRef]
- Barcik, J.; Ernst, M.; Dlaska, C.E.; Drenchev, L.; Zeiter, S.; Epari, D.R.; Windolf, M. Programable Active Fixator System for Systematic In Vivo Investigation of Bone Healing Processes. Sensors 2021, 21, 17. [Google Scholar] [CrossRef]
- Goodship, A.E.; Lawes, T.J.; Rubin, C.T. Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: Preliminary evidence of efficacy. J. Orthop. Res. 2009, 27, 922–930. [Google Scholar] [CrossRef]
- Suzuki, T.; Matsuura, Y.; Yamazaki, T.; Akasaka, T.; Ozone, E.; Matsuyama, Y.; Mukai, M.; Ohara, T.; Wakita, H.; Taniguchi, S. Biomechanics of callus in the bone healing process, determined by specimen-specific finite element analysis. Bone 2020, 132, 115212. [Google Scholar] [CrossRef] [PubMed]
- Epari, D.; Duda, G.; Thompson, M. Mechanobiology of bone healing and regeneration: In vivo models. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1543–1553. [Google Scholar] [CrossRef]
- Pivonka, P.; Dunstan, C.R. Role of mathematical modeling in bone fracture healing. BoneKEy Rep. 2012, 1, 221. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Lacroix, D.; Prendergast, P. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Garcia-Aznar, J.; Kuiper, J.; Doblaré, M. Influence of fracture gap size on the pattern of long bone healing: A computational study. J. Theor. Biol. 2005, 235, 105–119. [Google Scholar] [CrossRef]
- Miramini, S.; Zhang, L.; Richardson, M.; Pirpiris, M.; Mendis, P.; Oloyede, K.; Edwards, G. Computational simulation of the early stage of bone healing under different configurations of locking compression plates. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 900–913. [Google Scholar] [CrossRef]
- Wehner, T.; Steiner, M.; Ignatius, A.; Claes, L. Prediction of the time course of callus stiffness as a function of mechanical parameters in experimental rat fracture healing studies-a numerical study. PLoS ONE 2014, 9, e115695. [Google Scholar] [CrossRef][Green Version]
- Steiner, M.; Claes, L.; Ignatius, A.; Simon, U.; Wehner, T. Numerical simulation of callus healing for optimization of fracture fixation stiffness. PLoS ONE 2014, 9, e101370. [Google Scholar] [CrossRef]
- Fu, R.; Feng, Y.; Liu, Y.; Willie, B.M.; Yang, H. The combined effects of dynamization time and degree on bone healing. J. Orthop. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M. Evolution of internal fixation of long bone fractures: The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84-B, 1093–1110. [Google Scholar] [CrossRef]
- Burny, F.; Donkerwolcke, M.; Moulart, F.; Bourgois, R.; Puers, R.; Van Schuylenbergh, K.; Barbosa, M.; Paiva, O.; Rodes, F.; Bégueret, J.B.; et al. Concept, design and fabrication of smart orthopedic implants. Med. Eng. Phys. 2000, 22, 469–479. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcik, J.; Epari, D.R. Can Optimizing the Mechanical Environment Deliver a Clinically Significant Reduction in Fracture Healing Time? Biomedicines 2021, 9, 691. https://doi.org/10.3390/biomedicines9060691
Barcik J, Epari DR. Can Optimizing the Mechanical Environment Deliver a Clinically Significant Reduction in Fracture Healing Time? Biomedicines. 2021; 9(6):691. https://doi.org/10.3390/biomedicines9060691
Chicago/Turabian StyleBarcik, Jan, and Devakara R. Epari. 2021. "Can Optimizing the Mechanical Environment Deliver a Clinically Significant Reduction in Fracture Healing Time?" Biomedicines 9, no. 6: 691. https://doi.org/10.3390/biomedicines9060691
APA StyleBarcik, J., & Epari, D. R. (2021). Can Optimizing the Mechanical Environment Deliver a Clinically Significant Reduction in Fracture Healing Time? Biomedicines, 9(6), 691. https://doi.org/10.3390/biomedicines9060691





